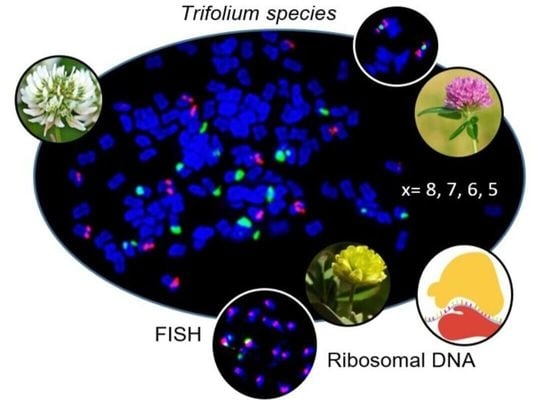
Variation in Ribosomal DNA in the Genus Trifolium (Fabaceae)
Abstract
:1. Introduction
2. Results
2.1. rDNA Localization on Chromosomes and Range of Variation in Trifolium
2.2. Reconstruction of the Ancestral Karyotype in Trifolium
2.3. ITS1 Sequence Variability
3. Discussion
3.1. rDNA Localization on Chromosomes and Range of Variation in Trifolium
3.2. Cytological and Sequence Diversity
4. Materials and Methods
4.1. Plant Material and Chromosome Preparation
4.2. Probe DNA Isolation and Labelling
4.3. Fluorescence In Situ Hybridization
4.4. Phylogenetic Tree Construction
4.5. Identification of Specific Polymorphisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zohary, M.; Heller, D. The Genus Trifolium; Publications of the Israel Academy of Sciences and Humanities. Section of Sciences; The Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1984. [Google Scholar]
- Gillett, J.M.; Taylor, N.L.; Collins, M. The World of Clovers, 1st ed.; Iowa State University Press: Ames, IA, USA, 2001. [Google Scholar]
- Ellison, N.W.; Liston, A.; Steiner, J.J.; Williams, W.M.; Taylor, N.L. Molecular phylogenetics of the clover genus (Trifolium-Leguminosae). Mol. Phylogenet. Evol. 2006, 39, 688–705. [Google Scholar] [CrossRef]
- Nosrati, H.; Feizi, M.H.; Razban-Haghighi, A.; Seyet-Tarrah, S. Impact of life history on genetic variation in Trifolium (Fabaceae) estimated by ISSR. Environ. Exp. Biol. 2015, 13, 83–88. [Google Scholar]
- Sprent, J.I. Nodulation in Legumes; Royal Botanic Gardens: Kew, UK, 2001. [Google Scholar]
- Taylor, N.L.; Quesenberry, K.H.; Anderson, M.K. Genetic system relationships in Trifolium. Econ. Bot. 1979, 33, 431–441. [Google Scholar] [CrossRef]
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for science and breeding. Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef] [Green Version]
- Panitsa, M.; Trigas, P.; Iatrou, G.; Sfenthourakis, S. Factors effecting plant species richness and endemism on land-bridge islands—An example from the East Aegean archipelago. Acta Oecol. 2010, 36, 431–437. [Google Scholar] [CrossRef]
- Scoppola, A.; Tirado, J.L.; Gutiérrez, F.M.; Magrini, S. The genus Trifolium (Fabaceae) in south Europe: A critical review on species richness and distribution. Nord. J. Bot. 2018, 36, njb-01723. [Google Scholar] [CrossRef]
- Falistocco, E.; Marconi, G.; Falcinelli, M. Comparative cytogenetic study on Trifolium subterraneum (2n = 16) and Trifolium israeliticum (2n = 12). Genome 2013, 56, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Vižintin, L.; Javornik, B.; Bohanec, B. Genetic characterization of selected Trifolium species as revealed by nuclear DNA content and ITS rDNA region analysis. Plant Sci. 2006, 170, 859–866. [Google Scholar] [CrossRef]
- Goldblatt, P. Cytology and Phylogeny of Leguminosae. In Advances in Legume Systematics, Part 2; Polhill, R.M., Raven, P.H., Eds.; Royal Botanic Gardens: Kew, UK, 1981; pp. 427–463. [Google Scholar]
- Cleveland, R.W. Reproductive Cycle and Cytogenetics. In Agronomy Monographs; Taylor, N.L., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1985; pp. 71–110. [Google Scholar]
- Plant DNA C-Value Database. Available online: https://cvalues.science.kew.org/search/angiosperm (accessed on 10 May 2021).
- Ištvánek, J.; Jaroš, M.; Křenek, A.; Řepková, J. Genome assembly and annotation for red clover (Trifolium pratense; Fabaceae). Am. J. Bot. 2014, 101, 327–337. [Google Scholar] [CrossRef]
- De Vega, J.J.; Ayling, S.; Hegarty, M.; Kudrna, D.; Goicoechea, J.L.; Ergon, Å.; Rognli, O.A.; Jones, C.; Swain, M.; Geurts, R.; et al. Red clover (Trifolium pratense L.) draft genome provides a platform for trait improvement. Sci. Rep. 2015, 5, 17394. [Google Scholar] [CrossRef]
- Griffiths, A.G.; Moraga, R.; Tausen, M.; Gupta, V.; Bilton, T.P.; Campbell, M.A.; Ashby, R.; Nagy, I.; Khan, A.; Larking, A.; et al. Breaking Free: The genomics of allopolyploidy-facilitated niche expansion in white clover. Plant Cell 2019, 31, 1466–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dluhošová, J.; Ištvánek, J.; Nedělník, J.; Řepková, J. Red clover (Trifolium pratense) and zigzag clover (T. medium)—A picture of genomic similarities and differences. Front. Plant. Sci. 2018, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Kaur, P.; Shirasawa, K.; Nichols, P.; Nagano, S.; Appels, R.; Erskine, W.; Isobe, S.N. Draft genome sequence of subterranean clover, a reference for genus Trifolium. Sci. Rep. 2016, 6, 30358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Bayer, P.E.; Milec, Z.; Vrána, J.; Yuan, Y.; Appels, R.; Edwards, D.; Batley, J.; Nichols, P.; Erskine, W.; et al. An advanced reference genome of Trifolium subterraneum L. reveals genes related to agronomic performance. Plant Biotechnol. J. 2017, 15, 1034–1046. [Google Scholar] [CrossRef] [Green Version]
- Sveinsson, S.; Cronk, Q. Evolutionary origin of highly repetitive plastid genomes within the clover genus (Trifolium). BMC Evol. Biol. 2014, 14, 228. [Google Scholar] [CrossRef] [Green Version]
- Biscotti, M.A.; Olmo, E.; Heslop-Harrison, J.S. Repetitive DNA in eukaryotic genomes. Chromosome Res. 2015, 23, 415–420. [Google Scholar] [CrossRef]
- Sastri, D.C.; Hilu, K.; Appels, R.; Lagudah, E.S.; Playford, J.; Baum, B.R. An overview of evolution in plant 5S DNA. Plant Syst. Evol. 1992, 183, 169–181. [Google Scholar] [CrossRef]
- Garcia, S.; Garnatje, T.; Kovařík, A. Plant rDNA database: Ribosomal DNA loci information goes online. Chromosoma 2012, 121, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Roa, F.; Guerra, M. Distribution of 45S rDNA sites in chromosomes of plants: Structural and evolutionary implications. BMC Evol. Biol. 2012, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roa, F.; Guerra, M. Non-Random Distribution of 5S rDNA sites and its association with 45S rDNA in plant chromosomes. Cytogenet. Genome Res. 2015, 146, 243–249. [Google Scholar] [CrossRef]
- Sato, S.; Isobe, S.; Asamizu, E.; Ohmido, N.; Kataoka, R.; Nakamura, Y.; Kaneko, T.; Sakurai, N.; Okumura, K.; Klimenko, I.; et al. Comprehensive structural analysis of the genome of red clover (Trifolium pratense L.). DNA Res. 2005, 12, 301–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulikova, O.; Gualtieri, G.; Geurts, R.; Kim, D.J.; Cook, D.; Huguet, T.; de Jong, J.H.; Fransz, P.F.; Bisseling, T. Integration of the FISH pachytene and genetic maps of Medicago truncatula. Plant J. 2001, 27, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cerbah, M.; Kevei, Z.; Siljak-Yakovlev, S.; Kondorosi, E.; Kondorosi, A.; Trinh, T.H. FISH chromosome mapping allowing karyotype analysis in Medicago truncatula lines Jemalong J5 and R-108-1. Mol. Plant Microbe. Interact. 1999, 12, 947–950. [Google Scholar] [CrossRef] [Green Version]
- Falistocco, E.; Falcinelli, M. Genomic organization of rDNA loci in natural populations of Medicago truncatula Gaertn. Hereditas 2003, 138, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Schneeweiss, H.; Schneeweiss, G.M.; Stuessy, T.F.; Mabuchi, T.; Park, J.; Jang, C.; Sun, B. Chromosomal stasis in diploids contrasts with genome restructuring in auto- and allopolyploid taxa of Hepatica (Ranunculaceae). New Phytol. 2007, 174, 669–682. [Google Scholar] [CrossRef]
- Jang, T.S.; McCann, J.; Parker, J.S.; Takayama, K.; Hong, S.P.; Schneeweiss, G.M.; Weiss-Schneeweiss, H. rDNA loci evolution in the genus Glechoma (Lamiaceae). PLoS ONE 2016, 11, e0167177. [Google Scholar] [CrossRef] [Green Version]
- Falistocco, E.; Torricelli, R.; Falcinelli, M. Genomic relationships between Medicago murex Willd. and Medicago lesinsii E. Small. investigated by in situ hybridization. Theor. Appl. Genet. 2002, 105, 829–833. [Google Scholar] [CrossRef]
- Ansari, H.A.; Ellison, N.W.; Williams, W.M. Molecular and cytogenetic evidence for an allotetraploid origin of Trifolium dubium (Leguminosae). Chromosoma 2008, 117, 159–167. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, X.M.; Gao, X.F.; Jin, D.P.; Zhang, L.B. ITS Non-concerted evolution and rampant hybridization in the legume genus Lespedeza (Fabaceae). Sci. Rep. 2017, 7, 40057. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, Q.-F.; Dong, W.-S.; Bi, Y.-H.; Zhou, Z.-G. Characterization and physical mapping of nuclear ribosomal RNA (rRNA) genes in the haploid gametophytes of Saccharina japonica (Phaeophyta). J. Appl. Phycol. 2017, 29, 2695–2706. [Google Scholar] [CrossRef] [Green Version]
- Ansari, H. Molecular cytogenetic organization of 5S and 18S-26S rDNA loci in white clover (Trifolium repens L.) and related species. Ann. Bot. 1999, 83, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Dluhošová, J.; Řepková, J.; Jakešová, H.; Nedělník, J. Impact of interspecific hybridization of T. pratense × T. medium and backcrossing on genetic variability of progeny. Czech J. Genet. Plant. 2016, 52, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Garcia, S.; Kovařík, A. Dancing together and separate again: Ribosomal RNA multigene loci: Nomads of the Triticeae genomes. Heredity 2013, 111, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubcovsky, J.; Dvorák, J. Ribosomal RNA multigene loci: Nomads of the Triticeae genomes. Genetics 1995, 140, 1367–1377. [Google Scholar] [CrossRef]
- Rosato, M.; Kovařík, A.; Garilleti, R.; Rosselló, J.A. Conserved organisation of 45S rDNA sites and rDNA gene copy number among major clades of early land plants. PLoS ONE 2016, 11, e0162544. [Google Scholar] [CrossRef]
- She, C.W.; Wei, L.; Jiang, X.H. Molecular cytogenetic characterization and comparison of the two cultivated Canavalia species (Fabaceae). Comp. Cytogenet. 2017, 11, 579–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.P.; Wu, Y.X.; Zhao, H.; Wang, Y.; Lü, X.M.; Wang, J.M.; Xu, Y.; Li, Z.Y.; Han, Y.H. Cytogenetic relationships among Citrullus species in comparison with some genera of the tribe Benincaseae (Cucurbitaceae) as inferred from rDNA distribution patterns. BMC Evol. Biol. 2016, 16, 85. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.D.; Gardner, R.C.; Murray, B.G. Cytological characterization of heterochromatin and rDNA in Pinus tadiata and P. taeda. Plant Syst. Evol. 2000, 223, 71–79. [Google Scholar] [CrossRef]
- Hizume, M.; Shibata, F.; Matsusaki, Y.; Garajova, Z. Chromosome identification and comparative karyotypic analyses of four Pinus species. Theor. Appl. Genet. 2002, 105, 491–497. [Google Scholar] [CrossRef]
- Martínez, J.; Vargas, P.; Luceño, M.; Cuadrado, Á. Evolution of Iris subgenus Xiphium based on chromosome numbers, FISH of nrDNA (5S, 45S) and trnL–trnF sequence analysis. Plant Syst. Evol. 2010, 289, 223–235. [Google Scholar] [CrossRef]
- Mizuochi, H.; Marasek, A.; Okazaki, K. Molecular cloning of Tulipa fosteriana rDNA and subsequent FISH analysis yields cytogenetic organization of 5S rDNA and 45S rDNA in T. gesneriana and T. fosteriana. Euphytica 2007, 155, 235–248. [Google Scholar] [CrossRef]
- Winterfeld, G.; Becher, H.; Voshell, S.; Hilu, K.; Röser, M. Karyotype evolution in Phalaris (Poaceae): The role of reductional dysploidy, polyploidy and chromosome alteration in a wide-spread and diverse genus. PLoS ONE 2018, 13, e0192869. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Imamura, K.; Nagano, K.; Hoshi, Y. Contrasting patterns of the 5S and 45S rDNA evolutions in the Byblis liniflora complex (Byblidaceae). J. Plant Res. 2011, 124, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Olanj, N.; Garnatje, T.; Sonboli, A.; Vallès, J.; Garcia, S. The striking and unexpected cytogenetic diversity of genus Tanacetum L. (Asteraceae): A cytometric and fluorescent in situ hybridisation study of Iranian taxa. BMC Plant Biol. 2015, 15, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vozárová, R.; Herklotz, V.; Kovařík, A.; Tynkevich, Y.O.; Volkov, R.A.; Ritz, C.M.; Lunerová, J. Ancient origin of two 5S rDNA families dominating in the genus Rosa and their behavior in the canina-type meiosis. Front. Plant Sci. 2021, 12, 643548. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Davis, T.M. Conservation and loss of ribosomal RNA gene sites in diploid and polyploid Fragaria (Rosaceae). BMC Plant Biol. 2011, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Garcia, S.; Kovařík, A.; Leitch, A.R.; Garnatje, T. Cytogenetic features of rRNA genes across land plants: Analysis of the plant rDNA database. Plant J. 2017, 89, 1020–1030. [Google Scholar] [CrossRef] [Green Version]
- Weiss-Schneeweiss, H.; Tremetsberger, K.; Schneeweiss, G.M.; Parker, J.S.; Stuessy, T.F. Karyotype diversification and evolution in diploid and polyploid South American Hypochaeris (Asteraceae) inferred from rDNA localization and genetic fingerprint data. Ann. Bot. 2008, 101, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Dadejová, M.; Lim, K.Y.; Soucková-Skalická, K.; Matyášek, R.; Grandbastien, M.; Leitch, A.; Kovařík, A. Transcription activity of rRNA genes correlates with a tendency towards intergenomic homogenization in Nicotiana allotetraploids. New Phytol. 2007, 174, 658–668. [Google Scholar] [CrossRef]
- Rosato, M.; Álvarez, I.; Nieto Feliner, G.; Rosselló, J.A. High and uneven levels of 45S rDNA site-number variation across wild populations of a diploid plant genus (Anacyclus, Asteraceae). PLoS ONE 2017, 12, e0187131. [Google Scholar] [CrossRef] [Green Version]
- Matyášek, R.; Renny-Byfield, S.; Fulneček, J.; Macas, J.; Grandbastien, M.-A.; Nichols, R.; Leitch, A.; Kovařík, A. Next generation sequencing analysis reveals a relationship between rDNA unit diversity and locus number in Nicotiana diploids. BMC Genom. 2012, 13, 722. [Google Scholar] [CrossRef] [Green Version]
- Lunerová, J.; Renny-Byfield, S.; Matyášek, R.; Leitch, A.; Kovařík, A. Concerted evolution rapidly eliminates sequence variation in rDNA coding regions but not in intergenic spacers in Nicotiana tabacum allotetraploid. Plant Syst. Evol. 2017, 303, 1043–1060. [Google Scholar] [CrossRef]
- Marques, A.; Moraes, L.; Aparecida dos Santos, M.; Costa, I.; Costa, L.; Nunes, T.; Melo, N.; Simon, M.F.; Leitch, A.R.; Almeida, C.; et al. Origin and parental genome characterization of the allotetraploid Stylosanthes scabra Vogel (Papilionoideae, Leguminosae), an important legume pasture crop. Ann. Bot. 2018, 122, 1143–1159. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.H.; Mudge, J.; Kim, D.-J.; Shoemaker, R.C.; Cook, D.R.; Young, N.D. Comparative physical mapping reveals features of microsynteny between Glycine max, Medicago truncatula, and Arabidopsis thaliana. Genome 2004, 47, 141–155. [Google Scholar] [CrossRef]
- Fonsêca, A.; Ferreira, J.; dos Santos, T.R.B.; Mosiolek, M.; Bellucci, E.; Kami, J.; Gepts, P.; Geffroy, V.; Schweizer, D.; dos Santos, K.G.B.; et al. Cytogenetic map of common bean (Phaseolus vulgaris L.). Chromosome Res. 2010, 18, 487–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susek, K.; Bielski, W.K.; Hasterok, R.; Naganowska, B.; Wolko, B. A first glimpse of wild lupin karyotype variation as revealed by comparative cytogenetic mapping. Front. Plant Sci. 2016, 7, 1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyrwa, K.; Książkiewicz, M.; Szczepaniak, A.; Susek, K.; Podkowiński, J.; Naganowska, B. Integration of Lupinus angustifolius L. (narrow-leafed lupin) genome maps and comparative mapping within legumes. Chromosome Res. 2016, 24, 355–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; Li, L.; Liu, H.; Fu, L.; Qin, L.; Zhang, Z.; Cui, C.; Sun, Z.; Han, S.; Xu, J.; et al. High-resolution chromosome painting with repetitive and single-copy oligonucleotides in Arachis species identifies structural rearrangements and genome differentiation. BMC Plant Biol. 2018, 18, 240. [Google Scholar] [CrossRef]
- Devi, J.; Ko, J.M.; Seo, B.B. FISH and GISH: Modern cytogenetic techniques. Indian J. Biotechnol. 2005, 4, 307–315. [Google Scholar]
- Pellerin, R.J.; Waminal, N.E.; Kim, H.H. FISH mapping of rDNA and telomeric repeats in 10 Senna species. Hortic. Environ. Biotechnol. 2019, 60, 253–260. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lysák, M.A.; Mandáková, T. Analysis of plant meiotic chromosomes by chromosome painting. Methods Mol. Biol. 2013, 990, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kirov, I.; Divashuk, M.; Van Laere, K.; Soloviev, A.; Khrustaleva, L. An easy “SteamDrop” method for high quality plant chromosome preparation. Mol. Cytogenet. 2014, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Harpke, D.; Peterson, A. 5.8S motifs for the identification of pseudogenic ITS regions. Botany 2008, 86, 300–305. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- UNAFold. Available online: http://unafold.rna.albany.edu/?q=mfold/RNA-Folding-Form2.3 (accessed on 12 November 2020).
- Hřibová, E.; Čížková, J.; Christelová, P.; Taudien, S.; de Langhe, E.; Doležel, J. The ITS1-5.8S-ITS2 sequence region in the Musaceae: Structure, diversity and use in molecular phylogeny. PLoS ONE 2011, 6, e17863. [Google Scholar] [CrossRef] [Green Version]




| SNP Polymorphism | Indel Polymorphism | |||
|---|---|---|---|---|
| Diploid (ancestral basic chromosome number) | k | 11.811 | k(i) | 1.570 |
| Pi | 0.0642 | Pi(i) | 0.0063 | |
| Diploid (reduced basic chromosome numbers) | k | 17.464 | k(i) | 4.307 |
| Pi | 0.0939 | Pi(i) | 0.0176 | |
| Polyploid | k | 19.864 | k(i) | 1.303 |
| Pi | 0.0867 | Pi(i) | 0.0055 | |
| Subgenus/ Section | Trifolium Species | Accession Number | Gene Bank | Subgenus/ Section | Trifolium Species | Accession Number | Gene Bank |
|---|---|---|---|---|---|---|---|
| Chronosemium | Trichocephalum | T. subterraneum subsp. subterraneum L. | TRIF259 | G-DE | |||
| T. aureum L. | 13T0500014 | PR-CZ | Vesicastrum | T. fragiferum L. | TRIF1140 | G-DE | |
| T. badium Schreb. | AZ4518, AZ159 | AR-NZ | T. resupinatum L. | TRIF1134 | G-DE | ||
| Trifolium | T. spumosum L. | AZ198, 13T0500086 | AR-NZ, PR-CZ | ||||
| Trifolium | T. alpestre L. | TRIF210 | G-DE | Trifoliastrum | T. glomeratum L. | TRIF136, TRIF142 | G-DE |
| T. arvense L. | 13T0500032 | PR-CZ | T. montanum L. | TRIF152 | G-DE | ||
| T. bocconei Savi. | TRIF81, TRIF93, TRIF40 | G-DE | T. occidentale Coombe | OCD1210, OCD50 | AR-NZ | ||
| T. cherleri L. | TRIF135 | G-DE | T. pallescens Schreb. | AZ6429 | AR-NZ | ||
| T. diffusum Ehrh. | TRIF250 | G-DE | |||||
| T. hirtum All. | AZ6762, TRIF213 | AR-NZ, G-DE | T. thalii Vill. | AZ6833 | AR-NZ | ||
| T. ligusticum Balb. Ex Loisel | TRIF137 | G-DE | Involucrarium | T. chilense Hook. & Arn. | AZ1759 | AR-NZ | |
| T. pallidum Waldst&Kit | TRIF253 | G-DE | T. microdon Hook. & Arn. | AZ6256 | AR-NZ | ||
| T. purpureum Loisel. | TRIF143 | G-DE | T. microcephalum Pursh. | TRIF244 | G-DE | ||
| T. rubens L. | TRIF33, TRIF211 | G-DE | Paramesus | T. glanduliferum Boiss. | AZ6880 | AR-NZ | |
| T. squamosum L. | TRIF68 | G-DE | T. strictum L. | TRIF109 | G-DE | ||
| T. stellatum L. | TRIF252 | G-DE | Lupinaster | T. lupinaster L. | TRIF272 | G-DE | |
| T. pannonicum Jacq. | TRIF8 | G-DE | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vozárová, R.; Macková, E.; Vlk, D.; Řepková, J. Variation in Ribosomal DNA in the Genus Trifolium (Fabaceae). Plants 2021, 10, 1771. https://doi.org/10.3390/plants10091771
Vozárová R, Macková E, Vlk D, Řepková J. Variation in Ribosomal DNA in the Genus Trifolium (Fabaceae). Plants. 2021; 10(9):1771. https://doi.org/10.3390/plants10091771
Chicago/Turabian StyleVozárová, Radka, Eliška Macková, David Vlk, and Jana Řepková. 2021. "Variation in Ribosomal DNA in the Genus Trifolium (Fabaceae)" Plants 10, no. 9: 1771. https://doi.org/10.3390/plants10091771