Influence of Drying Method and Argon Plasma Modification of Bacterial Nanocellulose on Keratinocyte Adhesion and Growth
Abstract
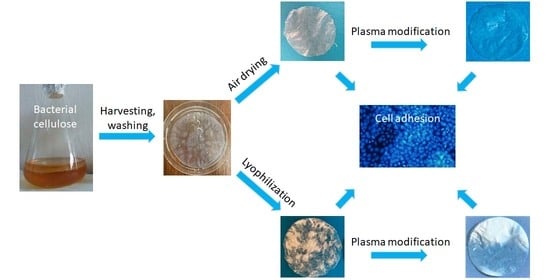
:1. Introduction
2. Materials and Methods
2.1. Preparation of BC Foils
2.2. Plasma Modification of BC Foils
2.3. Methods of Analysis
2.4. Cell Model and Culture Conditions
2.5. Evaluation of the Cell Number, Morphology, and Viability
2.6. Statistics
3. Results and Discussion
3.1. Production of BC Pellicles
3.2. Surface Modification of BC
3.3. Chemical Composition of BC
3.4. Surface Morphology of BC
3.5. Gas Sorption Analysis of Porosity and Specific Surface Area
3.6. Contact Angle and Hydrophilicity
3.7. In Vitro Tests of Cell Cultivation on BC Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Matsutani, M.; Ito, K.; Azuma, Y.; Ogino, H.; Shirai, M.; Yakushi, T.; Matsushita, K. Adaptive mutation related to cellulose producibility in Komagataeibacter medellinensis (Gluconacetobacter xylinus) NBRC 3288. Appl. Microbiol. Biotechnol. 2015, 99, 7229–7240. [Google Scholar] [CrossRef] [PubMed]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Yamada, Y.; Yukphan, P.; Vu, H.T.L.; Muramatsu, Y.; Ochaikul, D.; Tanasupawat, S.; Nakagawa, Y. Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J. Gen. Appl. Microbiol. 2012, 58, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Younesi, M.; Akkus, A.; Akkus, O. Microbially-derived nanofibrous cellulose polymer for connective tissue regeneration. Mater. Sci. Eng. C 2019, 99, 96–102. [Google Scholar] [CrossRef]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial cellulose films: Influence of bacterial strain and drying route on film properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Q.; Cao, X.; Li, Z.; Zhu, J.; Li, L. Effect of lyophilization on the bacterial cellulose produced by different Komagataeibacter strains to adsorb epicatechin. Carbohydr. Polym. 2020, 246, 116632. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Nakagaito, A.; Yano, H. Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl. Phys. A 2005, 80, 155–159. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Rydzkowski, T.; Thakur, V.K.; Borysiak, S. Enzymatic engineering of nanometric cellulose for sustainable polypropylene nanocomposites. Ind. Crop. Prod. 2021, 161, 113188. [Google Scholar] [CrossRef]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.; Boukherroub, R. Cellulose Nanocrystals/Graphene Hybrids—A Promising New Class of Materials for Advanced Applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Husemann, V.E.; Werner, R. Cellulose synthesis by Acetobacter xylinum. I. The molecular weight of bacterial cellulose and molecular weight distribution during the synthesis. Die Makromol. Chem. 1963, 59, 43–60. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Kiruba, T. Bacterial cellulose-A potential material for sustainable eco-friendly fashion products. J. Nat. Fibers 2020, 1–13. [Google Scholar] [CrossRef]
- Buruaga-Ramiro, C.; Valenzuela, S.V.; Valls, C.; Roncero, M.B.; Pastor, F.J.; Díaz, P.; Martinez, J. Development of an antimicrobial bioactive paper made from bacterial cellulose. Int. J. Biol. Macromol. 2020, 158, 587–594. [Google Scholar] [CrossRef]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial Cellulose: A Sustainable Source to Develop Value-Added Products—A Review. BioResources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Uryu, M.; Kurihara, N. Acoustic Diaphragm and Method for Producing Same. U.S. Patent US5274199A, 20 April 1993. [Google Scholar]
- Liu, X.; Souzandeh, H.; Zheng, Y.; Xie, Y.; Zhong, W.-H.; Wang, C. Soy protein isolate/bacterial cellulose composite membranes for high efficiency particulate air filtration. Compos. Sci. Technol. 2017, 138, 124–133. [Google Scholar] [CrossRef]
- Hasan, N.; Biak, D.R.A.; Kamarudin, S. Application of Bacterial Cellulose (BC) in Natural Facial Scrub. Int. J. Adv. Sci. Eng. Inf. Technol. 2012, 2, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Picheth, G.F.; Pirich, C.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bodhibukkana, C.; Srichana, T.; Kaewnopparat, S.; Tangthong, N.; Bouking, P.; Martin, G.P.; Suedee, R. Composite membrane of bacterially-derived cellulose and molecularly imprinted polymer for use as a transdermal enantioselective controlled-release system of racemic propranolol. J. Control. Release 2006, 113, 43–56. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.; Pinto, P.; Almeida, I.; da Costa, P.J.C.; Silvestre, A.; Neto, C.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; León, I.E.; Gonzalez, J.S.; Porto, L.M.; Álvarez, V.; Castro, G.R. Modified bacterial cellulose scaffolds for localized doxorubicin release in human colorectal HT-29 cells. Colloids Surf. B Biointerfaces 2016, 140, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gao, C.; Zhang, Y.; Wan, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. C 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Jia, H.; Jia, Y.; Wang, J.; Hu, Y.; Zhang, Y.; Jia, S. Potentiality of Bacterial Cellulose as the Scaffold of Tissue Engineering of Cornea. In Proceedings of the 2009 2nd International Conference on Biomedical Engineering and Informatics, Tianjin, China, 17–19 October 2009; pp. 1–5. [Google Scholar]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, R.; Chen, H.; Lu, Y.; Zhou, J.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Investigation on artificial blood vessels prepared from bacterial cellulose. Mater. Sci. Eng. C 2015, 46, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Xu, C.; Ma, X.; Chen, S.; Tao, M.; Yuan, L.; Jing, Y. Bacterial Cellulose Membranes Used as Artificial Substitutes for Dural Defection in Rabbits. Int. J. Mol. Sci. 2014, 15, 10855–10867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374–605393. [Google Scholar] [CrossRef]
- Bionext®. Available online: http://www.bennetthealth.net/Bionext/#Clinical (accessed on 22 April 2021).
- Aung, B.J. Does A New Cellulose Dressing Have Potential in Chronic Wounds? Podiatry Today 2004, 17, 20–26. [Google Scholar]
- Axcelon®. Available online: https://axcelonbp.com/nanoderm-ag/ (accessed on 22 April 2021).
- Nexfill®. Available online: https://nexfill.com.br/ (accessed on 22 April 2021).
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S. Bacterial Cellulose as a Versatile Platform for Research and Development of Biomedical Materials. Processes 2020, 8, 624. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2005, 76, 431–438. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [Green Version]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Vosmanska, V.; Kolarova, K.; Rimpelova, S.; Svorcik, V. Surface modification of oxidized cellulose haemostat by argon plasma treatment. Cellulose 2014, 21, 2445–2456. [Google Scholar] [CrossRef]
- Pertile, R.; Andrade, F.K.; Alves, C.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Kurniawan, H.; Lai, J.-T.; Wang, M.-J. Biofunctionalized bacterial cellulose membranes by cold plasmas. Cellulose 2012, 19, 1975–1988. [Google Scholar] [CrossRef]
- Vasconcellos, V.M.; Farinas, C.S. The effect of the drying process on the properties of bacterial cellulose films from Gluconacetobacter hansenii. Chem. Eng. Trans. 2018, 64, 145–150. [Google Scholar] [CrossRef]
- Illa, M.P.; Sharma, C.S.; Khandelwal, M. Tuning the physiochemical properties of bacterial cellulose: Effect of drying conditions. J. Mater. Sci. 2019, 54, 12024–12035. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J. Taiwan Inst. Chem. Eng. 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, J.-N.; Wee, Y.-J.; Park, D.-H.; Ryu, H.-W. Production of Bacterial Cellulose by Gluconacetobacter sp. RKY5 Isolated from Persimmon Vinegar. Appl. Biochem. Biotechnol. 2006, 131, 705–715. [Google Scholar] [CrossRef]
- Bae, S.; Sugano, Y.; Shoda, M. Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J. Biosci. Bioeng. 2004, 97, 33–38. [Google Scholar] [CrossRef]
- Wee, Y.-J. Isolation and characterization of a bacterial cellulose-producing bacterium derived from the persimmon vinegar. Afr. J. Biotechnol. 2011, 10, 16267–16276. [Google Scholar] [CrossRef] [Green Version]
- Švorčík, V.; Kolářová, K.; Slepička, P.; Mackova, A.; Novotná, M.; Hnatowicz, V. Modification of surface properties of high and low density polyethylene by Ar plasma discharge. Polym. Degrad. Stab. 2006, 91, 1219–1225. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing cellulose structures with vibrational spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Hofstetter, K.; Hinterstoisser, B.; Salmén, L. Moisture uptake in native cellulose—The roles of different hydrogen bonds: A dynamic FT-IR study using Deuterium exchange. Cellulose 2006, 13, 131–145. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform-Materials Analysis, 1st ed.; Salih, S., Ed.; InTech: Rijeka, Croatia, 2012; pp. 45–68. [Google Scholar]
- Maréchal, Y.; Chanzy, H. The hydrogen bond network in I β cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (d,l-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Slepička, P.; Trostová, S.; Kasálková, N.S.; Kolská, Z.; Sajdl, P.; Švorčík, V. Surface Modification of Biopolymers by Argon Plasma and Thermal Treatment. Plasma Process. Polym. 2011, 9, 197–206. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Steinerova, M.; Matejka, R.; Stepanovska, J.; Filova, E.; Stankova, L.; Rysova, M.; Martinova, L.; Dragounova, H.; Domonkos, M.; Artemenko, A.; et al. Human osteoblast-like SAOS-2 cells on submicron-scale fibers coated with nanocrystalline diamond films. Mater. Sci. Eng. C 2021, 121, 111792. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Tolde, Z.; Vanek, P.; Nehasil, V.; Doubková, M.; Trávníčková, M.; Drahokoupil, J.; Buixaderas, E.; Borodavka, F.; Novakova, J.; et al. Beta-Titanium Alloy Covered by Ferroelectric Coating–Physicochemical Properties and Human Osteoblast-Like Cell Response. Coatings 2021, 11, 210. [Google Scholar] [CrossRef]
- Grausova, L.; Kromka, A.; Bacakova, L.; Potocký, S.; Vanecek, M.; Lisa, V. Bone and vascular endothelial cells in cultures on nanocrystalline diamond films. Diam. Relat. Mater. 2008, 17, 1405–1409. [Google Scholar] [CrossRef]
- Bacakova, M.; Pajorova, J.; Stranska, D.; Hadraba, D.; Lopot, F.; Riedel, T.; Brynda, E.; Zaloudkova, M.; Bacakova, L. Protein nanocoatings on synthetic polymeric nanofibrous membranes designed as carriers for skin cells. Int. J. Nanomed. 2017, 12, 1143–1160. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, M.; Pajorova, J.; Broz, A.; Hadraba, D.; Lopot, F.; Zavadakova, A.; Vistejnova, L.; Beno, M.; Kostic, I.; Jencova, V.; et al. A two-layer skin construct consisting of a collagen hydrogel reinforced by a fibrin-coated polylactide nanofibrous membrane. Int. J. Nanomed. 2019, 14, 5033–5050. [Google Scholar] [CrossRef] [Green Version]
- Stankova, L.; Fraczek-Szczypta, A.; Blazewicz, M.; Filova, E.; Blazewicz, S.; Lisa, V.; Bacakova, L. Human osteoblast-like MG 63 cells on polysulfone modified with carbon nanotubes or carbon nanohorns. Carbon 2014, 67, 578–591. [Google Scholar] [CrossRef]
- Engler, A.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]










| Sample | Weight Loss [%] |
|---|---|
| BC-AD 60 s | 3.20 ± 0.36 |
| BC-AD 240 s | 14.26 ± 0.61 |
| BC-AD 480 s | 27.89 ± 2.88 |
| BC-L 60 s | 2.40 ± 0.45 |
| BC-L 240 s | 6.80 ± 1.27 |
| BC-L 480 s | 12.64 ± 3.00 |
| Sample | C (%) | O (%) | O/C |
|---|---|---|---|
| BC-AD | 55.80 | 44.20 | 0.6124 |
| BC-AD 60 s | 58.39 | 41.61 | 0.7126 |
| BC-AD 240 s | 59.49 | 40.51 | 0.6809 |
| BC-L | 57.24 | 42.76 | 0.6595 |
| BC-L 60 s | 59.36 | 40.64 | 0.6847 |
| BC-L 240 s | 49.69 | 50.31 | 1.0125 |
| Sample | SBET [m2·g−1] | Vp [cm3·g−1] |
|---|---|---|
| BC-AD | 9.9 ± 1.6 | 0.011 ± 0.002 |
| BC-AD 240 s | 140.5 ± 4.8 | 0.142 ± 0.008 |
| BC-L | 45.0 ± 0.7 | 0.056 ± 0.001 |
| BC-L 240 s | 156.3 ± 1.5 | 0.308 ± 0.015 |
| Sample | Contact Angle [°] |
|---|---|
| BC-AD | 63.91 ± 2.69 |
| BC-AD 60 s | 25.42 ± 2.86 |
| BC-AD 240 s | 32.79 ± 2.00 |
| BC-L | 34.74 ± 6.80 |
| BC-L 60 s | 27.00 ± 2.80 |
| BC-L 240 s | 20.90 ± 1.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutová, A.; Staňková, L.; Vejvodová, K.; Kvítek, O.; Vokatá, B.; Fajstavr, D.; Kolská, Z.; Brož, A.; Bačáková, L.; Švorčík, V. Influence of Drying Method and Argon Plasma Modification of Bacterial Nanocellulose on Keratinocyte Adhesion and Growth. Nanomaterials 2021, 11, 1916. https://doi.org/10.3390/nano11081916
Kutová A, Staňková L, Vejvodová K, Kvítek O, Vokatá B, Fajstavr D, Kolská Z, Brož A, Bačáková L, Švorčík V. Influence of Drying Method and Argon Plasma Modification of Bacterial Nanocellulose on Keratinocyte Adhesion and Growth. Nanomaterials. 2021; 11(8):1916. https://doi.org/10.3390/nano11081916
Chicago/Turabian StyleKutová, Anna, Lubica Staňková, Kristýna Vejvodová, Ondřej Kvítek, Barbora Vokatá, Dominik Fajstavr, Zdeňka Kolská, Antonín Brož, Lucie Bačáková, and Václav Švorčík. 2021. "Influence of Drying Method and Argon Plasma Modification of Bacterial Nanocellulose on Keratinocyte Adhesion and Growth" Nanomaterials 11, no. 8: 1916. https://doi.org/10.3390/nano11081916