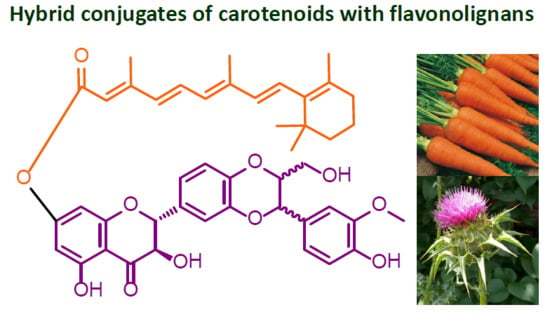
Preparation of Retinoyl-Flavonolignan Hybrids and Their Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Nuclear Magnetic Resonance (NMR) and Mass Spectrometry (MS) Methodology
2.3. HPLC
2.4. Chemical Synthesis
2.4.1. General Procedures for the Synthesis of Conjugates
2.4.2. Synthesis of Conjugates
2.5. Antioxidant Activity
2.5.1. Determination of Log P Values
2.5.2. DPPH Radical Scavenging
2.5.3. Antioxidant Activity
2.5.4. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Conjugates
3.2. Antioxidant and Biophysical Testing of Conjugates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-Azo-bis(2-amidinopropane) dihydrochloride |
| BHT | Butylated hydroxytoluene |
| CAA | Cellular antioxidant activity |
| CUPRAC | Cupric reducing antioxidant capacity |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| DCU | Dicyclohexylurea |
| DMAP | 4-Dimethylaminopyridine |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| FRAP | Ferric reducing antioxidant power |
| NF-κb | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ORAC | Oxygen radical absorbance capacity |
| ROS | Reactive oxygen species |
| THF | Tetrahydrofuran |
References
- Brambilla, D.; Mancuso, C.; Scuderi, M.R.; Bosco, P.; Cantarella, G.; Lempereur, L.; Di Benedetto, G.; Pezzino, S.; Bernardini, R. The role of antioxidant supplement in immune system, neoplastic and neurodegenerative disorders: A point of view for an assessment of the risk/benefit profile. Nutrition 2008, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Hernández Blanco, I.; Hoffmann, T.; Martin, H.-D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: Carotenoids, flavonoids, phenols and indigoids. The role of β-carotene in antioxidant functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.A.R.; Russell, R.M. β-Carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, M.; Calogeropoulou, T.; Rekka, E.; Chryselis, M.; Papazafiri, P.; Gaitanaki, C.; Makriyannis, A. Bifunctional agents for reperfusion arrhythmias: Novel hybrid vitamin E/Class I antiarrhythmics. Bioorg. Med. Chem. 2003, 11, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Bebbington, D.; Dawson, C.E.; Gaur, S.; Spencer, J. Prodrug and covalent linker strategies for the solubilization of dual-action antioxidants/iron chelators. Bioorg. Med. Chem. Lett. 2002, 12, 3297–3300. [Google Scholar] [CrossRef]
- Gažák, R.; Marhol, P.; Purchartová, K.; Monti, D.; Biedermann, D.; Riva, S.; Cvak, L.; Křen, V. Large-scale separation of silybin diastereoisomers using lipases. Process Biochem. 2010, 45, 1657–1663. [Google Scholar] [CrossRef]
- Vavříková, E.; Křen, V.; Ježová-Kalachová, L.; Biler, M.; Chantemargue, B.; Pyszková, M.; Riva, S.; Kuzma, M.; Valentová, K.; Ulrichová, J.; et al. Novel flavonolignan hybrid antioxidants: From enzymatic preparation to molecular rationalization. Eur. J. Med. Chem. 2017, 127, 263–274. [Google Scholar] [CrossRef]
- Papa, T.B.R.; Pinho, V.D.; do Nascimento, E.S.P.; Santos, W.G.; Burtoloso, A.C.B.; Skibsted, L.H.; Cardoso, D.R. Astaxanthin diferulate as a bifunctional antioxidant. Free Radic. Res. 2015, 49, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Hundsdörfer, C.; Stahl, W.; Müller, T.J.J.; De Spirt, S. UVA photoprotective properties of an artificial carotenylflavonoid hybrid molecule. Chem. Res. Toxicol. 2012, 25, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Beutner, S.; Frixel, S.; Ernst, H.; Hoffmann, T.; Hernandez-Blanco, I.; Hundsdoerfer, C.; Kiesendahl, N.; Kock, S.; Martin, H.D.; Mayer, B.; et al. Carotenylflavonoids, a novel group of potent, dual-functional antioxidants. Arkivoc 2007, 8, 279–295. [Google Scholar]
- Han, R.M.; Zhang, J.P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Fuksová, K.; Marhol, P.; Kuzma, M.; Agarwal, R.; Křen, V. Preparative method for isosilybin isolation based on enzymatic kinetic resolution of silymarin mixture. Process Biochem. 2013, 48, 184–189. [Google Scholar] [CrossRef]
- Džubák, P.; Hajdúch, M.; Gažák, R.; Svobodová, A.; Psotová, J.; Walterová, D.; Sedmera, P.; Křen, V. New derivatives of silybin and 2,3-dehydrosilybin and their cytotoxic and p-glycoprotein modulatory activity. Bioorg. Med. Chem. 2006, 14, 3793–3810. [Google Scholar] [CrossRef] [PubMed]
- Pyka, A.; Babuska, M.; Zachariasz, M. A comparison of theoretical methods of calculation of partition coefficients for selected drugs. Acta Pol. Pharm. 2006, 63, 159–167. [Google Scholar] [PubMed]
- Joyeux, M.; Mortier, F.; Fleurentin, J. Screening of antiradical, antilipoperoxidant and hepatoprotective effect of nine plant extracts used in Caribbean folk medicine. Phythother. Res. 1995, 9, 228–230. [Google Scholar] [CrossRef]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J.; Shalliker, R.A. Ferric reducing antioxidant potential (FRAP) of antioxidants using reaction flow chromatography. Anal. Chim. Acta 2017, 967, 93–101. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gažák, R.; Sedmera, P.; Marzorati, M.; Riva, S.; Křen, V. Laccase-mediated dimerization of the flavonolignan silybin. J. Mol. Catal. B 2008, 50, 87–92. [Google Scholar] [CrossRef]
- Vavříková, E.; Vacek, J.; Valentová, K.; Marhol, P.; Ulrichová, J.; Kuzma, M.; Křen, V. Chemo-enzymatic synthesis of silybin and 2,3-dehydrosilybin dimers. Molecules 2014, 19, 4115–4134. [Google Scholar] [CrossRef] [PubMed]
- Maugard, T.; Legoy, M.D. Enzymatic synthesis of derivatives of vitamin a in organic media. J. Mol. Catal. B Enzym. 2000, 8, 275–280. [Google Scholar] [CrossRef]
- Háda, M.; Nagy, V.; Takátsy, A.; Deli, J.; Agócs, A. Dicarotenoid esters of bivalent acids. Tetrahedron Lett. 2008, 49, 3524–3526. [Google Scholar] [CrossRef]
- Rejasse, B.; Maugard, T.; Legoy, M.D. Enzymatic procedures for the synthesis of water-soluble retinol derivatives in organic media. Enzyme Microb. Technol. 2003, 32, 312–320. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Kim, H.; Um, S.; Lee, J.; Ryoo, H.; Jung, H. Synthesis and in vitro biological activity of retinyl retinoate, a novel hybrid retinoid derivative. Bioorg. Med. Chem. 2008, 16, 6387–6393. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhou, L.M.; Liu, P.; Baker, P.J.; Liu, S.S.; Xue, Y.P.; Xu, M.; Zheng, Y.G. Efficient two-step chemo-enzymatic synthesis of all-trans-retinyl palmitate with high substrate concentration and product yield. Appl. Microbiol. Biotechnol. 2015, 99, 8891–8902. [Google Scholar] [CrossRef]
- Akono Ntonga, P.; Baldovini, N.; Mouray, E.; Mambu, L.; Belong, P.; Grellier, P. Activity of Ocimum basilicum, Ocimum canum and Cymbopogon citratus essential oils against Plasmodium falciparum and mature-stage larvae of Anopheles funestus s.s. Parasite 2014, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Malik, A.; Sharma, S.; Dhiman, R.C. Larvicidal potential of essential oils against Musca domestica and Anopheles stephensi. Parasitol. Res. 2016, 115, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 23, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Biedermann, D.; Křen, V. 2,3-Dehydroderivatives of silymarin flavonolignans: Prospective natural compounds for the prevention of chronic diseases. Proceedings 2019, 11, 21. [Google Scholar] [CrossRef]
- Valentová, K.; Purchartová, K.; Rydlová, L.; Roubalová, L.; Biedermann, D.; Petrásková, L.; Křenková, A.; Pelantová, H.; Holečková-Moravcová, V.; Tesařová, E.; et al. Sulfated metabolites of flavonolignans and 2,3-dehydroflavonolignans: Preparation and properties. Int. J. Mol. Sci. 2018, 19, 2349. [Google Scholar] [CrossRef] [PubMed]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Blasa, M.; Angelino, D.; Gennari, L.; Ninfali, P. The cellular antioxidant activity in red blood cells (CAA-RBC): A new approach to bioavailability and synergy of phytochemicals and botanical extracts. Food Chem. 2011, 125, 685–691. [Google Scholar] [CrossRef]
- Gažák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Křen, V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity—Role of individual hydroxyl groups. Free Radic. Biol. Med. 2009, 46, 745–758. [Google Scholar] [CrossRef]


| Parent Compound | Conjugate with Retinoic Acid | Mixture 1:1 Eq with Retinoic Acid | |
|---|---|---|---|
| Retinol (1) | 745 ± 11 | − | − |
| Retinoic acid (2) | 1485 ± 110 | − | − |
| Silybin (3ab) | 472 ± 16 | (6ab) 666 ± 16 * | 499 ± 6 |
| Silybin A (3a) | 818 ± 22 | (6a) 379 ± 19 * | 750 ± 33 |
| Silybin B (3b) | 659 ± 29 | (6b) 540 ± 24 * | 773 ± 7 |
| 2,3-Dehydrosilybin (4) | 19.2 ± 0.3 | (7) 734 ± 35 * | 15.1 ± 0.3 |
| Isosilybin A (5a) | 783 ± 9 | (8a) 2361 ± 152 * | 610 ± 18 |
| FRAP [TE] a | CUPRAC b [TE] | ORAC c (IC50 [µM]) | CAA d (IC50 [µM]) | LogP e | |
|---|---|---|---|---|---|
| Retinol (1) | 1.76 ± 0.04 | 0.09 ± 0.01f | 169 ± 12 | 1271 ± 147 | 5.92 |
| Retinoic acid (2) | 0.62 ± 0.02 | 0.10 ± 0.02f | 13 ± 2 | 460 ± 211 | 5.80 |
| Silybin (3ab) | 0.335 ± 0.006 | 0.17 ± 0.00 | 7.8 ± 0.7 | 11.8 ± 0.3 | 1.47 |
| Silybin A (3a) | 0.278 ± 0.005 | 0.20 ± 0.00 | 8.5 ± 0.4 | 10.0 ± 0.7 | 1.47 |
| Silybin B (3b) | 0.268 ± 0.009 | 0.16 ± 0.02 | 8.0 ± 0.3 | 6.8 ± 0.5 | 1.47 |
| 2,3-Dehydrosilybin (4) | 4.06 ± 0.05 | 0.25 ± 0.00 | 9.6 ± 0.4 | 10.9 ± 0.5 | 2.44 |
| Isosilybin A (5a) | 0.280 ± 0.006 | 0.16 ± 0.02 | 4.3 ± 1.1 | >100 h | 1.47 |
| Silybin AB-7-O-retinoate (6ab) | 0.03 ± 0.02 *,# | 0.22 ± 0.01# | 91 ± 4 *,# | >500 h | 7.53 |
| Silybin A-7-O-retinoate (6a) | 0.038 ± 0.002 *,# | 0.21 ± 0.02# | 9.0 ± 0.7 j | >50 g,h | 7.53 |
| Silybin B-7-O-retinoate (6b) | 0.010 ± 0.001 *,# | 0.04 ± 0.01f | 230 ± 8 *,# | >500 g | 7.53 |
| 2,3-Dehydrosilybin-3-O-retinoate (7) | 0.034 ± 0.005 *,# | 0.07 ± 0.02f | 130 ± 7 *,# | >500 g | 8.21 |
| Isosilybin A-7-O-retinoate (8a) | 0.022 ± 0.003 *,# | 0.11 ± 0.01f | 174 ± 7 *,# | >500 g | 7.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambers, C.S.; Biedermann, D.; Valentová, K.; Petrásková, L.; Viktorová, J.; Kuzma, M.; Křen, V. Preparation of Retinoyl-Flavonolignan Hybrids and Their Antioxidant Properties. Antioxidants 2019, 8, 236. https://doi.org/10.3390/antiox8070236
Chambers CS, Biedermann D, Valentová K, Petrásková L, Viktorová J, Kuzma M, Křen V. Preparation of Retinoyl-Flavonolignan Hybrids and Their Antioxidant Properties. Antioxidants. 2019; 8(7):236. https://doi.org/10.3390/antiox8070236
Chicago/Turabian StyleChambers, Christopher S., David Biedermann, Kateřina Valentová, Lucie Petrásková, Jitka Viktorová, Marek Kuzma, and Vladimír Křen. 2019. "Preparation of Retinoyl-Flavonolignan Hybrids and Their Antioxidant Properties" Antioxidants 8, no. 7: 236. https://doi.org/10.3390/antiox8070236