Synapse-Mimicking Memristors Based on 3,6-Di(tpy)-9-Phenylcarbazole Unimer and Its Copolymer with Cobalt(II) Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
3. Procedures
4. Results
4.1. Characterization of Studied Materials
4.2. Electrical Characteristics
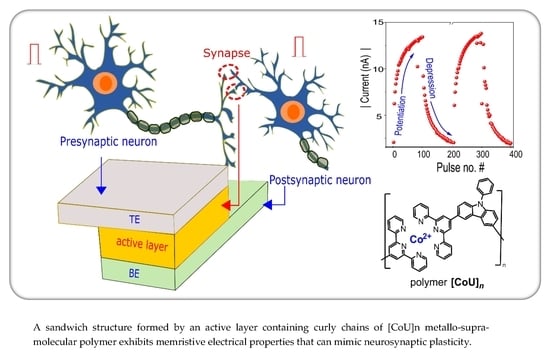
4.3. Synaptic Plasticity
4.3.1. Potentiation and Depression
4.3.2. Memory Modulation by Paired-Pulse Facilitation (PPF) and Depression (PPD)
4.3.3. Short- and Long-Term Memory
4.4. Analysis of the Resistive Changes in U
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The Missing Memristor Found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Strukov, D.B.; Stewart, D.R. Memristive Devices for Computing. Nat. Nanotechnol. 2013, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Foulger, S.H.; Bandera, Y.; Grant, B.; Vilčáková, J.; Sáha, P.; Foulger, S.H. Exploiting Multiple Percolation in Two-Terminal Memristor to Achieve a Multitude of Resistive States. J. Mater. Chem. C 2021, 9, 8975–8986. [Google Scholar] [CrossRef]
- Cafferty, B.J.; Ten, A.S.; Fink, M.J.; Morey, S.; Preston, D.J.; Mrksich, M.; Whitesides, G.M. Storage of Information Using Small Organic Molecules. ACS Cent. Sci. 2019, 5, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Jo, S.H.; Lu, W. Short-Term Memory to Long-Term Memory Transition in a Nanoscale Memristor. ACS Nano 2011, 5, 7669–7676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Che, Q.; Wang, K.; El-Khouly, M.E.; Liu, J.; Fu, Y.; Zhang, B.; Chen, Y. Donor-Acceptor-Type Poly[Chalcogenoviologen-Alt-Triphenylamine] for Synaptic Biomimicking and Neuromorphic Computing. iScience 2022, 25, 103640. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Bae, J.; Lee, C.H.; Shin, S.; Kobayashi, N.P. Ultra-Low Power Non-Volatile Resistive Crossbar Memory Based on Pull up Resistors. Org. Electron. 2017, 41, 73–78. [Google Scholar] [CrossRef]
- Prezioso, M.; Merrikh-Bayat, F.; Hoskins, B.D.; Adam, G.C.; Likharev, K.K.; Strukov, D.B. Training and Operation of an Integrated Neuromorphic Network Based on Metal-Oxide Memristors. Nature 2015, 521, 61–64. [Google Scholar] [CrossRef]
- Goswami, S.; Matula, A.J.; Rath, S.P.; Hedström, S.; Saha, S.; Annamalai, M.; Sengupta, D.; Patra, A.; Ghosh, S.; Jani, H.; et al. Robust Resistive Memory Devices Using Solution-Processable Metal-Coordinated Azo Aromatics. Nat. Mater. 2017, 16, 1216–1224. [Google Scholar] [CrossRef]
- Park, S.; Lee, T.J.; Kim, D.M.; Kim, J.C.; Kim, K.; Kwon, W.; Ko, Y.G.; Choi, H.; Chang, T.; Ree, M. Electrical Memory Characteristics of a Nondoped π-Conjugated Polymer Bearing Carbazole Moieties. J. Phys. Chem. B 2010, 114, 10294–10301. [Google Scholar] [CrossRef]
- Li, W.; Guo, F.; Ling, H.; Liu, H.; Yi, M.; Zhang, P.; Wang, W.; Xie, L.; Huang, W.; Li, W.; et al. Solution-Processed Wide-Bandgap Organic Semiconductor Nanostructures Arrays for Nonvolatile Organic Field-Effect Transistor Memory. Small 2018, 14, 1701437. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, T.; Bandera, Y.; Grant, B.; Zdyrko, B.; Foulger, S.H.; Vilčáková, J.; Sáha, P.; Pfleger, J. Carbazole Derivatized N-Alkyl Methacrylate Polymeric Memristors as Flexible Synaptic Substitutes. Adv. Electron. Mater. 2020, 6, 2000042. [Google Scholar] [CrossRef]
- Xie, L.H.; Ling, Q.D.; Hou, X.Y.; Huang, W. An Effective Friedel-Crafts Postfunctionalization of Poly(N-Vinylcarbazole) to Tune Carrier Transportation of Supramolecular Organic Semiconductors Based on π-Stacked Polymers for Nonvolatile Flash Memory Cell. J. Am. Chem. Soc. 2008, 130, 2120–2121. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Q.; Ling, S.; Fan, T.; Zhang, C.; Zhu, X.; Zhang, Q.; Zhang, Y.; Zhang, J.; Yu, S.; et al. A Benzothiadiazole-Containing π-Conjugated Small Molecule as Promising Element for Nonvolatile Multilevel Resistive Memory Device. J. Solid State Chem. 2021, 294, 121850. [Google Scholar] [CrossRef]
- Zhao, F.J.; Wang, H.; Li, K.; Wang, X.D.; Zhang, N.; Zhu, X.; Dou, F.; Zhao, X.; Zhang, W.W.; Zhang, Y.; et al. Non-Volatile Ternary Memristors Based on a Polymer Containing a Carbazole Donor with CuO NPs Embedded. Inorg. Chem. 2019, 46, 11925–11928. [Google Scholar] [CrossRef]
- Yu, Y.; Bian, L.; Zhang, Y.; Liu, Z.; Li, Y.; Zhang, R.; Ju, R.; Yin, C.; Yang, L.; Yi, M.; et al. Synthesis of Donor-Acceptor Gridarenes with Tunable Electronic Structures for Synaptic Learning Memristor. ACS Omega 2019, 4, 5863–5869. [Google Scholar] [CrossRef]
- Hong, E.Y.H.; Poon, C.T.; Yam, V.W.W. A Phosphole Oxide-Containing Organogold(III) Complex for Solution-Processable Resistive Memory Devices with Ternary Memory Performances. J. Am. Chem. Soc. 2016, 138, 6368–6371. [Google Scholar] [CrossRef]
- Hu, B.; Wang, C.; Wang, J.; Gao, J.; Wang, K.; Wu, J.; Zhang, G.; Cheng, W.; Venkateswarlu, B.; Wang, M.; et al. Inorganic–Organic Hybrid Polymer with Multiple Redox for High-Density Data Storage. Chem. Sci. 2014, 5, 3404–3408. [Google Scholar] [CrossRef]
- Min Yoon, S.; Warren, S.C.; Grzybowski, B.A.; Yoon, S.M.; Warren, S.C.; Grzybowski, B.A. Storage of Electrical Information in Metal–Organic-Framework Memristors. Angew. Chemie Int. Ed. 2014, 53, 4437–4441. [Google Scholar] [CrossRef]
- Pradhan, B.; Das, S. Role of New Bis(2,2′-Bipyridyl)(Triazolopyridyl)Ruthenium(II) Complex in the Organic Bistable Memory Application. Chem. Mater. 2008, 20, 1209–1211. [Google Scholar] [CrossRef]
- Tang, J.H.; Sun, T.G.; Shao, J.Y.; Gong, Z.L.; Zhong, Y.W. Resistive Memory Devices Based on a Triphenylamine-Decorated Non-Precious Cobalt(II) Bis-Terpyridine Complex. Chem. Commun. 2017, 53, 11925–11928. [Google Scholar] [CrossRef]
- Vohlídal, J.; Hissler, M. Chapter 6, Metallo-Supramolecular Polymers. In Smart Inorganic Polymers: Synthesis, Properties, and Emerging Applications in Materials and Life Sciences; Hey-Hawkins, E., Hissler, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 141–162. [Google Scholar]
- Chernyshev, A.; Acharya, U.; Pfleger, J.; Trhlíková, O.; Zedník, J.; Vohlídal, J. Iron (II) Metallo-Supramolecular Polymers Based on Thieno [3,2-b]Thiophene for Electrochromic Applications. Polymers 2021, 13, 362. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, C.; Chakraborty, C. Metallo-Macrocycle Camouflages: Multicolored Electrochromism in a Fe(II) Based Metallo-Supramolecular Macrocycle Utilizing the Redox of Metal Centers and Carbazole Containing Ligand. ACS Appl. Electron. Mater. 2019, 1, 2531–2540. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N. From 1 → 3 Dendritic Designs to Fractal Supramacromolecular Constructs: Understanding the Pathway to the Sierpiński Gasket. Chem. Soc. Rev. 2015, 44, 3954–3967. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newkome, G.R. Terpyridine-Based Metallosupramolecular Constructs: Tailored Monomers to Precise 2D-Motifs and 3D-Metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef]
- Ding, B.; Solomon, M.B.; Leong, C.F.; D’Alessandro, D.M. Redox-Active Ligands: Recent Advances towards Their Incorporation into Coordination Polymers and Metal-Organic Frameworks. Coord. Chem. Rev. 2021, 439, 213891. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Shang, J.; Zhang, C.; Tan, H.; Yi, X.; Pan, L.; Zhang, W.; Fan, F.; Liu, Y.; et al. An Organic Terpyridyl-Iron Polymer Based Memristor for Synaptic Plasticity and Learning Behavior Simulation. RSC Adv. 2016, 6, 25179–25184. [Google Scholar] [CrossRef]
- Winter, A.; Friebe, C.; Chiper, M.; Hager, M.D.; Schubert, U.S. Self-Assembly of π-Conjugated Bis(Terpyridine) Ligands with Zinc(II) Ions: New Metallosupramolecular Materials for Optoelectronic Applications. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4083–4098. [Google Scholar] [CrossRef]
- Crispini, A.; Cretu, C.; Aparaschivei, D.; Andelescu, A.A.; Sasca, V.; Badea, V.; Aiello, I.; Szerb, E.I.; Costisor, O. Influence of the Counterion on the Geometry of Cu(I) and Cu(II) Complexes with 1,10-Phenanthroline. Inorganica Chim. Acta 2018, 470, 342–351. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, S.; Kamilya, S.; Mandal, S.; Mehta, S.; Mondal, A. Impact of Counteranion on Reversible Spin-State Switching in a Series of Cobalt(II) Complexes Containing a Redox-Active Ethylenedioxythiophene-Based Terpyridine Ligand. Inorg. Chem. 2022, 61, 17080–17088. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalt. Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Hou, X.; Xiao, X.; Zhou, Q.H.; Cheng, X.F.; He, J.H.; Xu, Q.F.; Li, H.; Li, N.J.; Chen, D.Y.; Lu, J.M. Surface Engineering to Achieve Organic Ternary Memory with a High Device Yield and Improved Performance. Chem. Sci. 2017, 8, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ye, K.; Sun, J.; Zhan, Y.; Jia, J.; Xue, P.; Zhang, G.; Zhang, Z.; Lu, R. Branched Benzothiadiazole-Cored Oligomers with Terminal Carbazoles: Synthesis and Fluorescence Probing Nitroaromatics. Dye. Pigment. 2015, 116, 36–45. [Google Scholar] [CrossRef]
- Hu, Q.; Ma, K.; Mei, Y.; He, M.; Kong, J.; Zhang, X. Metal-to-Ligand Charge-Transfer: Applications to Visual Detection of β-Galactosidase Activity and Sandwich Immunoassay. Talanta 2017, 167, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sakamoto, R.; Ho, C.L.; Nishihara, H.; Wong, W.Y. Electrochromic Triphenylamine-Based Cobalt(II) Complex Nanosheets. J. Mater. Chem. C 2019, 7, 9159–9166. [Google Scholar] [CrossRef]
- Pan, L.; Hu, B.; Zhu, X.; Chen, X.; Shang, J.; Tan, H.; Xue, W.; Zhu, Y.; Liu, G.; Li, R.W. Role of Oxadiazole Moiety in Different D–A Polyazothines and Related Resistive Switching Properties. J. Mater. Chem. C 2013, 1, 4556–4564. [Google Scholar] [CrossRef]
- Gu, Q.-F.; He, J.-H.; Chen, D.-Y.; Dong, H.-L.; Li, Y.-Y.; Li, H.; Xu, Q.-F.; Lu, J.-M.; Gu, Q.; He, J.; et al. Multilevel Conductance Switching of a Memory Device Induced by Enhanced Intermolecular Charge Transfer. Adv. Mater. 2015, 27, 5968–5973. [Google Scholar] [CrossRef]
- Wang, G.; Miao, S.; Zhang, Q.; Liu, H.; Li, H.; Li, N.; Xu, Q.; Lu, J.; Wang, L. Effect of a P-Spacer between a Donor and an Acceptor on Small Molecule-Based Data-Storage Device Performance. Chem. Commun 2013, 49, 9470. [Google Scholar] [CrossRef]
- Nguyen, H.A.D.; Yu, J.; Xie, L.; Taouil, M.; Hamdioui, S.; Fey, D. Memristive Devices for Computing: Beyond CMOS and beyond von Neumann. In Proceedings of the 2017 IFIP/IEEE International Conference on Very Large Scale Integration (VLSI-SoC), Abu Dhabi, United Arab Emirates, 23–25 October 2017; pp. 1–10. [Google Scholar] [CrossRef]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G.M. Synaptic Plasticity and Memory: An Evaluation of the Hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; Mclean, R.S.; Onoa, G.B.; et al. Non-Volatile Polymer Memory Device Based on a Novel Copolymer of N-Vinylcarbazole and Eu-Complexed Vinylbenzoate. Adv. Mater. 2005, 17, 455–459. [Google Scholar] [CrossRef]
- Sokolov, A.S.; Abbas, H.; Abbas, Y.; Choi, C.; Sokolov, A.S.; Abbas, H.; Abbas, Y.; Choi, C. Towards Engineering in Memristors for Emerging Memory and Neuromorphic Computing: A Review. J. Semicond. 2021, 42, 013101. [Google Scholar] [CrossRef]
- Hu, S.G.; Liu, Y.; Chen, T.P.; Liu, Z.; Yu, Q.; Deng, L.J.; Yin, Y.; Hosaka, S. Emulating the Paired-Pulse Facilitation of a Biological Synapse with a NiOx-Based Memristor. Appl. Phys. Lett. 2013, 102, 183510. [Google Scholar] [CrossRef]
- Yu, R.; Li, E.; Wu, X.; Yan, Y.; He, W.; He, L.; Chen, J.; Chen, H.; Guo, T. Electret-Based Organic Synaptic Transistor for Neuromorphic Computing. ACS Appl. Mater. Interfaces 2020, 12, 15446–15455. [Google Scholar] [CrossRef]
- Hui Liu, Y.; Qiang Zhu, L.; Feng, P.; Shi, Y.; Wan, Q.; Liu, Y.H.; Zhu, L.Q.; Feng, P.; Shi, Y.; Wan, Q. Freestanding Artificial Synapses Based on Laterally Proton-Coupled Transistors on Chitosan Membranes. Adv. Mater. 2015, 27, 5599–5604. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Shin, S.; Kang, D.C.; Kim, K.; Jeong, Y.; Kim, J.; Lee, S.; Kwak, J.Y.; Park, J.; Hwang, G.W.; Lee, K.S.; et al. Emulating the Short-Term Plasticity of a Biological Synapse with a Ruthenium Complex-Based Organic Mixed Ionic–Electronic Conductor. Mater. Adv. 2022, 3, 2827–2837. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, L.; Feng, J.; Yang, L.; Xu, W. Flexible Organic Artificial Synapse with Ultrashort-Term Plasticity for Tunable Time-Frequency Signal Processing. Chin. Chem. Lett. 2023, 34, 108419. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, X.; Xu, Z.; Wang, M.; Liu, Q.; Ma, Y.; Chen, J.; Meng, K.; Wu, Y.; Miao, J.; et al. Self-Rectifying and Forming-Free Resistive Switching Behaviors in Pt/La2Ti2O7/Pt Structure. Ceram. Int. 2022, 48, 4693–4698. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.-Q.; Mao, J.-Y.; Wang, Z.-P.; Wu, S.; Zhou, M.; Chen, T.; Zhou, Y.; Han, S.-T. Recent Advances of Volatile Memristors: Devices, Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000055. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, S.; Sun, L.; Liu, L.; Wei, H.; Xu, Z.; Xu, W.; Xu, W. A Low-Dimensional Hybrid p-i-n Heterojunction Neuromorphic Transistor with Ultra-High UV Sensitivity and Immediate Switchable Plasticity. Appl. Mater. Today 2021, 25, 101223. [Google Scholar] [CrossRef]
- Zhu, X.; Li, D.; Liang, X.; Lu, W.D. Ionic Modulation and Ionic Coupling Effects in MoS2 Devices for Neuromorphic Computing. Nat. Mater. 2019, 18, 141–148. [Google Scholar] [CrossRef]
- Wang, L.; Wen, D. Nonvolatile Bio-Memristor Based on Silkworm Hemolymph Proteins. Sci. Rep. 2017, 7, 17418. [Google Scholar] [CrossRef]
- Panthi, Y.R.; Pfleger, J.; Výprachtický, D.; Pandey, A.; Thottappali, M.A.; Šeděnková, I.; Konefał, M.; Foulger, S.H. Rewritable Resistive Memory Effect in Poly[N-(3-(9H-Carbazol-9-Yl)Propyl)-Methacrylamide] Memristor. J. Mater. Chem. C 2023, 11, 17093–17105. [Google Scholar] [CrossRef]
- Lim, Z.X.; Sreenivasan, S.; Wong, Y.H.; Zhao, F.; Cheong, K.Y. Effects of Electrode Materials on Charge Conduction Mechanisms of Memory Device Based on Natural Aloe Vera. MRS Adv. 2016, 1, 2513–2518. [Google Scholar] [CrossRef]
- Li, F.; Hu, Z.; Qiao, H.; Liu, L.; Hu, J.; Chen, X.; Li, J. Terpyridine-Based Donor–Acceptor Metallo-Supramolecular Polymers with Tunable Band Gaps: Synthesis and Characterization. Dye. Pigment. 2016, 132, 142–150. [Google Scholar] [CrossRef]
- Matteucci, E.; Baschieri, A.; Sambri, L.; Monti, F.; Pavoni, E.; Bandini, E.; Armaroli, N. Carbazole-Terpyridine Donor-Acceptor Dyads with Rigid π-Conjugated Bridges. ChemPlusChem 2019, 84, 1353–1365. [Google Scholar] [CrossRef]
- Ma, Y.; She, P.; Zhang, K.Y.; Yang, H.; Qin, Y.; Xu, Z.; Liu, S.; Zhao, Q.; Huang, W. Dynamic Metal-Ligand Coordination for Multicolour and Water-Jet Rewritable Paper. Nat. Commun. 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hu, J.Y.; Luo, J.; Liao, W.M.; He, J. Conductive Metal–Organic Frameworks: Mechanisms, Design Strategies and Recent Advances. Top. Curr. Chem. 2020, 378, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yasseri, A.A.; Lindsey, J.S.; Bocian, D.F. Molecular Memories That Survive Silicon Device Processing and Real-World Operation. Science 2003, 302, 1543–1545. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2019; Available online: https://www.wiley.com/en-sg/Infrared+and+Raman+Characteristic+Group+Frequencies%3A+Tables+and+Charts%2C+3rd+Edition-p-9780470093078 (accessed on 16 December 2023).












| Compound | ELUMO (eV) | EHOMO (eV) | Eg (eV) |
|---|---|---|---|
| U | −3.43 | −5.74 | 2.31 |
| [CoU]n | −3.92 | −5.95 | 2.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Chernyshev, A.; Panthi, Y.R.; Zedník, J.; Šturcová, A.; Konefał, M.; Kočková, O.; Foulger, S.H.; Vohlídal, J.; Pfleger, J. Synapse-Mimicking Memristors Based on 3,6-Di(tpy)-9-Phenylcarbazole Unimer and Its Copolymer with Cobalt(II) Ions. Polymers 2024, 16, 542. https://doi.org/10.3390/polym16040542
Pandey A, Chernyshev A, Panthi YR, Zedník J, Šturcová A, Konefał M, Kočková O, Foulger SH, Vohlídal J, Pfleger J. Synapse-Mimicking Memristors Based on 3,6-Di(tpy)-9-Phenylcarbazole Unimer and Its Copolymer with Cobalt(II) Ions. Polymers. 2024; 16(4):542. https://doi.org/10.3390/polym16040542
Chicago/Turabian StylePandey, Ambika, Andrei Chernyshev, Yadu Ram Panthi, Jiří Zedník, Adriana Šturcová, Magdalena Konefał, Olga Kočková, Stephen H. Foulger, Jiří Vohlídal, and Jiří Pfleger. 2024. "Synapse-Mimicking Memristors Based on 3,6-Di(tpy)-9-Phenylcarbazole Unimer and Its Copolymer with Cobalt(II) Ions" Polymers 16, no. 4: 542. https://doi.org/10.3390/polym16040542