Ethylene-Octene-Copolymer with Embedded Carbon and Organic Conductive Nanostructures for Thermoelectric Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of Fillers and Composites
3.2. XPS Data
3.3. FTIR Measurements
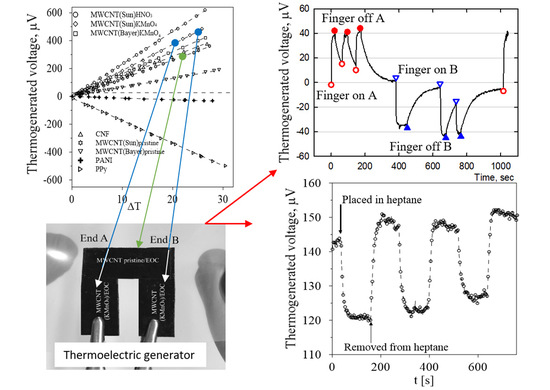
3.4. Thermoelectric Power Measurement
3.5. Self-Powered Signaling Sensor of Temperature Change
3.6. Self-Powered Vapor Sensor
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petsagkourakis, I.; Tybrant, K.; Crispin, X.; Ohkubo, I.; Satoh, N.; Mori, T. Thermoelectric materials and applications for energy harvesting power generation. Sci. Technol. Adv. Mater. 2018, 19, 836–862. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Epstein, A.J. Polyaniline—A new concept in conducting polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Hao, B.; Li, L.C.; Wang, Y.P.; Qian, H.S.; Tong, G.X.; Chen, H.F.; Chen, K.Y. Electrical and microwave absorbing properties of polypyrrole synthesized by optimum strategy. J. Appl. Polym. Sci. 2013, 127, 4273–4279. [Google Scholar] [CrossRef]
- Trivedi, D. Polyanilines. In Handbook of Organic Conductive Molecules and Polymers; Nalwa, H.S., Ed.; Wiley: Chichester, UK, 1997; Volume 2, pp. 505–572. [Google Scholar]
- Wang, W.J.; Sun, S.P.; Gu, S.J.; Shen, H.W.; Zhang, Q.H.; Zhu, J.J.; Wang, L.J.; Jiang, W. One-pot fabrication and thermoelectric properties of Ag nanoparticles-polyaniline hybrid nanocomposites. RSC Adv. 2014, 51, 26810–26816. [Google Scholar] [CrossRef]
- Mitra, M.; Kulsi, C.; Kargupta, K.; Ganguly, S.; Banerjee, D. Composite of polyaniline-bismuth selenide with enhanced thermoelectric performance. J. Appl. Polym. Sci. 2018, 135, 46887. [Google Scholar] [CrossRef]
- Ube, T.; Koyanagi, J.; Kosaki, T.; Fujimoto, K.; Yokozeki, T.; Ishiguro, T.; Nishio, K. Fabrication of well-isolated graphene and evaluation of thermoelectric performance of polyaniline-graphene composite film. J. Mat. Sci. 2019, 54, 3904–3913. [Google Scholar] [CrossRef]
- Liang, L.R.; Chen, G.M.; Guo, C.Y. Polypyrrole nanostructures and their thermoelectric performance. Mat. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Misra, S.; Bharti, M.; Singh, A.; Debnath, A.K.; Aswal, D.K.; Hayakawa, Y. Nanostructured polypyrrole: Enhancement in thermoelectric figure of merit through suppression of thermal conductivity. Mat. Res. Exp. 2017, 4, 085007. [Google Scholar] [CrossRef]
- Du, Y.; Niu, H.; Li, J.; Dou, Y.C.; Shen, S.Z.; Jia, R.P.; Xu, J.Y. Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites. Polymers 2018, 10, 1143. [Google Scholar] [CrossRef] [Green Version]
- Aghelinejad, M.; Zhang, Y.C.; Leung, S.N. Processing parameters to enhance the electrical conductivity and thermoelectric power factor of polypyrrole/multi-walled carbon nanotubes nanocomposites. Synt. Met. 2019, 247, 59–66. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Debnath, A.K.; Aswal, D.K.; Muthe, K.P.; Gadkari, S.C. Flexo-green Polypyrrole–Silver nanocomposite films for thermoelectric power generation. Energ. Convers. Manage. 2017, 144, 143–152. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, J.; Wang, L.Y.; Du, K.; Yin, Q.; Yin, Q.J. Polypyrrole/Graphene/Polyaniline Ternary Nanocomposite with High Thermoelectric Power Factor. ACS Appl. Mat. Interf. 2017, 9, 20124–20131. [Google Scholar] [CrossRef] [PubMed]
- Slobodian, P.; Riha, P.; Olejnik, R.; Benlikaya, R. Analysis of sensing properties of thermoelectric vapor sensor made of carbon nanotubes/ethylene-octene copolymer composites. Carbon 2016, 110, 257–266. [Google Scholar] [CrossRef]
- Nonoguchi, Y.; Ohashi, K.; Kanazawa, R.; Ashiba, K.; Hata, K.; Nakagawa, T.; Adachi, C.; Tanase, T.; Kawai, T. Systematic conversion of single walled carbon nanotubes into n-type thermoelectric materials by molecular dopants. Sci. Rep. 2013, 3, 3344. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.F.; Chen, S.; Wang, H.; Shen, S.Z.; Donelson, R.; Lin, T. Thermoelectric fabrics: Toward power generating clothing. Sci. Rep. 2015, 5, 6144. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.H.; Hou, T.; Zhang, F.; Wang, Z.L. Nanowure-composite based flexible thermoelectrics nanogenerqators and self-powered temperature sensors. Nano Res. 2012. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.; Paul, B.; Eklund, P. Review: Flexible thermoelectric materials and devices. Appl. Mater. Today 2018, 18, 366–388. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Lengalova, A.; Svoboda, P.; Saha, P. Multi-wall carbon nanotube networks as potential resistive gas sensors for organic vapor detection. Carbon 2011, 49, 2499–2507. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Benlikaya, R.; Slobodian, P.; Riha, P. Enhanced strain-dependent electrical resistance of polyurethane composites with embedded oxidized multiwalled carbon nanotube networks. J. Nanomat. 2013, 2013, 327597. [Google Scholar] [CrossRef]
- Jansson, P.A. (Ed.) Deconvolution of Spectra and Images; Academic Press: San Diego, CA, USA, 1997; pp. 119–134. [Google Scholar]
- Hernadi, K.; Siska, A.; Thien-Nga, L.; Forro, L.; Kiricsi, I. Reactivity of different kinds of carbon during oxidative purification of catalytically prepared carbon nanotubes. Solid State Ionics 2001, 141, 203–209. [Google Scholar] [CrossRef]
- Rasheed, A.; Howe, J.Y.; Dadmun, M.D.; Britt, P.F. The efficiency of the oxidation of carbon nanofibers with various oxidizing agents. Carbon 2007, 45, 1072–1080. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrothe, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Ros, T.G.; Van Dillen, A.J.; Geus, J.W.; Koningsberger, D.C. Surface oxidation of carbon nanofibres. Chem. Eur. J. 2002, 8, 1151–1162. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Ma, Q. Influence of surface functionalization via chemical oxidation on the properties of carbon nanotubes. J. Coll. Int. Sci. 2012, 370, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Abuilaiwi, F.A.; Laoui, T.; Al-Harthi, M.; Atieh, M.A. Modification and functionalization of multiwalled carbon nanotubes (MWCNT) via Fischer esterification. Arab. J. Sci. Eng. 2010, 35, 37–48. [Google Scholar]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Lopez-Ramon, M.V.; Carrasco-Marın, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Kim, U.J.; Furtado, C.A.; Liu, X.; Chen, G.; Eklund, P.C. Raman and IR spectroscopy of chemically processed single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 15437–15445. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of chemical oxidation on the structure of sigle-walled nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Yi, B.; Zhang, Z.; Tan, Z. Preparation and characterization of multi-walled carbon nanotubes supported Pt Ru catalysts for proton exchange membrane fuel cells. Carbon 2005, 43, 3144–3152. [Google Scholar] [CrossRef]
- Tang, J.S.; Jing, X.B.; Wang, B.C.; Wang, F. Infrared-spectra of soluble polyaniline. Synth. Met. 1988, 24, 231–238. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Chiang, J.C.; Huang, W.S.; Humphery, B.D.; Somasiri, N.L.D. Polyaniline: Protonic acid doping to the metallic regime. Mol. Cryst. Liquid Cryst. 1985, 25, 309–318. [Google Scholar] [CrossRef]
- Brožová, L.; Holler, P.; Kovářová, J.; Stejskal, J.; Trchová, M. The stability of polyaniline in strongly alkaline or acidic aqueous media. Polym. Degrad. Stabil. 2008, 93, 592–600. [Google Scholar] [CrossRef]
- Wang, J.G.; Neoh, K.G.; Kang, E.T. Comparative study of chemically synthesized and plasma polymerized pyrrole and thiophene thin films. Thin Solid Films 2004, 446, 205–217. [Google Scholar] [CrossRef]
- Cheah, K.; Forsyth, M.; Truong, V.T. Ordering and stability in conducting polypyrrole. Synth. Met. 1998, 94, 215–219. [Google Scholar] [CrossRef]
- Krause, B.; Barbier, C.; Levente, J.; Klaus, M.; Pötschke, P. Screening of different carbon nanotubes in melt-mixed polymer composites with different polymer matrices for their thermoelectrical properties. J. Composit. Sci. 2019, 3, 106. [Google Scholar] [CrossRef] [Green Version]












| Wavenumber (cm−1) | |||
|---|---|---|---|
| Possible Assignments | MWCNT | MWCNT | MWCNT |
| (HNO3) | (KMnO4) | ||
| OH stretch | 3435 | 3428 | 3427 |
| C–H stretch (CH2, CH3) | 2908,2840 | 2980,2880 | 2978,2890 |
| C=O stretch (carboxyl or ketone) | 1705 | 1726 | 1710 |
| Intermediate oxidized products—quinone groups | 1652 | 1661,1635 | 1641 |
| C=C stretch | 1559 | 1580 | 1569 |
| CH2/CH3 bending | 1460 | 1437 | 1440 |
| Skeletal C-C tangential motions +C–O stretch | 1222 | 1184 | 1190 |
| C–O stretch | 1082 | 1084,1049 | 1087,1046 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slobodian, P.; Riha, P.; Olejnik, R.; Sedlacik, M. Ethylene-Octene-Copolymer with Embedded Carbon and Organic Conductive Nanostructures for Thermoelectric Applications. Polymers 2020, 12, 1316. https://doi.org/10.3390/polym12061316
Slobodian P, Riha P, Olejnik R, Sedlacik M. Ethylene-Octene-Copolymer with Embedded Carbon and Organic Conductive Nanostructures for Thermoelectric Applications. Polymers. 2020; 12(6):1316. https://doi.org/10.3390/polym12061316
Chicago/Turabian StyleSlobodian, Petr, Pavel Riha, Robert Olejnik, and Michal Sedlacik. 2020. "Ethylene-Octene-Copolymer with Embedded Carbon and Organic Conductive Nanostructures for Thermoelectric Applications" Polymers 12, no. 6: 1316. https://doi.org/10.3390/polym12061316