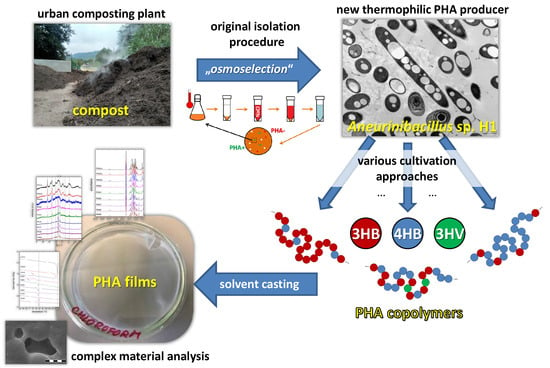
Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers–1. Isolation and Characterization of the Bacterium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of the Thermophilic PHA Producer
2.2. Identification and Metabolic and Morphological Characterization of the Isolate
2.3. Production of PHA in Shaking Flasks by Aneurinibacillus sp. H1
3. Results and Discussion
3.1. Taxonomic, Metabolic and Morphological Description the Isolate
3.2. Production of PHA on Various Carbon and Nitrogen Substrates
3.3. Influence of Temperature on Production of PHA
3.4. Biosynthesis of PHA Copolymers and Terpolymer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zeldes, B.M.; Keller, M.W.; Loder, A.J.; Straub, C.T.; Adams, M.W.W.; Kelly, R.M. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front. Microbiol. 2015, 6, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranawat, P.; Rawat, S. Stress response physiology of thermophiles. Arch. Microbiol. 2017, 199, 391–414. [Google Scholar] [PubMed]
- Chen, G.-Q.; Jiang, X.-R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [PubMed]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018, 36, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Maršálek, L.; Miranda de Sousa Dias, M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Kunioka, M.; Tamaki, A.; Doi, Y. Crystalline and thermal properties of bacterial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1989, 22, 694–697. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [Green Version]
- Koller, M. Chemical and biochemical engineering approaches in manufacturing Polyhydroxyalkanoate (PHA) biopolyesters of tailored structure with focus on the diversity of building blocks. Chem. Biochem. Eng. Q. 2019, 32, 413–438. [Google Scholar] [CrossRef]
- Koller, M. Switching from petro-plastics to microbial polyhydroxyalkanoates (PHA): The biotechnological escape route of choice out of the plastic predicament? Eur. Biotechnol. J. 2019, 3, 32–44. [Google Scholar]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Sustainable PHA production in integrated lignocellulose biorefineries. New Biotechnol. 2019, 49, 161–168. [Google Scholar]
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuel Bioprod. Biorefin. 2019, 13, 208–227. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.H.; Willems, A.; Steinbüchel, A. Isolation and characterization of new poly (3HB)-accumulating star-shaped cell aggregates-forming thermophilic bacteria. J. Appl. Microbiol. 2010, 109, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Pantazaki, A.A.; Tambaka, M.G.; Langlois, V.; Guerin, P.; Kyriakidis, D.A. Polyhydroxyalkanoate (PHA) biosynthesis in Thermus thermophilus: Purification and biochemical properties of PHA synthase. Mol. Cell. Biochem. 2003, 254, 173–183. [Google Scholar] [CrossRef]
- Sheu, D.S.; Chen, W.M.; Yang, J.Y.; Chang, R.C. Thermophilic bacterium Caldimonas taiwanensis produces poly (3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzyme Microb. Technol. 2009, 44, 289–294. [Google Scholar]
- Pernicova, I.; Novackova, I.; Sedlacek, P.; Kourilova, X.; Koller, M.; Obruca, S. Application of osmotic challenge for enrichment of microbial consortia in polyhydroxyalkanoates producing thermophilic and thermotolerant bacteria and their subsequent isolation. Int. J. Biol. Macromol. 2020, 144, 698–704. [Google Scholar]
- Nováková, D.; Švec, P.; Zeman, M.; Busse, H.-J.; Mašlaňová, I.; Pantůček, R.; Králová, S.; Krištofová, L.; Sedláček, I. Pseudomonas leptonychotis sp. nov., isolated from weddell seals in Antarctica. Int. J. Syst. Evol. Microbiol. 2020, 70, 302–308. [Google Scholar]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Krzyzanek, V.; Nebesarova, J.; Samek, O.; Kucera, D.; Benesova, P.; Hrubanova, K.; Milerova, M.; et al. The presence of PHB granules in cytoplasm protects non-halophilic bacterial cells against the harmful impact of hypertonic environments. New Biotechnol. 2017, 39, 68–80. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Oborna, J.; Marova, I. Application of protease-hydrolyzed whey as a complex nitrogen source to increase poly(3-hydroxybutyrate) production from oils by Cupriavidus necator. Biotechnol. Lett. 2014, 36, 775–781. [Google Scholar]
- Novackova, I.; Kucera, D.; Porizka, J.; Pernicova, I.; Sedlacek, P.; Koller, M.; Kovalcik, A.; Obruca, S. Adaptation of Cupriavidus necator to levulinic acid for enhanced production of P(3HB-co-3HV) copolyesters. Biochem. Eng. J. 2019, 151, 107350. [Google Scholar]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Ilieva, V.I.; Sikorska, W.; Musioł, M.; Ziȩba, M.; Marek, A.A.; Keddie, D.; et al. The microbial production of Polyhydroxyalkanoates from waste polystyrene fragments attained using oxidative degradation. Polymers 2018, 10, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadzil, F.I.M.; Mizuno, S.; Hiroe, A.; Nomura, C.T.; Tsuge, T. Low Carbon concentration feeding improves medium-chain-length polyhydroxyalkanoate production in Escherichia coli strains with defective β-oxidation. Front. Bioeng. Biotechnol. 2018, 6, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernicova, I.; Kucera, D.; Nebesarova, J.; Kalina, M.; Novackova, I.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates on waste frying oil employing selected Halomonas strains. Bioresour. Technol. 2019, 292, 122028. [Google Scholar] [PubMed]
- Ye, J.; Hu, D.; Yin, J.; Huang, W.; Xiang, R.; Zhang, L.; Wang, X.; Han, J.; Chen, G.-Q. Stimulus response-based fine-tuning of polyhydroxyalkanoate pathway in Halomonas. Metab. Eng. 2020, 57, 85–95. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar]
- Kumar, P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol. Adv. 2013, 31, 1543–1561. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, B.S. Valorization of polyhydroxyalkanoates production process by co-synthesis of value-added products. Bioresour. Technol. 2018, 269, 544–556. [Google Scholar] [CrossRef]
- Kumar, P.; Ray, S.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int. J. Biol. Macromol. 2015, 78, 9–16. [Google Scholar] [CrossRef]
- Shida, O.; Takagi, H.; Kadowaki, K.; Komagata, K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. Int. J. Syst. Bacteriol. 1996, 46, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Zhang, Y.; Xi, L.; Huo, F.; Zhao, J.-Y.; Li, J. Thermophilic production of polyhydroxyalkanoates by a novel Aneurinibacillus strain isolated from Gudao oilfield, China. J. Basic Microb. 2015, 55, 1125–1133. [Google Scholar] [CrossRef]
- Mravec, F.; Obruca, S.; Krzyzanek, V.; Sedlacek, P.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Nebesarova, J. Accumulation of PHA granules in Cupriavidus necator as seen by confocal fluorescence microscopy. FEMS Microbiol. Lett. 2016, 363, fnw094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucera, D.; Pernicová, I.; Kovalcik, A.; Koller, M.; Mullerova, L.; Sedlacek, P.; Mravec, F.; Nebesarova, J.; Kalina, M.; Marova, I.; et al. Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Bioresour. Technol. 2018, 256, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, M.R.; Ahn, J.-S.; Widhelm, T.J.; Eckrich, V.M.; Endres, J.L.; Driks, A.; Rutkowski, G.E.; Wingerd, K.L.; Bayles, K.W. Poly(3-hydroxybutyrate) fuels the tricarboxylic acid cycle and de novo lipid biosynthesis during Bacillus anthracis sporulation. Mol. Microbiol. 2017, 104, 793–803. [Google Scholar]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Keshavarz, T.; Bucke, C.; Roy, I. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J. Biotechnol. 2007, 132, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar]
- Mohandas, S.P.; Balan, L.; Jayanath, G.; Anoop, B.S.; Philip, R.; Cubelio, S.S.; Bright Singh, I.S. Biosynthesis and characterization of polyhydroxyalkanoate from marine Bacillus cereus MCCB 281 utilizing glycerol as carbon source. Int. J. Biol. Macromol. 2018, 119, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Soni, S.K. Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour. Technol. 2017, 243, 492–501. [Google Scholar] [CrossRef]
- Zain, N.F.M.; Abdullah, W.N.W.; Shun, T.J.; Keong, L.C.; Samian, M.R. Optimization of polyhydroxyalkanoate (PHA) production by Burkholderia cepacia BPT1213 utilizing waste glycerol as the sole carbon source. Malaysian J. Microbiol. 2018, 14, 164–171. [Google Scholar]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co- and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, L.-J.; Lee, M.-C.; Chuang, P.-J.; Kuo, Y.-Y.; Lin, J.-H.; Wu, T.-M.; Li, S.-Y. The production of poly(3-hydroxybutyrate) by thermophilic Caldimonas manganoxidans from glycerol. J. Polym. Res. 2018, 25, 85. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Steinbüchel, A. High-cell-density cyclic fed-batch fermentation of a poly (3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl. Environ. Microbiol. 2010, 76, 7890–7895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneswari, S.; Vijaya, S.; Majid, M.I.A.; Sudesh, K.; Sipaut, C.S.; Azizan, M.N.M.; Amirul, A.A. Enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer with manipulated variables and its properties. J. Ind. Microbiol. Biotechnol. 2009, 36, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Huong, K.-H.; Mohd Yahya, A.R.; Amirul, A.A. Pronounced synergistic influence of mixed substrate cultivation on single step copolymer P(3HB-co-4HB) biosynthesis with a wide range of 4HB monomer composition. J. Chem. Technol. Biotechnol. 2014, 89, 1023–1029. [Google Scholar] [CrossRef]
- Lee, W.-H.; Azizan, M.N.M.; Sudesh, K. Efects of culture conditions on the composition of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Comamonas acidovorans. Polym. Degrad. Stab. 2004, 84, 129–134. [Google Scholar] [CrossRef]
- Kucera, D.; Novackova, I.; Pernicova, I.; Sedlacek, P.; Obruca, S. Biotechnological Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate-co-3-hydroxyvalerate) terpolymer by Cupriavidus sp. DSM 19379. Bioengineering 2019, 6, 74. [Google Scholar] [CrossRef] [Green Version]


| Substrate | CDM [g/L] | P(3HB) [% per CDM] | P(3HB) [g/L] |
|---|---|---|---|
| Sucrose | 0.41 ± 0.10 | 16.52 ± 0.31 | 0.07 ± 0.02 |
| Mannose | 0.19 ± 0.08 | 14.85 ± 0.05 | 0.03 ± 0.01 |
| Galactose | 0.22 ± 0.04 | 8.94 ± 0.13 | 0.02 ± 0.00 |
| Glucose | 2.00 ± 0.36 | 27.74 ± 0.18 | 0.55 ± 0.10 |
| Fructose | 0.24 ± 0.01 | 14.32 ± 0.21 | 0.03 ± 0.00 |
| Lactose | 0.20 ± 0.01 | 9.06 ± 0.20 | 0.02 ± 0.00 |
| Glycerol | 2.19 ± 0.07 | 45.95 ± 0.10 | 1.00 ± 0.04 |
| WFO 1 | 0.01 ± 0.00 | n.d. | n.d. |
| Substrate | Concentration [g/L] | Biomass [g/L] | P(3HB) [% per CDM] | P(3HB) [g/L] |
|---|---|---|---|---|
| Glycerol | 10 | 1.60 ± 0.50 | 13.00 ± 1.20 | 0.21 ± 0.03 |
| 20 | 3.27 ± 0.13 | 51.97 ± 2.28 | 1.70 ± 0.11 | |
| 30 | 2.72 ± 0.07 | 53.40 ± 4.82 | 1.45 ± 0.19 | |
| Waste/crude Glycerol | 10 | 1.08 ± 0.08 | 10.78 ± 1.33 | 0.12 ± 0.02 |
| 20 | 2.88 ± 0.13 | 49.45 ± 0.65 | 1.42 ± 0.03 | |
| 30 | 1.67 ± 0.32 | 46.10 ± 5.55 | 0.77 ± 0.13 |
| Temperature [°C] | Biomass [g/L] | P(3HB) [% per CDM] | P(3HB) [g/L] |
|---|---|---|---|
| 35 | 0.17 ± 0.01 | 30.20 ± 0.01 | 0.05 ± 0.01 |
| 40 | 2.66 ± 0.14 | 32.12 ± 4.49 | 0.92 ± 0.08 |
| 45 | 3.68 ± 0.63 | 55.31 ± 5.81 | 2.03 ± 0.41 |
| 50 | 3.23 ± 0.03 | 46.01 ± 3.55 | 1.49 ± 0.37 |
| 55 | 1.46 ± 0.05 | 50.18 ± 1.35 | 0.73 ± 0.03 |
| 60 | 0.80 ± 0.01 | 30.60 ± 1.35 | 0.24 ± 0.01 |
| 65 | 0.89 ± 0.06 | 32.66 ± 0.24 | 0.29 ± 0.02 |
| Desired Monomer | Precursor | Biomass [g/L] | PHA [% per CDM] | PHA [g/L] | 3HV [mol %] | 4HB [mol %] | 3HB [mol %] |
|---|---|---|---|---|---|---|---|
| 3HV 1 | Levulinate | 1.08 ± 0.06 | 36.70 ± 0.72 | 0.39 ± 0.02 | n.d. | n.d. | 100.00 ± 0.00 |
| Propionate | 1.11 ± 0.04 | 28.15 ± 0.27 | 0.31 ± 0.01 | 32.10 ± 0.02 | n.d. | 67.90 ± 0.02 | |
| Propanol | 1.47 ± 0.04 | 45.11 ± 0.05 | 0.66 ± 0.02 | 3.66 ± 0.04 | n.d. | 96.34 ± 0.04 | |
| Valerate | 1.90 ± 0.02 | 36.29 ± 0.01 | 0.69 ± 0.01 | 66.62 ± 0.83 | n.d. | 33.38 ± 0.83 | |
| 4HB 2 | 1,6-hexandiol | 0.37 ± 0.08 | 9.08 ± 0.31 | 0.03 ± 0.01 | n.d. | n.d. | 100 ± 0.00 |
| γ-butyrolactone | 0.52 ± 0.00 | 85.84 ± 1.99 | 0.45 ± 0.01 | n.d. | 63.35 ± 3.27 | 36.65 ± 3.27 | |
| 1,4-butandiol | 1.02 ± 0.04 | 75.48 ± 2.36 | 0.77 ± 0.04 | n.d. | 79.91 ± 1.84 | 20.09 ± 1.84 | |
| 3HV + 4HB 3 | Valerate + 1,4-butanediol | 1.44 ± 0.06 | 40.27 ± 2.08 | 0.58 ± 0.03 | 33.13 ± 1.03 | 54.18 ± 1.23 | 12.69 ± 2.26 |
| 1,4-BD [g/L] | Glycerol [g/L] | Biomass [g/L] | PHA in CDM [%] | PHA [g/L] | 4HB in PHA [mol %] |
|---|---|---|---|---|---|
| 3 | 0 | 1.22 ± 0.11 | 50.14 ± 2.65 | 0.61 ± 0.07 | 90.56 ± 0.70 |
| 4 | 0 | 1.67 ± 0.02 | 54.78 ± 1.21 | 0.91 ± 0.02 | 90.89 ± 1.19 |
| 5 | 0 | 1.60 ± 0.23 | 45.73 ± 0.65 | 0.73 ± 0.11 | 88.31 ± 0.29 |
| 6 | 0 | 1.23 ± 0.15 | 56.32 ± 0.56 | 0.69 ± 0.08 | 92.81 ± 0.04 |
| 7 | 0 | 1.13 ± 0.01 | 51.80 ± 0.71 | 0.58 ± 0.01 | 88.02 ± 0.48 |
| 8 | 0 | 0.96 ± 0.00 | 44.00 ± 2.76 | 0.42 ± 0.03 | 86.87 ± 1.32 |
| 12 | 0 | 0.72 ± 0.06 | 43.90 ± 2.28 | 0.32 ± 0.03 | 83.61 ± 0.94 |
| 16 | 0 | 0.56 ± 0.05 | 43.38 ± 1.26 | 0.24 ± 0.02 | 85.99 ± 4.40 |
| 4 | 2 | 2.69 ± 0.15 | 68.25 ± 0.62 | 1.83 ± 0.10 | 83.56 ± 1.48 |
| 4 | 4 | 2.79 ± 0.03 | 65.23 ± 2.64 | 1.82 ± 0.08 | 74.43 ± 1.70 |
| 4 | 6 | 2.28 ± 0.26 | 50.37 ± 0.87 | 1.15 ± 0.13 | 42.45 ± 7.88 |
| 4 | 8 | 2.43 ± 0.01 | 40.74 ± 3.35 | 0.99 ± 0.08 | 36.42 ± 1.63 |
| 4 | 20 | 2.56 ± 0.11 | 44.69 ± 1.23 | 1.14 ± 0.06 | 4.59 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pernicova, I.; Novackova, I.; Sedlacek, P.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers–1. Isolation and Characterization of the Bacterium. Polymers 2020, 12, 1235. https://doi.org/10.3390/polym12061235
Pernicova I, Novackova I, Sedlacek P, Kourilova X, Kalina M, Kovalcik A, Koller M, Nebesarova J, Krzyzanek V, Hrubanova K, et al. Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers–1. Isolation and Characterization of the Bacterium. Polymers. 2020; 12(6):1235. https://doi.org/10.3390/polym12061235
Chicago/Turabian StylePernicova, Iva, Ivana Novackova, Petr Sedlacek, Xenie Kourilova, Michal Kalina, Adriana Kovalcik, Martin Koller, Jana Nebesarova, Vladislav Krzyzanek, Kamila Hrubanova, and et al. 2020. "Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers–1. Isolation and Characterization of the Bacterium" Polymers 12, no. 6: 1235. https://doi.org/10.3390/polym12061235