On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characteristics
2.2. Textural Characteristics
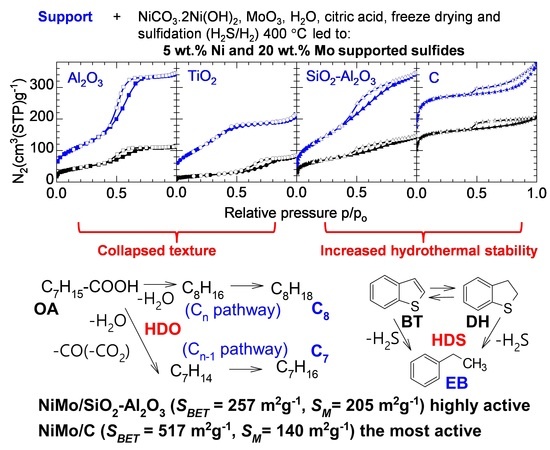
2.2.1. Supports
2.2.2. NiMo Sulfide Catalysts
2.3. Catalytic Activity
2.4. Textural Stability during HDO/HDS Reactions
2.5. Selectivity in HDO/HDS Reactions
2.5.1. HDO/HDS Selectivity
2.5.2. Selectivity to Reaction Intermediates
2.6. Recapitulative Discussion and Outlook
- The method of catalyst preparation should be tailored to the specific character of each individual support.
- The supports on the base of SiO2-Al2O3 represent a promising alternative to single oxides because they could be tuned in a wide range of acid-base properties [38,39,40,41,42,43,44] and because they increase dispersion of MoS2 compared to SiO2 [37]. Acidity seems crucial for the HDO of fatty acid containing feeds because it influences the formation of linear or branched hydrocarbons [21,41].
- The active phase should hydrogenate olefins, the reaction intermediates of the HDO of fatty-acid-containing feeds. In this respect, the most promising support of the NiMo phase was found to be the activated carbon. Activated carbon as well as the use of citric acid contributes to low metal-support interaction and stability of Type II NiMoS phase containing a high number of corner sites [36,37].
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing challenges in biofuels production. Catal. Today 2013, 217, 13–56. [Google Scholar] [CrossRef]
- Platanitis, P.; Panagiotou, G.D.; Bourikas, K.; Kodulis, C.; Lycourghiotis, A. Hydrodeoxygenation of phenol over hydrotreatment catalysts in their reduced and sulfided states. Open Catal. J. 2014, 7, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Xia, Z.; Tang, W.; Wang, H.; Fang, M. Hydrogenation of Model Compounds Catalyzed by MCM-41-Supported Nickel Phosphide. Adv. Mater. Res. 2014, 864–867, 366–372. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, G.; Li, L.; Tan, S.; Wu, K.; Zhang, X.; Yang, Y. Facile hydrothermal synthesis of flower-like Co–Mo–S catalysts and their high activities in the hydrodeoxygenation of p-cresol and hydrodesulfurization of benzothiophene. Fuel 2016, 174, 1–8. [Google Scholar] [CrossRef]
- Dhandapani, B.; Clair, T.; Oyama, S.T. Simultaneous hydrodesulfurization, hydrodeoxygenation, and hydrogenation with molybdenum carbide. Appl. Catal. A 1998, 168, 219–228. [Google Scholar] [CrossRef]
- Odebunmi, E.O.; Ollis, D.F. Catalytic hydrodeoxygenation: II. Interactions between catalytic hydrodeoxygenation of m-cresol and hydrodesulfurization of benzothiophene and dibenzothiophene. J. Catal. 1983, 80, 65–75. [Google Scholar] [CrossRef]
- Pstrowska, K.; Walendziewski, J.; Łuzny, R.; Stolarski, M. Hydroprocessing of rapeseed pyrolysis bio-oil over NiMo/Al2O3 catalyst. Catal. Today 2014, 223, 54–65. [Google Scholar] [CrossRef]
- Pstrowska, K.; Walendziewski, J.; Stolarski, M. Hydrorefining of oil from rapeseed cake pyrolysis over NiMo/Al2O3 catalyst. Fuel Process. Technol. 2014, 128, 191–198. [Google Scholar] [CrossRef]
- Pinheiro, A.; Hudebine, D.; Dupassieux, N.; Geantet, C. Impact of oxygenated compounds from lignocellulosic biomass pyrolysis oils on gas oil hydrotreatment. Eng. Fuel. 2009, 23, 1007–1014. [Google Scholar] [CrossRef]
- Bui, V.N.; Toussaint, G.; Laurenti, D.; Mirodatos, C.; Geantet, C. Co-processing of pyrolisis bio oils and gas oil for new generation of bio-fuels: Hydrodeoxygenation of guaïacol and SRGO mixed feed. Catal. Today 2009, 143, 172–178. [Google Scholar] [CrossRef]
- Vonortas, A.; Kubicka, D.; Papayannakos, N. Catalytic co-hydroprocessing of gasoil–palm oil/AVO mixtures over a NiMo/γ-Al2O3 catalyst. Fuel 2014, 116, 49–55. [Google Scholar] [CrossRef]
- Varakin, A.N.; Salnikov, V.A.; Nikulshina, M.S.; Maslakov, K.I.; Mozhaev, A.V.; Nikulshin, P.A. Beneficial role of carbon in Co(Ni)MoS catalysts supported on carbon-coated alumina for co-hydrotreating of sunflower oil with straight-run gas oil. Catal. Today 2017, 292, 110–120. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Salnikov, V.A.; Pimerzin, A.A.; Eremina, Y.V.; Koklyukhin, A.S.; Tsvetkov, V.S.; Pimerzin, A.A. Co-hydrotreating of straight-run diesel fraction and vegetable oil on Co(Ni)-PMo/Al2O3 catalysts. Petrol. Chem. 2016, 56, 56–61. [Google Scholar] [CrossRef]
- Eller, Z.; Varga, Z.; Hancsók, J. Renewable Jet Fuel from Kerosene/Coconut Oil Mixtures with Catalytic Hydrogenation. Energ. Fuel. 2019, 33, 6444–6453. [Google Scholar] [CrossRef]
- Mercader, F.M.; Groeneveld, M.J.; Kersten, S.R.A.; Geantet, C.; Toussaint, G.; Way, N.W.J.; Schaverien, C.J.; Hogendoorn, K.J.A. Hydrodeoxygenation of pyrolysis oil fractions: Process understanding and quality assessment through co-processing in refinery units. Eng. Environ. Sci. 2011, 4, 985–997. [Google Scholar]
- Sepúlveda, C.; Escalona, N.; García, R.; Laurenti, D.; Vrinat, M. Hydrodeoxygenation and hydrodesulfurization co-processing over ReS2 supported catalysts. Catal. Today 2012, 195, 101–105. [Google Scholar] [CrossRef]
- Vonortas, A.; Papayannakos, N. Hydrodesulphurization and hydrodeoxygenation of gasoil-vegetable oil mixtures over a Pt/γ-Al2O3 catalyst. Fuel Process. Technol. 2016, 150, 126–131. [Google Scholar] [CrossRef]
- Morales-Hernández, G.; Pacheco-Sosa, J.G.; Escobar-Aguilar, J.; Torres, J.G.T.; Pérez-Vidal, H.; Lunagómez-Rocha, M.A.; De La Cruz-Romero, D.; Del Ángel-Vicente, P. Improving platinum dispersion on sba-15 by titania addition. Rev. Mex. Ing. Quim. 2020, 19, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Kaluža, L.; Karban, J.; Gulková, D. Activity and selectivity of Co(Ni)Mo sulfides supported on MgO, Al2O3, ZrO2, TiO2, MCM-41 and activated carbon in parallel hydrodeoxygenation of octanoic acid and hydrodesulfurization of 1-benzothiophene Reac. Kinet. Mech. Cat. 2019, 127, 887–902. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Sato, T.; Shimada, H.; Matsubayashi, N.; Nishijima, A. Influences of oxygen-containing substances on deactivation of sulfided molybdate catalysts. Appl. Catal. 1991, 73, 55–63. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Influence of water in the deactivation of a sulfided NiMoγ-Al2O3 catalyst during hydrodeoxygenation. J. Catal. 1994, 146, 281–291. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Chang, L. Study of reactions over sulfide catalysts in CO–CO2–H2–H2O system. Catal. Today 1999, 51, 25–38. [Google Scholar] [CrossRef]
- Senol, O.I.; Viljava, T.R.; Krause, A.O.I. Hydrodeoxygenation of aliphatic esters on sulphided NiMo/γ-Al2O3 and CoMo/γ-Al2O3 catalyst: The effect of water. Catal. Today 2005, 106, 186–189. [Google Scholar]
- Pinheiro, A.; Dupassieux, N.; Hudebine, D.; Geantet, C. Impact of the Presence of Carbon Monoxide and Carbon Dioxide on Gas Oil Hydrotreatment: Investigation on Liquids from Biomass Cotreatment with Petroleum Cuts. Eng. Fuel. 2011, 25, 804–812. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A model compound (methyl oleate, oleic acid, triolein) study of triglycerides hydrodeoxygenation over alumina-supported NiMo sulfide. Appl. Catal. B Environ. 2017, 201, 290–301. [Google Scholar] [CrossRef]
- Mejias, J.A.; Berry, A.J.; Refson, K.; Fraser, D.G. The kinetics and mechanism of MgO dissolution Chem. Phys. Lett. 1999, 314, 558–563. [Google Scholar]
- Klicpera, T.; Zdražil, M. Preparation of high-activity MgO-supported Co–Mo and Ni–Mo sulfide hydrodesulfurization catalysts. J. Catal. 2002, 206, 314–320. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Bukhtiyarova, G.A.; Deliy, I.V.; Aleksandrov, P.V.; Porsin, A.A.; Panafidin, M.A.; Gerasimov, E.Y.; Bukhtiyarov, V.I. The effect of rapeseed oil and carbon monoxide on SRGO hydrotreating over sulfide CoMo/Al2O3 and NiMo/Al2O3 catalysts. Catal. Today 2020, 357, 526–533. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Yatsenko, D.A.; Gerasimov, E.Y.; Vlasova, E.; Bukhtiyarova, G.A.; Tsybulya, S.V. Total Scattering Debye Function Analysis: Effective Approach for Structural Studies of Supported MoS2-Based Hydrotreating Catalysts. Ind. Eng. Chem. Res. 2020, 59, 10914–10922. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; pp. 1–303. [Google Scholar]
- Kaluža, L.; Gulková, D.; Šolcová, O.; Žilková, N.; Čejka, J. Hydrotreating catalysts supported on organized mesoporous alumina: Optimization of Mo deposition and promotional effects of Co and Ni. Appl. Catal. A Gen. 2008, 351, 93–101. [Google Scholar] [CrossRef]
- Kaluža, L.; Zdražil, M.; Žilková, N.; Čejka, J. High activity of highly loaded MoS2 hydrodesulfurization catalysts supported on organised mesoporous alumina. Catal. Commun. 2002, 3, 151–157. [Google Scholar] [CrossRef]
- Čejka, J.; Žilková, N.; Kaluža, L.; Zdražil, M. Mesoporous alumina as a support for hydrodesulfurization catalysts. Stud. Surf. Sci. Catal. 2002, 141, 243–250. [Google Scholar]
- Escobar, J.; Barrera, M.C.; Gutiérrez, A.W.; Terrazas, J.E. Benzothiophene hydrodesulfurization over NiMo/alumina catalysts modified by citric acid. Effect of addition stage of organic modifier. Fuel Process. Technol. 2017, 156, 33–42. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Kooyman, P.J.; Van der Meer, Y.; Van der Kraan, A.M.; De Beer, V.H.J.; Van Veen, J.A.R.; Van Santen, R.A. The Relation between Morphology and Hydrotreating Activity for Supported MoS2 Particles. J. Catal. 2001, 199, 224–235. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Tayakout-Fayolle, M.; Chainet, F.; Pirngruber, G.D.; Geantet, C. Use of kinetic modeling for investigating support acidity effects of NiMo sulfide catalysts on quinoline hydrodenitrogenation. C. Appl. Catal. A Gen. 2017, 530, 132–144. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A real support effect on the hydrodeoxygenation of methyl oleate by sulfided NiMo catalysts. Catal. Today 2017, 298, 181–189. [Google Scholar] [CrossRef]
- Escalona, G.; Rai, A.; Betancourt, P.; Sinha, A.K. Selective poly-aromatics saturation and ring opening during hydroprocessing of light cycle oil over sulfided Ni-Mo/SiO2-Al2O3 catalyst. Fuel 2018, 219, 270–278. [Google Scholar] [CrossRef]
- Ramesh, A.; Tamizhdurai, P.; Krishnan, P.S.; Ponnusamy, V.K.; Sakthinathan, S.; Shanthi, K. Catalytic transformation of non-edible oils to biofuels through hydrodeoxygenation using Mo-Ni/mesoporous alumina-silica catalysts. Fuel 2020, 262, 116494. [Google Scholar] [CrossRef]
- Ramesh, A.; Tamizhdurai, P.; Mangesh, V.L.; Palanichamy, K.; Gopinath, S.; Sureshkumar, K.; Shanthi, K. Mg/SiO2–Al2O3 supported nickel catalysts for the production of naphthenic hydrocarbon fuel by hydro-de-oxygenation of eugenol. Int. J. Hydrogen. Energ. 2019, 44, 25607–25620. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Danilova, I.G.; Faizullin, A.V.; Amarantov, S.V.; Bubennov, S.V.; Prosochkina, T.R.; Grigoreva, N.G.; Paukshtis, E.A.; Kutepov, B.I. Sol-gel synthesis of mesoporous aluminosilicates with a narrow pore size distribution and catalytic activity thereof in the oligomerization of dec-1-ene. Micropor. Mesopor. Mat. 2016, 230, 118–127. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Briones, L.; Arroyo, M. Selective hydrodecarboxylation of fatty acids into long-chain hydrocarbons catalyzed by Pd/Al-SBA-15. Micropor. Mesopor. Mat. 2019, 280, 88–96. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Louis, C.; Breysse, M.; Cattenot, M.; Bellière, V.; Geantet, C.; Vrinat, M.; Blanchard, P.; Payen, E.; Inoue, S.; et al. New generation of titanium dioxide support for hydrodesulfurization. Appl. Catal. B: Environ. 2003, 41, 181–191. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, B.; Cheng, X.; Wang, C.; Zhao, L.; Zhu, Y.; Zhu, J.; Lu, X. NiMo catalysts supported on graphene-modified mesoporous TiO2 toward highly efficient hydrodesulfurization of dibenzothiophene. Appl. Catal. A Gen. 2015, 502, 157–165. [Google Scholar] [CrossRef]
- Platanitis, P.; Panagiotou, G.D.; Bourikas, K.; Kordulis, C.; Fierro, J.L.G.; Lycourghiotis, A. Preparation of un-promoted molybdenum HDS catalysts supported on titania by equilibrium deposition filtration: Optimization of the preparative parameters and investigation of the promoting action of titania. J. Mol. Catal. A Chem. 2016, 412, 1–12. [Google Scholar] [CrossRef]
- Naboulsi, I.; Lebeau, B.; Aponte, C.F.L.; Brunet, S.; Mallet, M.; Michelin, L.; Bonne, M.; Carteret, C.; Blin, J.L. Selective direct desulfurization way (DDS) with CoMoS supported over mesostructured titania for the deep hydrodesulfurization of 4, 6-dimethydibenzothiophene. Appl. Catal. A Gen. 2018, 563, 91–97. [Google Scholar] [CrossRef]
- Li, M.; Song, J.; Yue, F.; Pan, F.; Yan, W.; Hua, Z.; Li, L.; Yang, Z.; Li, L.; Wen, G.; et al. Complete Hydrodesulfurization of Dibenzothiophene via Direct Desulfurization Pathway over Mesoporous TiO2-Supported NiMo Catalyst Incorporated with Potassium. Catalysts 2019, 9, 448. [Google Scholar] [CrossRef] [Green Version]
- Vít, Z.; Šolcová, O. Synthesis and properties of mesoporous silica–alumina with narrow pore size distribution obtained without use of pore-regulating agents. Micropor. Mesopor. Mater. 2006, 96, 197–204. [Google Scholar] [CrossRef]
- Kaluža, L.; Gulková, D.; Vít, Z.; Zdražil, M. Water-assisted spreading of MoO3 onto SiO2–Al2O3 supports for preparation of sulfide CoMo hydrodesulfurization catalysts. Fuel 2013, 112, 272–276. [Google Scholar] [CrossRef]
- Carrier, X.; Lambert, J.F.; Kuba, S.; Knözinger, H.; Che, M. Influence of ageing on MoO3 formation in the preparation of alumina-supported Mo catalysts. J. Mol. Struct. 2003, 656, 231–238. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Lecloux, A.; Pirard, J.P. The importance of standard isotherms in the analysis of adsorption isotherms for determining the porous texture of solids. J. Colloid Interface Sci. 1979, 70, 265–281. [Google Scholar] [CrossRef]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A Gen. 1995, 129, 157–165. [Google Scholar] [CrossRef]



| Surface Area SBET (m2 g−1) | Catalyst Activity k | ||||||
|---|---|---|---|---|---|---|---|
| Support a | Catalysts | (mmol g−1 h−1) | |||||
| Fresh b | Spent c | kHDO | kHDS | ||||
| Per Gram of Support | Per Gram of Catalyst | Per Gram of Support | Per Gram of Catalyst | Per Gram of Support | |||
| Al2O3 | 413 | 165 | 275 | 154 | 257 | 19 | 51 |
| TiO2 | 417 | 72 | 120 | 86 | 143 | 126 | 66 |
| SiO2-Al2O3 | 500 | 239 | 398 | 257 | 428 | 198 | 90 |
| C | 919 | 517 | 862 | 515 | 858 | 250 | 420 |
| Support | Fresh Catalyst | Spent Catalyst a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SBET m2 g−1 | SM m2 g−1 | VM mm3 g−1 | SBET m2 g−1 | SM m2 g−1 | VM mm3 g−1 | SBET m2 g−1 | SM m2 g−1 | VM mm3 g−1 | |
| Al2O3 | 413 | 399 | 2 | 165 | 164 | 2 | 154 | 143 | 4 |
| TiO2 | 417 | 415 | <1 | 72 | 72 | <1 | 86 | 86 | <1 |
| SiO2-Al2O3 | 500 | 500 | <1 | 239 | 205 | 15 | 257 | 229 | 11 |
| C | 919 | 224 | 336 | 517 | 140 | 194 | 515 | 139 | 193 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluža, L.; Soukup, K.; Koštejn, M.; Karban, J.; Palcheva, R.; Laube, M.; Gulková, D. On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene. Catalysts 2022, 12, 1559. https://doi.org/10.3390/catal12121559
Kaluža L, Soukup K, Koštejn M, Karban J, Palcheva R, Laube M, Gulková D. On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene. Catalysts. 2022; 12(12):1559. https://doi.org/10.3390/catal12121559
Chicago/Turabian StyleKaluža, Luděk, Karel Soukup, Martin Koštejn, Jindřich Karban, Radostina Palcheva, Marek Laube, and Daniela Gulková. 2022. "On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene" Catalysts 12, no. 12: 1559. https://doi.org/10.3390/catal12121559