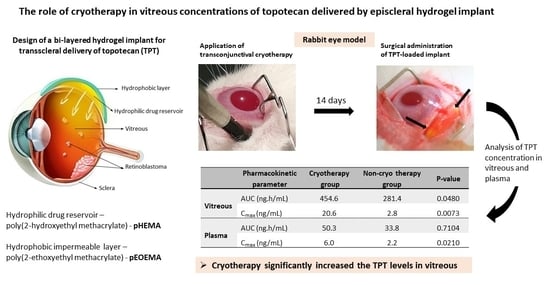
The Role of Cryotherapy in Vitreous Concentrations of Topotecan Delivered by Episcleral Hydrogel Implant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Implant Preparation and Drug Loading
2.3. In Vivo Studies and Sampling Procedures
2.4. Pharmacokinetic and Statistical Analysis
3. Results
3.1. Ophthalmic Surgery and Clinical Observations
3.2. Histopathology Findings
3.3. Pharmacokinetics of TPT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munier, F.L.; Beck-Popovic, M.; Chantada, G.L.; Cobrinik, D.; Kivelä, T.T.; Lohmann, D.; Maeder, P.; Moll, A.C.; Carcaboso, A.M.; Moulin, A.; et al. Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity”. Prog. Retin. Eye Res. 2019, 73, 100764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munier, F.L. Classification and Management of Seeds in Retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014, 35, 193–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munier, F.L.; Soliman, S.; Moulin, A.P.; Gaillard, M.-C.; Balmer, A.; Beck-Popovic, M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br. J. Ophthalmol. 2012, 96, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.H.; Brodie, S.E.; Marr, B.; Zabor, E.C.; Mondesire-Crump, I.; Abramson, D.H. Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience. Ophthalmology 2017, 124, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghassemi, F.; Shields, C.L.; Ghadimi, H.; Khodabandeh, A.; Roohipoor, R. Combined Intravitreal Melphalan and Topotecan for Refractory or Recurrent Vitreous Seeding From Retinoblastoma. JAMA Ophthalmol. 2014, 132, 936. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Douglass, A.M.; Beggache, M.; Say, E.A.T.; Shields, J.A. Intravitreous Chemotherapy for Active Vitreous Seeding from Retinoblastoma. Retina 2016, 36, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Koç, I.; Kiratli, H.; Chawla, B. Update on Intravitreal Chemotherapy for Retinoblastoma. Adv. Ophthalmol. Optom. 2021, 6, 101–118. [Google Scholar] [CrossRef]
- Francis, J.H.; Schaiquevich, P.; Buitrago, E.; Del Sole, M.J.; Zapata, G.; Croxatto, J.O.; Marr, B.P.; Brodie, S.E.; Berra, A.; Chantada, G.L.; et al. Local and Systemic Toxicity of Intravitreal Melphalan for Vitreous Seeding in Retinoblastoma. Ophthalmology 2014, 121, 1810–1817. [Google Scholar] [CrossRef]
- Munier, F.L.; Gaillard, M.-C.; Balmer, A.; Soliman, S.; Podilsky, G.; Moulin, A.P.; Beck-Popovic, M. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br. J. Ophthalmol. 2012, 96, 1078–1083. [Google Scholar] [CrossRef]
- Schaiquevich, P.; Fabius, A.W.; Francis, J.H.; Chantada, G.L.; Abramson, D.H. Ocular pharmacology of chemotherapy for retinoblastoma. Retina 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Buitrago, E.; Del Sole, M.J.; Torbidoni, A.; Fandino, A.; Asprea, M.; Croxatto, J.O.; Chantada, G.L.; Bramuglia, G.F.; Schaiquevich, P. Ocular and systemic toxicity of intravitreal topotecan in rabbits for potential treatment of retinoblastoma. Exp. Eye Res. 2013, 108, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, M.J.; Clausse, M.; Nejamkin, P.; Cancela, B.; Del Río, M.; Lamas, G.; Lubieniecki, F.; Francis, J.H.; Abramson, D.H.; Chantada, G.; et al. Ocular and systemic toxicity of high-dose intravitreal topotecan in rabbits: Implications for retinoblastoma treatment. Exp. Eye Res. 2022, 218, 109026. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Honavar, S.G.; Mulay, K.; Reddy, V.A.P. Eye salvage in diffuse anterior retinoblastoma using systemic chemotherapy with periocular and intravitreal topotecan. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 235–237.e2. [Google Scholar] [CrossRef] [PubMed]
- Bogan, C.M.; Kaczmarek, J.V.; Pierce, J.M.; Chen, S.; Boyd, K.L.; Calcutt, M.W.; Bridges, T.M.; Lindsley, C.W.; Nadelmann, J.B.; Liao, A.; et al. Evaluation of intravitreal topotecan dose levels, toxicity and efficacy for retinoblastoma vitreous seeds: A preclinical and clinical study. Br. J. Ophthalmol. 2022, 106, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Pasto, G.; Olaciregui, N.G.; Opezzo, J.A.W.; Castillo-Ecija, H.; Cuadrado-Vilanova, M.; Paco, S.; Rivero, E.M.; Vila-Ubach, M.; Restrepo-Perdomo, C.A.; Torrebadell, M.; et al. Increased delivery of chemotherapy to the vitreous by inhibition of the blood-retinal barrier. J. Control. Release 2017, 264, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Taich, P.; Moretton, M.A.; Del Sole, M.J.; Winter, U.; Bernabeu, E.; Croxatto, J.O.; Oppezzo, J.; Williams, G.; Chantada, G.L.; Chiappetta, D.A.; et al. Sustained-release hydrogels of topotecan for retinoblastoma. Colloids Surf. B Biointerfaces 2016, 146, 624–631. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, Q.; Liu, Y.; Wang, J.; Li, Q.; Li, Z.; Dong, Y.; Huang, Y.; Wang, L. A temperature-sensitive phase-change hydrogel of topotecan achieves a long-term sustained antitumor effect on retinoblastoma cells. Onco. Targets. Ther. 2019, 12, 6069–6082. [Google Scholar] [CrossRef] [Green Version]
- Delrish, E.; Jabbarvand, M.; Ghassemi, F.; Amoli, F.A.; Atyabi, F.; Lashay, A.; Soleimani, M.; Aghajanpour, L.; Dinarvand, R. Efficacy of topotecan nanoparticles for intravitreal chemotherapy of retinoblastoma. Exp. Eye Res. 2021, 204, 108423. [Google Scholar] [CrossRef]
- Francis, J.H.; Abramson, D.H.; Ji, X.; Shields, C.L.; Teixeira, L.F.; Schefler, A.C.; Cassoux, N.; Hadjistilianou, D.; Berry, J.L.; Frenkel, S.; et al. Risk of Extraocular Extension in Eyes With Retinoblastoma Receiving Intravitreous Chemotherapy. JAMA Ophthalmol. 2017, 135, 1426. [Google Scholar] [CrossRef]
- Carcaboso, A.M.; Chiappetta, D.A.; Opezzo, J.A.W.W.; Höcht, C.; Fandiño, A.C.; Croxatto, J.O.; Rubio, M.C.; Sosnik, A.; Abramson, D.H.; Bramuglia, G.F.; et al. Episcleral Implants for Topotecan Delivery to the Posterior Segment of the Eye. Investig. Opthalmology Vis. Sci. 2010, 51, 2126. [Google Scholar] [CrossRef]
- Pontes de Carvalho, R.A.; Krausse, M.L.; Murphree, A.L.; Schmitt, E.E.; Campochiaro, P.A.; Maumenee, I.H. Delivery from Episcleral Exoplants. Investig. Opthalmology Vis. Sci. 2006, 47, 4532. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, S.; Li, J.; Nan, K.; Lan, B.; Jin, Y.; Chen, H.; Cheng, L. Sustained release of triamcinolone acetonide from an episcleral plaque of multilayered poly-ε-caprolactone matrix. Acta Biomater. 2014, 10, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.-S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E.; Rittenhouse, K.D.; Wilson, C.G.; Weber, D.A.; Kuppermann, B.D.; et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: Basic research to clinical applications. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5403–5420. [Google Scholar] [CrossRef]
- Agban, Y.; Thakur, S.S.; Mugisho, O.O.; Rupenthal, I.D. Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov. Today 2019, 24, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.R.; Lee, S.S.; Kim, H.; Kim, S.; Lutz, R.J.; Galban, C.; Bungay, P.M.; Yuan, P.; Wang, N.S.; Kim, J.; et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 2006, 82, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W. Penetration of Chemotherapy Into Vitreous Is Increased by Cryotherapy and Cyclosporine in Rabbits. Arch. Ophthalmol. 1996, 114, 1390. [Google Scholar] [CrossRef]
- Dunker, S.; Faulborn, J.; Haller, E.-M.; Reich, M.-E. The effect of retinal cryoapplication on the vitreous. Retina 1997, 17, 338–343. [Google Scholar] [CrossRef]
- Steel, D.H.W.; West, J.; Cambell, W.G. A randomized controlled study of the use of the transscleral diode laser and cryotherapy in the management of rhegmatogenous retinal detachment. Retin. Jiurnal Retin. Vitr. Dis. 2000, 20, 346–357. [Google Scholar] [CrossRef]
- Abramson, D.; Ellsworth, R.; Rozakis, G. Cryotherapy for retinoblastoma. Arch. Ophthalmol. 1982, 100, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Parsons, H.; Shields, C.L.; Giblin, M.E. The role of cryotherapy in the management of retinoblastoma. Am. J. Ophthalmol. 1989, 108, 260–264. [Google Scholar] [CrossRef]
- Anagnoste, S.R.; Scott, I.U.; Murray, T.G.; Kramer, D.; Toledano, S. Rhegmatogenous retinal detachment in retinoblastoma patients undergoing chemoreduction and cryotherapy. Am. J. Ophthalmol. 2000, 129, 817–819. [Google Scholar] [CrossRef]
- Hamel, P.; Heon, E.; Gallie, B.L.; Budning, A.S. Focal therapy in the management of retinoblastoma: When to start and when to stop. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2000, 4, 334–337. [Google Scholar] [CrossRef]
- Abramson, D.H.; Schefler, A.C. Transpupillary thermotherapy as initial treatment for small intraocular retinoblastoma. Ophthalmology 2004, 111, 984–991. [Google Scholar] [CrossRef]
- Ancona-Lezama, D.; Dalvin, L.; Shields, C. Modern treatment of retinoblastoma: A 2020 review. Indian J. Ophthalmol. 2020, 68, 2356. [Google Scholar] [CrossRef]
- Khaqan, H.A.; Imtiaz, U.; Buksh, H.M.; Ur Rehman, H.A.; Naz, R. Outcomes of intravitreal melphalan for vitreous seedings in retinoblastoma resistant to systemic chemotherapy. Pediatr. Hematol. Oncol. J. 2021, 6, 22–25. [Google Scholar] [CrossRef]
- Cocarta, A.-I.; Hobzova, R.; Sirc, J.; Cerna, T.; Hrabeta, J.; Svojgr, K.; Pochop, P.; Kodetova, M.; Jedelska, J.; Bakowsky, U.; et al. Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater. Sci. Eng. C 2019, 103, 109799. [Google Scholar] [CrossRef]
- Cocarta, A.I.; Hobzova, R.; Trchova, M.; Svojgr, K.; Kodetova, M.; Pochop, P.; Uhlik, J.; Sirc, J. 2-Hydroxyethyl Methacrylate Hydrogels for Local Drug Delivery: Study of Topotecan and Vincristine Sorption/Desorption Kinetics and Polymer-Drug Interaction by ATR-FTIR Spectroscopy. Macromol. Chem. Phys. 2021, 2100086, 1–11. [Google Scholar] [CrossRef]
- Hobzova, R.; Kodetova, M.; Pochop, P.; Uhlik, J.; Dunovska, K.; Svojgr, K.; Hrabeta, J.; Feriancikova, B.; Cocarta, A.I.; Sirc, J. Hydrogel implants for transscleral diffusion delivery of topotecan: In vivo proof of concept in a rabbit eye model. Int. J. Pharm. 2021, 606, 120832. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Govoni, P.; Antopolsky, M.; Murtomaki, L.; Padula, C.; Santi, P.; Nicoli, S. Permeation of Proteins, Oligonucleotide and Dextrans Across Ocular Tissues: Experimental Studies and a Literature Update. J. Pharm. Sci. 2015, 104, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Durairaj, C.; Kadam, R.S.; Lee, S.J.; Mo, Y.; Geroski, D.H.; Kompella, U.B.; Edelhauser, H.F. Human Scleral Diffusion of Anticancer Drugs from Solution and Nanoparticle Formulation. Pharm. Res. 2009, 26, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Ranta, V.-P.; Mannermaa, E.; Lummepuro, K.; Subrizi, A.; Laukkanen, A.; Antopolsky, M.; Murtomäki, L.; Hornof, M.; Urtti, A. Barrier analysis of periocular drug delivery to the posterior segment. J. Control. Release 2010, 148, 42–48. [Google Scholar] [CrossRef]
- Carcaboso, A.M.; Bramuglia, G.F.; Chantada, G.L.; Fandiño, A.C.; Chiappetta, D.A.; De Davila, M.T.G.; Rubio, M.C.; Abramson, D.H. Topotecan vitreous levels after periocular or intravenous delivery in rabbits: An alternative for retinoblastoma chemotherapy. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3761–3767. [Google Scholar] [CrossRef] [Green Version]
- Ghate, D.; Brooks, W.; McCarey, B.E.; Edelhauser, H.F. Pharmacokinetics of Intraocular Drug Delivery by Periocular Injections Using Ocular Fluorophotometry. Investig. Opthalmology Vis. Sci. 2007, 48, 2230. [Google Scholar] [CrossRef]
- Tsui, J.Y.; Dalgard, C.; Van Quill, K.R.; Lee, L.; Grossniklaus, H.E.; Edelhauser, H.F.; O’Brien, J.M. Subconjunctival Topotecan in Fibrin Sealant in the Treatment of Transgenic Murine Retinoblastoma. Investig. Opthalmology Vis. Sci. 2008, 49, 490. [Google Scholar] [CrossRef] [Green Version]
- Mallipatna, A.C.; Dimaras, H.; Chan, H.S.L.; Héon, E.; Gallie, B.L. Periocular Topotecan for Intraocular Retinoblastoma. Arch. Ophthalmol. 2011, 129, 738. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.J.; Li, S.K.; Tuitupou, A.L.; Kochambilli, R.P.; Papangkorn, K.; Mix, D.C., Jr.; Higuchi, W.I.; Higuchi, J.W. Passive and Oxymetazoline-Enhanced Delivery with a Lens Device: Pharmacokinetics and Efficacy Studies with Rabbits. J. Ocul. Pharmacol. Ther. 2008, 24, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yang, J.; Pan, H.; Tai, M.C.; Maher, M.H.; Jia, R.; Ge, S.; Lu, L. Dinutuximab Synergistically Enhances the Cytotoxicity of Natural Killer Cells to Retinoblastoma Through the Perforin-Granzyme B Pathway. Onco. Targets. Ther. 2020, 13, 3903–3920. [Google Scholar] [CrossRef]





| Non-Cryo Group | Cryo Group | |
|---|---|---|
| Corneal haze a | ||
| Grade 1 | 1 (11%) | - |
| Grade 2 | - | 1 (11%) |
| Grade 3 | - | 1 (11%) |
| Grade 4 | - | - |
| Corneal vascularization approximately 2 mm beyond the limbus in the upper quadrants | 1 (11%) | - |
| Implant uncovered with conjunctiva b | ||
| Grade 1 | - | - |
| Grade 2 | 1 (11%) | 1 (11%) |
| Grade 3 | - | - |
| Implant dislocated on the surface of the cornea and covers the entire cornea | - | 2 (22%) |
| Non-Cryo Group | Cryo Group | |
|---|---|---|
| Corneal haze a | ||
| Grade 1 | 2 (22%) | - |
| Grade 2 | - | - |
| Grade 3 | - | 2 (22%) |
| Grade 4 | - | - |
| Corneal vascularization over the upper limbus b | ||
| Grade 1 | 2 (22%) | 1 (11%) |
| Grade 2 | 3 (33%) | 4 (44%) |
| Grade 3 | - | - |
| Vitreous hemorrhage c | ||
| Grade 1 | 1 (11%) | - |
| Grade 2 | 1 (11%) | - |
| Grade 3 | 2(22%) | 1 (11%) |
| Implant uncovered with conjunctiva d | ||
| Grade 1 | - | - |
| Grade 2 | 1 (11%) | 2 (22%) |
| Grade 3 | - | - |
| Implant dislocated on the surface of the cornea and covers the entire cornea | 4 (44%) | 5 (56%) |
| Pharmacokinetic Parameter | Cryo Group | Non-Cryo Group | p-Value | |
|---|---|---|---|---|
| Vitreous | AUC0–∞ (ng·h/mL) | 454.6 (291.9–1260.0) | 281.4 (180.0–321.3) | 0.0480 |
| Cmax (ng/mL) | 20.6 (6.1–63.5) | 2.8 (2.2–8.2) | 0.0073 | |
| Tmax (h) | 8 (8–8) | 8 (8–8) | >0.9999 | |
| Plasma | AUC0–∞ (ng·h/mL) | 50.3 (44.3–70.6) | 33.8 (19.0–91.1) | 0.7104 |
| Cmax (ng/mL) | 6.0 (3.2–6.5) | 2.2 (1.4–4.8) | 0.0210 | |
| Tmax (h) | 2 (1.5–2) | 2 (2–2) | 0.1125 | |
| Vitreous/Plasma Ratio | AUC0–∞ | 8.7 (4.7–30.7) | 7.9 (4.2–17.6) | 0.8763 |
| Cmax | 4.3 (2.3–10.6) | 1.4 (0.9–3.1) | 0.0549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodetova, M.; Hobzova, R.; Sirc, J.; Uhlik, J.; Dunovska, K.; Svojgr, K.; Cocarta, A.-I.; Felsoova, A.; Slanar, O.; Sima, M.; et al. The Role of Cryotherapy in Vitreous Concentrations of Topotecan Delivered by Episcleral Hydrogel Implant. Pharmaceutics 2022, 14, 903. https://doi.org/10.3390/pharmaceutics14050903
Kodetova M, Hobzova R, Sirc J, Uhlik J, Dunovska K, Svojgr K, Cocarta A-I, Felsoova A, Slanar O, Sima M, et al. The Role of Cryotherapy in Vitreous Concentrations of Topotecan Delivered by Episcleral Hydrogel Implant. Pharmaceutics. 2022; 14(5):903. https://doi.org/10.3390/pharmaceutics14050903
Chicago/Turabian StyleKodetova, Martina, Radka Hobzova, Jakub Sirc, Jiri Uhlik, Katerina Dunovska, Karel Svojgr, Ana-Irina Cocarta, Andrea Felsoova, Ondrej Slanar, Martin Sima, and et al. 2022. "The Role of Cryotherapy in Vitreous Concentrations of Topotecan Delivered by Episcleral Hydrogel Implant" Pharmaceutics 14, no. 5: 903. https://doi.org/10.3390/pharmaceutics14050903