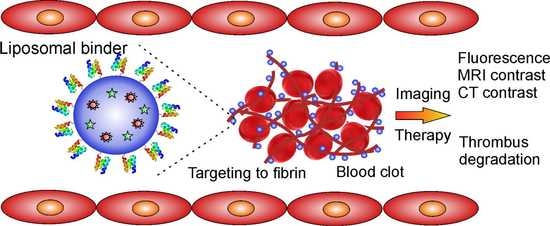
Targeting Human Thrombus by Liposomes Modified with Anti-Fibrin Protein Binders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Fusion Proteins Carrying Bβ Epitopes (BEP) Recognized by 102-10 mAb
2.2. Ribosome Display Selection of Binders
2.3. Binding of Protein Variants to Fibrin
2.4. Production and Purification of D7/E7-TolA-Avi Protein
2.5. Preparation of Layers of Fibrin Degradation Products and Fibrinogen
2.6. Binding of Protein Variants to Fibrin Layers Prepared from Lyophilized Fibrinogen or Human Plasma
2.7. Construction of Truncated D7 Proteins with C-Terminal His-Tag
2.8. Binding of D7 Protein Variants to Human Thrombus
2.9. Preparation of D7 Liposomes Modified by Different Variants of Anti-Fibrin Protein Binder D7
2.10. Characterization of D7 Liposomes
2.11. Confocal Microscopy of Thrombi Targeted with D7 Liposomes
2.12. Scanning Electron Microscopy of Whole Blood Thrombi
2.13. In Vitro Binding of D7F1 Liposomes under Flow Conditions Using MCA Model
3. Results
3.1. Production of Recombinant Protein Targets Carrying Bβ Epitope (BEP)
3.2. Ribosome Display and Screening of Protein Variants
3.3. Binding of D7 and E7 Protein Variants to Fibrin Layer
3.4. Binding of D7 Protein to Human Thrombus in Vitro
3.5. Production of Short Variants of D7 Binding Protein with C-Terminal His-Tag
3.6. Preparation and Characterization of Liposomes
3.7. Thermodynamic Characterization of Interaction between ABD-Protein Binders and Metallochelation Liposomes
3.8. Interaction of Fibrin-Specific Binders with Thrombi
3.9. Interaction of Targeted Liposomes with Thrombi
3.10. In Vitro Model of Thrombus Obstruction in Artery for the Evaluation of in Vitro Binding of Liposomes onto the Fibrin in Thrombi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skaf, E.; Stein, P.D.; Beemath, A.; Sanchez, J.; Bustamante, M.A.; Olson, R.E. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am. J. Cardiol. 2005, 96, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Silvain, J.; Bellemain, A.; Ecollan, P.; Montalescot, G.; Collet, J.P. Myocardial infarction: Role of new antiplatelet agents. Presse Med. 2011, 40, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S. Pulmonary embolism an update. Aust. Fam. Phys. 2017, 46, 816–820. [Google Scholar] [PubMed]
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Heidt, T.; Ehrismann, S.; Hovener, J.B.; Neudorfer, I.; Hilgendorf, I.; Reisert, M.; Hagemeyer, C.E.; Zirlik, A.; Reinohl, J.; Bode, C.; et al. Molecular imaging of activated platelets allows the detection of pulmonary embolism with magnetic resonance imaging. Sci. Rep. 2016, 6, 25044. [Google Scholar] [CrossRef]
- Von zur Muhlen, C.; Peter, K.; Ali, Z.A.; Schneider, J.E.; McAteer, M.A.; Neubauer, S.; Channon, K.M.; Bode, C.; Choudhury, R.P. Visualization of activated platelets by targeted magnetic resonance imaging utilizing conformation-specific antibodies against glycoprotein iib/iiia. J. Vasc. Res. 2009, 46, 6–14. [Google Scholar] [CrossRef]
- Zhang, N.; Li, C.; Zhou, D.; Ding, C.; Jin, Y.; Tian, Q.; Meng, X.; Pu, K.; Zhu, Y. Cyclic rgd functionalized liposomes encapsulating urokinase for thrombolysis. Acta Biomater. 2018, 70, 227–236. [Google Scholar] [CrossRef]
- Gargan, P.E.; Gaffney, P.J.; Pleasants, J.R.; Ploplis, V.A. A monoclonal antibody which recognises an epitopic region unique to the intact fibrin polymeric structure. Fibrinolysis 1993, 7, 275–283. [Google Scholar] [CrossRef]
- Soe, G.; Kohno, I.; Inuzuka, K.; Itoh, Y.; Matsuda, M. A monoclonal antibody that recognizes a neo-antigen exposed in the e domain of fibrin monomer complexed with fibrinogen or its derivatives: Its application to the measurement of soluble fibrin in plasma. Blood 1996, 88, 2109–2117. [Google Scholar] [CrossRef]
- Wada, H.; Kobayashi, T.; Abe, Y.; Hatada, T.; Yamada, N.; Sudo, A.; Uchida, A.; Nobori, T. Elevated levels of soluble fibrin or d-dimer indicate high risk of thrombosis. J. Thromb. Haemost. 2006, 4, 1253–1258. [Google Scholar] [CrossRef]
- Doh, H.J.; Song, K.S.; Kang, M.S.; Kim, D.S.; Kim, K.A.; Kang, J.; Jang, Y.; Chung, K.H. Novel monoclonal antibody that recognizes new neoantigenic determinant of d-dimer. Thromb. Res. 2006, 118, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, A.F.; Nair, S.A.; Graham, P.; McMurry, T.J.; Ladner, R.C.; Wescott, C.; Sexton, D.J.; Caravan, P. Fibrin specific peptides derived by phage display: Characterization of peptides and conjugates for imaging. Bioconjugate Chem. 2012, 23, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Overoye-Chan, K.; Koerner, S.; Looby, R.J.; Kolodziej, A.F.; Zech, S.G.; Deng, Q.; Chasse, J.M.; McMurry, T.J.; Caravan, P. Ep-2104r: A fibrin-specific gadolinium-based mri contrast agent for detection of thrombus. J. Am. Chem. Soc. 2008, 130, 6025–6039. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Spuentrup, E.; Cardenas-Molina, G.; Wiethoff, A.J.; Hartmann, M.G.; Caravan, P.; Parsons, E.C., Jr. Thrombus imaging with fibrin-specific gadolinium-based mr contrast agent ep-2104r: Results of a phase ii clinical study of feasibility. Investig. Radiol. 2009, 44, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Pilch, J.; Brown, D.M.; Komatsu, M.; Jarvinen, T.A.; Yang, M.; Peters, D.; Hoffman, R.M.; Ruoslahti, E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc. Natl. Acad. Sci. USA 2006, 103, 2800–2804. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Yasunaga, M.; Hanaoka, S.; Saijou, S.; Sugino, T.; Tsuji, A.; Saga, T.; Tsumoto, K.; Manabe, S.; Kuroda, J.; et al. Discovery of an uncovered region in fibrin clots and its clinical significance. Sci. Rep. 2013, 3, 2604. [Google Scholar] [CrossRef]
- Fuchigami, H.; Manabe, S.; Yasunaga, M.; Matsumura, Y. Chemotherapy payload of anti-insoluble fibrin antibody-drug conjugate is released specifically upon binding to fibrin. Sci. Rep. 2018, 8, 14211. [Google Scholar] [CrossRef]
- Obonai, T.; Fuchigami, H.; Furuya, F.; Kozuka, N.; Yasunaga, M.; Matsumura, Y. Tumour imaging by the detection of fibrin clots in tumour stroma using an anti-fibrin fab fragment. Sci. Rep. 2016, 6, 23613. [Google Scholar] [CrossRef]
- Tiukinhoy-Laing, S.D.; Buchanan, K.; Parikh, D.; Huang, S.; MacDonald, R.C.; McPherson, D.D.; Klegerman, M.E. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J. Drug Target. 2007, 15, 109–114. [Google Scholar] [CrossRef]
- Klegerman, M.E.; Zou, Y.; McPherson, D.D. Fibrin targeting of echogenic liposomes with inactivated tissue plasminogen activator. J. Liposome Res. 2008, 18, 95–112. [Google Scholar] [CrossRef]
- Yan, J.P.; Ko, J.H.; Qi, Y.P. Generation and characterization of a novel single-chain antibody fragment specific against human fibrin clots from phage display antibody library. Thromb. Res. 2004, 114, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Putelli, A.; Kiefer, J.D.; Zadory, M.; Matasci, M.; Neri, D. A fibrin-specific monoclonal antibody from a designed phage display library inhibits clot formation and localizes to tumors in vivo. J. Mol. Biol. 2014, 426, 3606–3618. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, S.; Mikulik, R.; Masek, J.; Raska, M.; Turanek Knotigova, P.; Miller, A.D.; Turanek, J. Liposomal nanocarriers for plasminogen activators. J. Control. Release 2016, 227, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef]
- Kumar, R.; Dogra, S.; Amarji, B.; Singh, B.; Kumar, S.; Sharma, S.; Vinay, K.; Mahajan, R.; Katare, O.P. Efficacy of novel topical liposomal formulation of cyclosporine in mild to moderate stable plaque psoriasis: A randomized clinical trial. JAMA Dermatol. 2016, 152, 807–815. [Google Scholar] [CrossRef]
- Bartheldyova, E.; Turanek Knotigova, P.; Zachova, K.; Masek, J.; Kulich, P.; Effenberg, R.; Zyka, D.; Hubatka, F.; Kotoucek, J.; Celechovska, H.; et al. N-oxy lipid-based click chemistry for orthogonal coupling of mannan onto nanoliposomes prepared by microfluidic mixing: Synthesis of lipids, characterisation of mannan-coated nanoliposomes and in vitro stimulation of dendritic cells. Carbohydr. Polym. 2019, 207, 521–532. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Bartheldyova, E.; Effenberg, R.; Masek, J.; Prochazka, L.; Knotigova, P.T.; Kulich, P.; Hubatka, F.; Velinska, K.; Zelnickova, J.; Zouharova, D.; et al. Hyaluronic acid surface modified liposomes prepared via orthogonal aminoxy coupling: Synthesis of nontoxic aminoxylipids based on symmetrically alpha-branched fatty acids, preparation of liposomes by microfluidic mixing, and targeting to cancer cells expressing cd44. Bioconjugate Chem. 2018, 29, 2343–2356. [Google Scholar]
- Ahmad, J.N.; Li, J.; Biedermannova, L.; Kuchar, M.; Sipova, H.; Semeradtova, A.; Cerny, J.; Petrokova, H.; Mikulecky, P.; Polinek, J.; et al. Novel high-affinity binders of human interferon gamma derived from albumin-binding domain of protein g. Proteins 2012, 80, 774–789. [Google Scholar] [CrossRef]
- Mareckova, L.; Petrokova, H.; Osicka, R.; Kuchar, M.; Maly, P. Novel binders derived from an albumin-binding domain scaffold targeting human prostate secretory protein 94 (psp94). Protein Cell 2015, 6, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Hlavnickova, M.; Kuchar, M.; Osicka, R.; Vankova, L.; Petrokova, H.; Maly, M.; Cerny, J.; Arenberger, P.; Maly, P. Abd-derived protein blockers of human il-17 receptor a as non-igg alternatives for modulation of il-17-dependent pro-inflammatory axis. Int. J. Mol. Sci. 2018, 19, 3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchar, M.; Vankova, L.; Petrokova, H.; Cerny, J.; Osicka, R.; Pelak, O.; Sipova, H.; Schneider, B.; Homola, J.; Sebo, P.; et al. Human interleukin-23 receptor antagonists derived from an albumin-binding domain scaffold inhibit il-23-dependent ex vivo expansion of il-17-producing t-cells. Proteins 2014, 82, 975–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krizova, L.; Kuchar, M.; Petrokova, H.; Osicka, R.; Hlavnickova, M.; Pelak, O.; Cerny, J.; Kalina, T.; Maly, P. P19-targeted abd-derived protein variants inhibit il-23 binding and exert suppressive control over il-23-stimulated expansion of primary human il-17+ t-cells. Autoimmunity 2017, 50, 102–113. [Google Scholar] [CrossRef]
- Mašek, J.; Bartheldyová, E.; Turánek-Knotigová, P.; Škrabalová, M.; Korvasová, Z.; Plocková, J.; Koudelka, Š.; Škodová, P.; Kulich, P.; Křupka, M.; et al. Metallochelating liposomes with associated lipophilised norabumdp as biocompatible platform for construction of vaccines with recombinant his-tagged antigens: Preparation, structural study and immune response towards rhsp90. J. Control. Release 2011, 151, 193–201. [Google Scholar] [CrossRef]
- Křupka, M.; Mašek, J.; Bartheldyová, E.; Knötigová, P.T.; Plocková, J.; Korvasová, Z.; Škrabalová, M.; Koudelka, Š.; Kulich, P.; Zachová, K.; et al. Enhancement of immune response towards non-lipidized borrelia burgdorferi recombinant ospc antigen by binding onto the surface of metallochelating nanoliposomes with entrapped lipophilic derivatives of norabumdp. J. Control. Release 2012, 160, 374–381. [Google Scholar] [CrossRef]
- Zuev, Y.F.; Litvinov, R.I.; Sitnitsky, A.E.; Idiyatullin, B.Z.; Bakirova, D.R.; Galanakis, D.K.; Zhmurov, A.; Barsegov, V.; Weisel, J.W. Conformational flexibility and self-association of fibrinogen in concentrated solutions. J. Phys. Chem. B 2017, 121, 7833–7843. [Google Scholar] [CrossRef]
- Koo, J.; Galanakis, D.; Liu, Y.; Ramek, A.; Fields, A.; Ba, X.; Simon, M.; Rafailovich, M.H. Control of anti-thrombogenic properties: Surface-induced self-assembly of fibrinogen fibers. Biomacromolecules 2012, 13, 1259–1268. [Google Scholar] [CrossRef]
- Lounes, K.C.; Lefkowitz, J.B.; Henschen-Edman, A.H.; Coates, A.I.; Hantgan, R.R.; Lord, S.T. The impaired polymerization of fibrinogen longmont (bbeta166arg-->cys) is not improved by removal of disulfide-linked dimers from a mixture of dimers and cysteine-linked monomers. Blood 2001, 98, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.L.; Pizzo, S.V.; Hill, R.L.; McKee, P.A. The effect of fibrin-stabilizing factor on the subunit structure of human fibrin. J. Clin. Investig. 1971, 50, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Zadravec, P.; Mareckova, L.; Petrokova, H.; Hodnik, V.; Perisic Nanut, M.; Anderluh, G.; Strukelj, B.; Maly, P.; Berlec, A. Development of recombinant lactococcus lactis displaying albumin-binding domain variants against shiga toxin 1 b subunit. PLoS ONE 2016, 11, e0162625. [Google Scholar] [CrossRef] [PubMed]
- Wahl, V.; Saurugger, E.; Khinast, J.; Laggner, P. Specific surface, crystallinity, and dissolution of lyophilized fibrinogen. A study by combined small- and wide-angle x-ray scattering (swaxs). Eur. J. Pharm. Biopharm. 2015, 89, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Wahl, V.; Leitgeb, S.; Laggner, P.; Pichler, H.; Liebminger, A.; Khinast, J. The influence of residual water on the solid-state properties of freeze-dried fibrinogen. Eur. J. Pharm. Biopharm. 2015, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Křupka, M.; Mašek, J.; Barkocziová, L.; Knotigová, P.T.; Kulich, P.; Plockova, J.; Lukac, R.; Bartheldyová, E.; Koudelka, S.; Chaloupková, R.; et al. The position of his-tag in recombinant ospc and application of various adjuvants affects the intensity and quality of specific antibody response after immunization of experimental mice. PLoS ONE 2016, 11, e0148497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mašek, J.; Bartheldyová, E.; Korvasová, Z.; Škrabalová, M.; Koudelka, Š.; Kulich, P.; Kratochvílová, I.; Miller, A.D.; Ledvina, M.; Raška, M.; et al. Immobilization of histidine-tagged proteins on monodisperse metallochelation liposomes: Preparation and study of their structure. Anal. Biochem. 2011, 408, 95–104. [Google Scholar] [CrossRef]
- Platt, V.; Huang, Z.; Cao, L.; Tiffany, M.; Riviere, K.; Szoka, F.C., Jr. Influence of multivalent nitrilotriacetic acid lipid-ligand affinity on the circulation half-life in mice of a liposome-attached his6-protein. Bioconjugate Chem. 2010, 21, 892–902. [Google Scholar] [CrossRef] [Green Version]








| Binder | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABDwt | Y | Y | K | N | L | I | N | N | A | K | T | V | E | G | V | K | A | L | I | D | E | I | L | A | A | L | P |
| D7 | A | Y | K | N | P | I | N | L | A | R | S | V | P | T | V | K | G | A | I | D | P | I | L | A | A | L | P |
| E7=F7 | F | Y | K | N | L | I | N | V | A | M | P | V | V | L | V | K | T | A | I | D | G | I | L | A | A | L | P |
| F11 | G | Y | K | N | W | I | N | P | A | D | G | V | A | G | V | K | S | A | I | D | A | I | L | A | A | L | P |
| Binder | Kd (M) | Δ°G (kJ/mol) |
|---|---|---|
| D7 F1 | 1.2 ± 0.1 × 10−7 | −39.7 ± 0.7 |
| ABD wt F2 | 1.06 ± 0.4 × 10−7 | −39.8 ± 1.9 |
| D7 F3 | 1.79 ± 0.55 × 10−7 | −38.5 ± 2.8 |
| D7 H2 | 2.21 ± 0.1 × 10−9 | −49.4 ± 1.0 |
| ABD-wt H2 | 2.35 ± 0.2 × 10−8 | −43.5 ± 1.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroková, H.; Mašek, J.; Kuchař, M.; Vítečková Wünschová, A.; Štikarová, J.; Bartheldyová, E.; Kulich, P.; Hubatka, F.; Kotouček, J.; Turánek Knotigová, P.; et al. Targeting Human Thrombus by Liposomes Modified with Anti-Fibrin Protein Binders. Pharmaceutics 2019, 11, 642. https://doi.org/10.3390/pharmaceutics11120642
Petroková H, Mašek J, Kuchař M, Vítečková Wünschová A, Štikarová J, Bartheldyová E, Kulich P, Hubatka F, Kotouček J, Turánek Knotigová P, et al. Targeting Human Thrombus by Liposomes Modified with Anti-Fibrin Protein Binders. Pharmaceutics. 2019; 11(12):642. https://doi.org/10.3390/pharmaceutics11120642
Chicago/Turabian StylePetroková, Hana, Josef Mašek, Milan Kuchař, Andrea Vítečková Wünschová, Jana Štikarová, Eliška Bartheldyová, Pavel Kulich, František Hubatka, Jan Kotouček, Pavlína Turánek Knotigová, and et al. 2019. "Targeting Human Thrombus by Liposomes Modified with Anti-Fibrin Protein Binders" Pharmaceutics 11, no. 12: 642. https://doi.org/10.3390/pharmaceutics11120642