Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
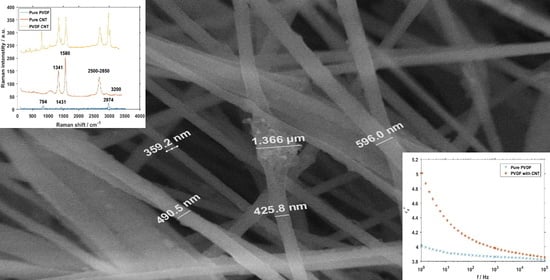
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, N.; He, Q.; Sun, J.; Xia, G.; Song, R. Crystallization behavior and electroactive properties of PVDF, P(VDF-TrFE) and their blend films. Polym. Test. 2017, 57, 302–306. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Shi, L.; Yang, X.; Lu, X. Effect of Nano-TiO2 Particles on the Performance of PVDF, PVDF-g-(Maleic anhydride), and PVDF-g-Poly(acryl amide) Membranes. Ind. Eng. Chem. Res. 2011, 50, 12113–12123. [Google Scholar] [CrossRef]
- Yang, J.; He, F.; Wu, H.; Liang, Y.; Wang, Y.; Sun, Z. Engineering Surface and Optical Properties of TiO2-Coated Electrospun PVDF Nanofibers Via Controllable Self-Assembly. Nanomaterials 2018, 8, 741. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Wang, G.; Rui, X.; Yang, F.; Wang, Y. Temperature compensation of a PVDF stress sensor and its application in the test of gun propellant charge compression stress. Smart Mater. Struct. 2019, 28, 025018. [Google Scholar] [CrossRef]
- Sharma, M.; Madras, G.; Bose, S. Unusual Fragility and Cooperativity in Glass-Forming and Crystalline PVDF/PMMA Blends in the Presence of Multiwall Carbon Nanotubes. Macromolecules 2015, 48, 2740–2750. [Google Scholar] [CrossRef]
- McKeen, L.W. Fluoropolymers. In Fatigue and Tribological Properties of Plastics and Elastomers; Rogers, M., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 291–315. [Google Scholar]
- Cardoso, V.F.; Minas, G.; Lanceros-Méndez, S. Multilayer spin-coating deposition of poly(vinylidene fluoride) films for controlling thickness and piezoelectric response. Sens. Actuators A Phys. 2013, 192, 76–80. [Google Scholar] [CrossRef]
- Shaik, H.; Rachith, S.N.; Rudresh, K.J.; Sheik, A.S.; Raman, K.H.T.; Kondaiah, P.; Rao, G.M. Towards β-phase formation probability in spin coated PVDF thin films. J. Polym. Res. 2017, 24, 35. [Google Scholar] [CrossRef]
- Wu, C.-M.; Chou, M.-H.; Zeng, W.-Y. Piezoelectric Response of Aligned Electrospun Polyvinylidene Fluoride/Carbon Nanotube Nanofibrous Membranes. Nanomaterials 2018, 8, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castkova, K.; Kastyl, J.; Sobola, D.; Petrus, J.; Stastna, E.; Riha, D.; Tofel, P. Structure-Properties Relationship of Electrospun PVDF Fibers. Nanomaterials 2020, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H. The Piezoelectricity of Poly (vinylidene Fluoride). Jpn. J. Appl. Phys. 1969, 8, 975–976. [Google Scholar] [CrossRef]
- Fukada, E.; Takashita, S. Piezoelectric Effect in Polarized Poly (vinylidene Fluoride). Jpn. J. Appl. Phys. 1969, 8, 960. [Google Scholar] [CrossRef]
- Nakamura, K.; Wada, Y. Piezoelectricity, pyroelectricity, and the electrostriction constant of poly(vinylidene fluoride). J. Polym. Sci. Part A-2 Polym. Phys. 1971, 9, 161–173. [Google Scholar] [CrossRef]
- Tamura, M.; Ogasawara, K.; Ono, N.; Hagiwara, S. Piezoelectricity in uniaxially stretched poly(vinylidene fluoride). J. Appl. Phys. 1974, 45, 3768–3771. [Google Scholar] [CrossRef]
- Oshiki, M.; Fukada, E. Inverse piezoelectric effect and electrostrictive effect in polarized poly(vinylidene fluoride) films. J. Mater. Sci. 1975, 10, 1–6. [Google Scholar] [CrossRef]
- Levi, N.; Czerw, R.; Xing, S.; Iyer, P.; Carroll, D.L. Properties of Polyvinylidene Difluoride−Carbon Nanotube Blends. Nano Lett. 2004, 4, 1267–1271. [Google Scholar] [CrossRef]
- Chatterjee, J.; Nash, N.; Cottinet, P.-J.; Wang, B. Synthesis and characterization of poly(vinylidene fluoride)/carbon nanotube composite piezoelectric powders. J. Mater. Res. 2012, 27, 2352–2359. [Google Scholar] [CrossRef]
- Mousa, M.S. Comparison between Single-Walled CNT, Multi-Walled CNT, and Carbon Nanotube-Fiber Pyrograf III. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012025. [Google Scholar] [CrossRef] [Green Version]
- Aqeel, S.M.; Huang, Z.; Walton, J.; Baker, C.; Falkner, D.; Liu, Z.; Wang, Z. Polyvinylidene fluoride (PVDF)/polyacrylonitrile (PAN)/carbon nanotube nanocomposites for energy storage and conversion. Adv. Compos. Hybrid Mater. 2017, 1, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.; Lim, J.Y.; Hong, S.M.; Lee, J.; Ha, J.; Choi, H.J.; Seo, Y. Enhanced Piezoelectric Properties of Electrospun Poly(vinylidene fluoride)/Multiwalled Carbon Nanotube Composites Due to High β-Phase Formation in Poly(vinylidene fluoride). J. Phys. Chem. C 2013, 117, 11791–11799. [Google Scholar] [CrossRef]
- Huang, S.; Yee, W.A.; Tjiu, W.C.; Liu, Y.; Kotaki, M.; Boey, Y.C.F.; Ma, J.; Liu, T.; Lu, X. Electrospinning of Polyvinylidene Difluoride with Carbon Nanotubes: Synergistic Effects of Extensional Force and Interfacial Interaction on Crystalline Structures. Langmuir 2008, 24, 13621–13626. [Google Scholar] [CrossRef] [PubMed]
- Mago, G.; Kalyon, D.M.; Fisher, F.T. Membranes of Polyvinylidene Fluoride and PVDF Nanocomposites with Carbon Nanotubes via Immersion Precipitation. J. Nanomater. 2008, 2008, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Peña-Álvarez, M.; Del Corro, E.; Langa, F.; Baonza, V.G.; Taravillo, M. Morphological changes in carbon nanohorns under stress: A combined Raman spectroscopy and TEM study. RSC Adv. 2016, 6, 49543–49550. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.H.M.; Moutinho, M.; Stavale, F.; Lucchese, M.M.; Capaz, R.B.; Achete, C.A.; Jorio, A. Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys. Rev. B 2010, 82, 125429. [Google Scholar] [CrossRef] [Green Version]
- NC7000TM—Technical Data Sheet. Available online: https://www.nanocyl.com/download/tds-nc7000/ (accessed on 13 November 2020).
- Singh, K.; Chaudhary, S.; Venugopal, R.; Gaurav, A. Bulk synthesis of multi-walled carbon nanotubes by AC arc discharge method. Proc. Inst. Mech. Eng. Part N J. Nanomater. Nanoeng. Nanosyst. 2017, 231, 141–151. [Google Scholar] [CrossRef]
- Satapathy, S.; Pawar, S.; Gupta, P.K.; Varma, K.B.R. Effect of annealing on phase transition in poly(vinylidene fluoride) films prepared using polar solvent. Bull. Mater. Sci. 2011, 34, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Boccaccio, T.; Bottino, A.; Capannelli, G.; Piaggio, P. Characterization of PVDF membranes by vibrational spectroscopy. J. Membr. Sci. 2002, 210, 315–329. [Google Scholar] [CrossRef]
- Constantino, C.J.L.; Job, A.E.; Simoes, R.D.; Giacometti, J.A.; Zucolotto, V.; Oliveira, O.N.; Gozzi, G.; Chinaglia, D.L. The Investigation of /spl alpha //spl rArr//spl beta/ Phase Transition in Poly(Vinylidene Fluoride) (PVDF). In Proceedings of the 2005 12th International Symposium on Electrets, IEEE, Salvador, Bahia, Brazil, 11–14 September 2005; pp. 178–181. [Google Scholar]
- Wang, G.-L.; Tian, Y.-M.; Cao, D.-X.; Yu, Y.-S.; Sun, W.-B. One-dimensional Salen-type Chain-like Lanthanide(III) Coordination Polymers: Syntheses, Crystal Structures, and Fluorescence Properties. Z. Für Anorg. Und Allg. Chem. 2010, 637, 583–588. [Google Scholar] [CrossRef]
- Li, Z.-F.; Cheng, X.-X.; Li, G.; Lu, H.-J.; Zhang, H.-F. Syntheses, structures, fluorescence and thermal properties of three lanthanide coordination polymers built by N-benzoyl-N′-(4-benzoxy)thiourea. J. Lumin. 2010, 130, 2192–2200. [Google Scholar] [CrossRef]
- Hartschuh, A.; Pedrosa, H.N.; Novotny, L.; Krauss, T.D. Simultaneous Fluorescence and Raman Scattering from Single Carbon Nanotubes. Science 2003, 301, 1354–1356. [Google Scholar] [CrossRef] [Green Version]
- Högele, A.; Galland, C.; Winger, M.; Imamoğlu, A. Photon Antibunching in the Photoluminescence Spectra of a Single Carbon Nanotube. Phys. Rev. Lett. 2008, 100, 217401. [Google Scholar] [CrossRef] [Green Version]
- Okpalugo, T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Duca, M.D.; Plosceanu, C.L.; Pop, T. Surface modifications of polyvinylidene fluoride (PVDF) under rf Ar plasma. Polym. Degrad. Stab. 1998, 61, 65–72. [Google Scholar] [CrossRef]
- Constantino, C.J.L.; Job, A.E.; Simões, R.D.; Giacometti, J.A.; Zucolotto, V.; Oliveira, O.N.; Gozzi, G.; Chinaglia, D.L. Phase Transition in Poly(Vinylidene Fluoride) Investigated with Micro-Raman Spectroscopy. Appl. Spectrosc. 2005, 59, 275–279. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Dallaev, R.; Tofel, P.; Trčka, T.; Grmela, L.; et al. Characterization of Polyvinylidene Fluoride (PVDF) Electrospun Fibers Doped by Carbon Flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.W.; Li, Y.; Zhang, X.F.; Chikkannanavar, S.B.; Zhao, Y.H.; Dangelewicz, A.M.; Zheng, L.X.; Doorn, S.K.; Jia, Q.X.; Peterson, D.E.; et al. Structure-Dependent Electrical Properties of Carbon Nanotube Fibers. Adv. Mater. 2007, 19, 3358–3363. [Google Scholar] [CrossRef]
- Puértolas, J.; García-García, J.; Pascual, F.; González-Domínguez, J.; Martínez, M.; Ansón-Casaos, A. Dielectric behavior and electrical conductivity of PVDF filled with functionalized single-walled carbon nanotubes. Compos. Sci. Technol. 2017, 152, 263–274. [Google Scholar] [CrossRef]
- Sedlak, P.; Gajdos, A.; Macku, R.; Majzner, J.; Holcman, V.; Sedlakova, V.; Kubersky, P. The effect of thermal treatment on ac/dc conductivity and current fluctuations of PVDF/NMP/[EMIM][TFSI] solid polymer electrolyte. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Šťastná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes. Materials 2021, 14, 1428. https://doi.org/10.3390/ma14061428
Kaspar P, Sobola D, Částková K, Dallaev R, Šťastná E, Sedlák P, Knápek A, Trčka T, Holcman V. Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes. Materials. 2021; 14(6):1428. https://doi.org/10.3390/ma14061428
Chicago/Turabian StyleKaspar, Pavel, Dinara Sobola, Klára Částková, Rashid Dallaev, Eva Šťastná, Petr Sedlák, Alexandr Knápek, Tomáš Trčka, and Vladimír Holcman. 2021. "Case Study of Polyvinylidene Fluoride Doping by Carbon Nanotubes" Materials 14, no. 6: 1428. https://doi.org/10.3390/ma14061428