Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Herbicide Treatment
2.2. Raman Spectroscopy
2.3. Chlorophyll Fluorescence Imaging
2.4. Thermal Imaging
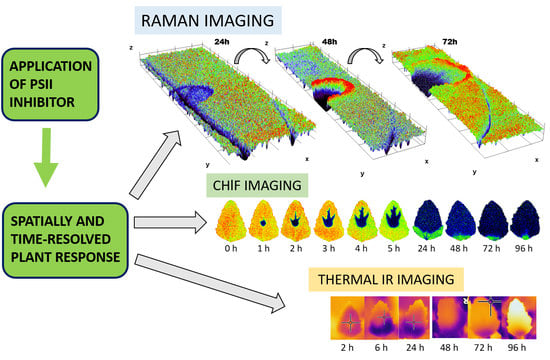
3. Results
3.1. Raman Imaging
3.2. Chlorophyll Fluorescence Imaging
3.3. Thermal Imaging
4. Discussion
5. Conclusions
- The combination of Raman, chlorophyll a fluorescence and thermal imaging allowed us to monitor the cascade of damage and defense responses following the application of PSII inhibiting herbicide with high spatial resolution, and also proved the usefulness for the monitoring of numerous abiotic and biotic stresses showing a similar cascade of response.
- ChlF imaging allowed us to monitor time-dependent metribuzin transport in acropetal direction through main veins and demonstrated the ability to distinguish between fast processes at the level of electron transport (1 − Vj) or non-photochemical energy dissipation (NPQ) and slower effect on maximum efficiency of PSII photochemistry (Fv/Fm).
- The high-resolution resonance Raman images of leaves show zones of local increase of carotenoid content relative to the rest of the leaf 48 and 72 h after the herbicide application, surrounding the damaged tissue in the zone of herbicide application, which is an indication of activation defense mechanisms in leaf.
- The substantial time-dependent variability in the carotenoid band position (from 1523 to 1517 cm−1) and also spatial variability of the band position after 48 h and 78 h (from 1517.5 to 1519 cm−1) indicates structural changes in the carotenoid composition.
- The increase of leaf temperature in the region surrounding the spot of herbicide application and expanding acropetally in the direction to the leaf tip proved the metribuzin effect on stomata closure, which was relatively slower compared to other responses.
Author Contributions
Funding
Conflicts of Interest
References
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, A.W.; Krieger-Liszkay, A. Herbicide-induced oxidative stress in photosystem II. Trends Biochem. Sci. 2001, 26, 648–653. [Google Scholar] [CrossRef]
- Powles, S.B. Photoinhibition of Photosynthesis Induced by Visible Light. Annu. Rev. Plant. Physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. BBA-Bioenergetics 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Harbinson, J.; Prinzenberg, A.E.; Kruijer, W.; Aarts, M.G. High throughput screening with chlorophyll fluorescence imaging and its use in crop improvement. Curr. Opin. Biotechnol. 2012, 23, 221–226. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Adams, W.W.; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398. [Google Scholar] [CrossRef]
- Lunch, C.K.; LaFountain, A.M.; Thomas, S.; Frank, H.A.; Lewis, L.A.; Cardon, Z.G. The xanthophyll cycle and NPQ in diverse desert and aquatic green algae. Photosynth. Res. 2013, 115, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin Has Enhanced Antioxidant Capacity with Respect to All Other Xanthophylls in Arabidopsis Leaves and Functions Independent of Binding to PSII Antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telfer, A.; Pascal, A.; Gall, A. Carotenoids in Photosynthesis. Carotenoids 2008, 4, 265–308. [Google Scholar]
- Omasa, K.; Takayama, K. Simultaneous Measurement of Stomatal Conductance, Non-photochemical Quenching, and Photochemical Yield of Photosystem II in Intact Leaves by Thermal and Chlorophyll Fluorescence Imaging. Plant. Cell Physiol. 2003, 44, 1290–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, L.F.; Fernandes, R.F.; Lobo-Hajdu, G.; de Oliveira, L.F.C. Conjugated polyenes as chemical probes of life signature: Use of Raman spectroscopy to differentiate polyenic pigments. Philos. Trans. R. Soc. A 2014, 72, 20140200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, D.; Kilponen, R.G.; Rimai, L. Resonance Raman scattering of laser radiation by vibrational modes of carotenoid pigment molecules in intact plant tissues. Nature 1970, 227, 743. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.C. Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure Appl. Chem. 1985, 57, 785–792. [Google Scholar] [CrossRef]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta A 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Marshall, C.P.; Leuko, S.; Coyle, C.M.; Walter, M.R.; Burns, B.P.; Neilan, B.A. Carotenoid Analysis of Halophilic Archaea by Resonance Raman Spectroscopy. Astrobiology 2007, 7, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Gierlinger, N.; Keplinger, T.; Harrington, M. Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 2012, 7, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Gierlinger, N. Revealing changes in molecular composition of plant cell walls on the micron-level by Raman mapping and vertex component analysis (VCA). Front. Plant. Sci. 2014, 5, 306. [Google Scholar] [CrossRef] [Green Version]
- Altangerel, N.; Ariunbold, G.O.; Gorman, C.; Alkahtani, M.H.; Borrego, E.J.; Bohlmeyer, D.; Hemmer, P.; Kolomiets, M.V.; Yuan, J.S.; Scully, M.O. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 3393. [Google Scholar] [CrossRef] [Green Version]
- Baranski, R.; Baranska, M.; Schulz, H. Changes in carotenoid content and distribution in living plant tissue can be observed and mapped in situ using NIR-FT-Raman spectroscopy. Planta 2005, 222, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Roman, M.; Marzec, K.M.; Grzebelus, E.; Simon, P.W.; Baranska, M.; Baranski, R. Composition and (in)homogeneity of carotenoid crystals in carrot cells revealed by high resolution Raman imaging. Spectrochim. Acta A 2015, 136, 1395–1400. [Google Scholar] [CrossRef]
- Vítek, P.; Novotná, K.; Hodaňová, P.; Rapantová, B.; Klem, K. Detection of herbicide effects on pigment composition and PSII photochemistry in Helianthus annuus by Raman spectroscopy and chlorophyll a fluorescence. Spectrochim. Acta A 2017, 170, 234–241. [Google Scholar] [CrossRef]
- Mehrotra, R.; Bhalothia, P.; Bansal, P.; Basantani, M.K.; Bharti, V.; Mehrotra, S. Abscisic acid and abiotic stress tolerance-Different tiers of regulation. J. Plant Physiol. 2014, 171, 486–496. [Google Scholar] [CrossRef]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef]
- Sobrino, J.A.; Frate, F.D.; Drusch, M.; Jiménez-Muñoz, J.C.; Manunta, P.; Regan, A. Review of Thermal Infrared Applications and Requirements for Future High-Resolution Sensors. IEEE Trans. Geosci. Remote 2016, 54, 2963–2972. [Google Scholar] [CrossRef]
- Jones, H.G. Thermal Imaging and Infrared Sensing in Plant Ecophysiology. In Advances in Plant. Ecophysiology Techniques; Springer: Cham, Switzerland, 2018; pp. 135–151. [Google Scholar]
- Mishra, K.B.; Mishra, A.; Klem, K.; Govindjee. Plant phenotyping: A perspective. Indian J. Plant Physiol. 2016, 21, 514–527. [Google Scholar] [CrossRef]
- Klem, K.; Mishra, K.B.; Novotná, K.; Rapantová, B.; Hodaňová, P.; Mishra, A.; Kováč, D.; Urban, O. Distinct growth and physiological responses of Arabidopsis thaliana natural accessions to drought stress and their detection using spectral reflectance and thermal imaging. Funct. Plant Biol. 2017, 44, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H.G. Estimating stomatal conductance with thermal imagery. Plant. Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef]
- Ketel, D.H.; van der Wielen, M.J.; Lotz, L.A.P. Prediction of a low dose herbicide effect from studies on binding of metribuzin to the chloroplasts of Chenopodium album L. Ann. Appl. Biol. 1996, 128, 519–531. [Google Scholar] [CrossRef]
- Nasdala, L.; Beyssac, O.; Schopf, J.W.; Bleisteiner, B. Application of Raman-based images in the Earth sciences. In Raman Imaging; Springer: Berlin/Heidelber, Germany, 2012; pp. 145–187. [Google Scholar]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of Photosystem II. BBA-Bioenergetics 2012, 1817, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef]
- Hess, F.D. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Song, Y.; Miao, Y.; Song, C.-P. Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytol. 2014, 201, 1121–1140. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuteja, N. Abscisic Acid and Abiotic Stress Signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanciullino, A.L.; Bidel, L.P.R.; Urban, L. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014, 37, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Pengmin, L.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [Green Version]
- Schoefs, B. Plant pigments: Properties, analysis, degradation. Adv. Food Nutr. Res. 2005, 49, 41–91. [Google Scholar]
- Wise, R.R.; Naylor, A.W. Chilling-Enhanced Photooxidation: Evidence for the Role of Singlet Oxygen and Superoxide in the Breakdown of Pigments and Endogenous Antioxidants. Plant Physiol. 1987, 83, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef]
- Schäfer, L.; Vioque, A.; Sandmann, G. Functional in situ evaluation of photosynthesis-protecting carotenoids in mutants of the cyanobacterium Synechocystis PCC6803. J. Photochem. Photobiol. B 2005, 78, 195–201. [Google Scholar] [CrossRef]
- Kusama, Y.; Inoue, S.; Jimbo, H.; Takaichi, S.; Sonoike, K.; Hihara, Y.; Nishiyama, Y. Zeaxanthin and Echinenone Protect the Repair of Photosystem II from Inhibition by Singlet Oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015, 56, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Miyabe, Y.; Ide, H.; Yamamoto, O. Hydroxyl radical scavenging ability of bacterioruberin. Radiat. Phys. Chem. 1997, 50, 267–269. [Google Scholar] [CrossRef]
- De Oliveira, V.E.; Castro, H.V.; Edwards, H.G.M.; de Oliveira, L.F.C. Carotenes and carotenoids in natural biological samples: A Raman spectroscopic analysis. J. Raman Spectrosc. 2010, 41, 642–650. [Google Scholar] [CrossRef]
- Vítek, P.; Ascaso, C.; Artieda, O.; Casero, M.C.; Wierzchos, J. Discovery of carotenoid red-shift in endolithic cyanobacteria from the Atacama Desert. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.G.M.; Moeller, R.; Villar, S.E.J.; Horneck, G.; Stackebrandt, E. Raman spectroscopic study of the photoprotection of extremophilic microbes against ultraviolet radiation. Int. J. Astrobiol. 2006, 5, 313–318. [Google Scholar] [CrossRef]
- Vítek, P.; Osterrothová, K.; Jehlička, J. Beta-carotene-A possible biomarker in the Martian evaporitic environment: Raman micro-spectroscopic study. Planet. Space Sci. 2009, 57, 454–459. [Google Scholar] [CrossRef]
- Ruban, A.V.; Pascal, A.A.; Robert, B.; Horton, P. Configuration and Dynamics of Xanthophylls in Light-harvesting Antennae of Higher Plants: Spectroscopic analysis of isolated light-harvesting complex of photosystem II and thylakoid membranes. J. Biol. Chem. 2001, 276, 24862–24870. [Google Scholar] [CrossRef] [Green Version]
- Ruban, A.V.; Pascal, A.; Lee, P.J.; Robert, B.; Horton, P. Molecular Configuration of Xanthophyll Cycle Carotenoids in Photosystem II Antenna Complexes. J. Biol. Chem. 2002, 277, 42937–42942. [Google Scholar] [CrossRef] [Green Version]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.M.; Grant, O.M.; Chaves, M.M. Thermography to explore plant–environment interactions. J. Exp. Bot. 2013, 64, 3937–3949. [Google Scholar] [CrossRef]
- Neill, S. Interactions between Abscisic Acid, Hydrogen Peroxide and Nitric Oxide Mediate Survival Responses during Water Stress. New Phytol. 2007, 175, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vítek, P.; Veselá, B.; Klem, K. Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging. Sensors 2020, 20, 1015. https://doi.org/10.3390/s20041015
Vítek P, Veselá B, Klem K. Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging. Sensors. 2020; 20(4):1015. https://doi.org/10.3390/s20041015
Chicago/Turabian StyleVítek, Petr, Barbora Veselá, and Karel Klem. 2020. "Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging" Sensors 20, no. 4: 1015. https://doi.org/10.3390/s20041015