Matriptase-2 and Hemojuvelin in Hepcidin Regulation: In Vivo Immunoblot Studies in Mask Mice
Abstract
:1. Introduction
2. Results
2.1. Absence of the Protease Domain of Matriptase-2 Results in Decreased Content of Hemojuvelin Protein
2.2. Hemojuvelin-Deficient Mice Display Significantly Decreased Content of Full-Length Matriptase-2
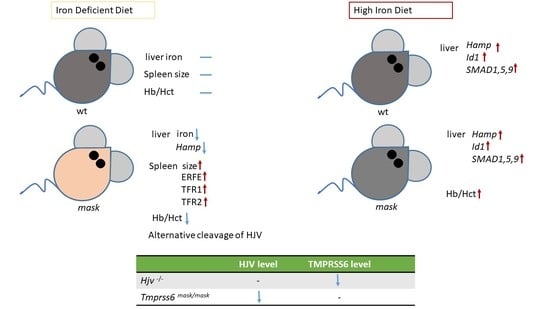
2.3. Mask Mice Upregulate Hepcidin When Fed an Iron-Enriched Diet
2.4. Erythropoietin Activates Erythropoiesis in Iron-Pretreated Mask Mice
2.5. Mask Mice Display Signs of Stress Erythropoiesis When Fed an Iron-Deficient Diet
2.6. Mask Mice Downregulate Hepcidin When Fed an Iron-Deficient Diet
2.7. Feeding an Iron-Deficient Diet Decreases Full-Length HJV Protein in Mask Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Immunoblotting
4.3. Gene Expression Analyses
4.4. Flow Cytometry
4.5. Hematological Parameters and Iron Content
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, J.D.; Campagnolo, L.; Goodarzi, G.; Truong, T.N.; Stuhlmann, H.; Quigley, J.P. Mouse matriptase-2: Identification, characterization and comparative mRNA expression analysis with mouse hepsin in adult and embryonic tissues. Biochem. J. 2003, 373, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Finberg, K.E.; Heeney, M.M.; Campagna, D.R.; Aydınok, Y.; Pearson, H.A.; Hartman, K.R.; Mayo, M.M.; Samuel, S.M.; Strouse, J.J.; Markianos, K.; et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat. Genet. 2008, 40, 569–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; She, E.; Gelbart, T.; Truksa, J.; Lee, P.; Xia, Y.; Khovananth, K.; Mudd, S.; Mann, N.; Moresco, E.M.Y.; et al. The Serine Protease TMPRSS6 Is Required to Sense Iron Deficiency. Science 2008, 320, 1088–1092. [Google Scholar] [CrossRef] [Green Version]
- Folgueras, A.R.; De Lara, F.M.; Pendás, J.A.M.; Garabaya, C.; Rodríguez, F.; Astudillo, A.; Bernal, T.; Cabanillas, R.; López-Otín, C.; Velasco, G. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood 2008, 112, 2539–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finberg, K.E.; Whittlesey, R.L.; Fleming, M.D.; Andrews, N.C. Down-regulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis. Blood 2010, 115, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Nai, A.; Rubio, A.; Campanella, A.; Gourbeyre, O.; Artuso, I.; Bordini, J.; Gineste, A.; Latour, C.; Besson-Fournier, C.; Céline, B.-F.; et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood 2016, 127, 2327–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestri, L.; Pagani, A.; Nai, A.; De Domenico, I.; Kaplan, J.; Camaschella, C. The Serine Protease Matriptase-2 (TMPRSS6) Inhibits Hepcidin Activation by Cleaving Membrane Hemojuvelin. Cell Metab. 2008, 8, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babitt, J.L.; Huang, F.W.; Wrighting, D.M.; Xia, Y.; Sidis, Y.; Samad, T.A.; Campagna, J.; Chung, R.T.; Schneyer, A.L.; Woolf, C.J.; et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 2006, 38, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Truksa, J.; Gelbart, T.; Peng, H.; Beutler, E.; Beutler, B.; Lee, P.L. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. Br. J. Haematol. 2009, 147, 571–581. [Google Scholar] [CrossRef]
- Nicolas, G.; Ramsay, A.J.; Mayeux, P.; Grandchamp, B.; Beaumont, C.; Velasco, G.; Vaulont, S.; Deschemin, J.-C.; Velasco, G. Is EPO therapy able to correct iron deficiency anaemia caused by matriptase-2 deficiency? Br. J. Haematol. 2011, 152, 498–500. [Google Scholar] [CrossRef]
- Nicolas, G.; Viattea, L.; Bennouna, M.; Beaumontb, C.; Kahna, A.; Vaulont, S.; Hepcidin, A. New Iron Regulatory Peptide. Blood Cells Mol. Dis. 2002, 29, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, M.; Krijt, J.; Sulc, K.; Necas, E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol. Res. 2006, 55, 667–674. [Google Scholar] [PubMed]
- Frýdlová, J.; Rychtarčíková, Z.; Gurieva, I.; Vokurka, M.; Truksa, J.; Krijt, J. Effect of erythropoietin administration on proteins participating in iron homeostasis in Tmprss6-mutated mask mice. PLoS ONE 2017, 12, e0186844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemetz, A.; Lenoir, A.; Deschemin, J.-C.; Lopez-Otin, C.; Ramsay, A.J.; Vaulont, S.; Nicolas, G. Matriptase-2 is essential for hepcidin repression during fetal life and postnatal development in mice to maintain iron homeostasis. Blood 2014, 124, 441–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, R.; Ganz, T. Erythroferrone: An Erythroid Regulator of Hepcidin and Iron Metabolism. HemaSphere 2018, 2, e35. [Google Scholar] [CrossRef]
- Arezes, J.; Foy, N.; McHugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; LaVallie, E.R.; Taylor, S.; et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef] [Green Version]
- Wahedi, M.; Wortham, A.M.; Kleven, M.D.; Zhao, N.; Jue, S.; Enns, C.A.; Zhang, A.-S. Matriptase-2 suppresses hepcidin expression by cleaving multiple components of the hepcidin induction pathway. J. Biol. Chem. 2017, 292, 18354–18371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enns, C.A.; Jue, S.; Zhang, A.-S. The ectodomain of matriptase-2 plays an important nonproteolytic role in suppressing hepcidin expression in mice. Blood 2020, 136, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, T.B. Cutting not the key to TMPRSS6 activity? Blood 2020, 136, 922–923. [Google Scholar] [CrossRef] [PubMed]
- Krijt, J.; Fujikura, Y.; Ramsay, A.J.; Velasco, G.; Nečas, E. Liver hemojuvelin protein levels in mice deficient in matriptase-2 (Tmprss6). Blood Cells Mol. Dis. 2011, 47, 133–137. [Google Scholar] [CrossRef]
- Zhang, A.-S.; West, A.P.; Wyman, A.E.; Bjorkman, P.J.; Enns, C.A. Interaction of Hemojuvelin with Neogenin Results in Iron Accumulation in Human Embryonic Kidney 293 Cells. J. Biol. Chem. 2005, 280, 33885–33894. [Google Scholar] [CrossRef] [Green Version]
- Frýdlová, J.; Přikryl, P.; Truksa, J.; Falke, L.L.; Du, X.; Gurieva, I.; Vokurka, M.; Krijt, J. Effect of Erythropoietin, Iron Deficiency and Iron Overload on Liver Matriptase-2 (TMPRSS6) Protein Content in Mice and Rats. PLoS ONE 2016, 11, e0148540. [Google Scholar] [CrossRef] [PubMed]
- Enns, C.A.; Ahmed, R.; Zhang, A.-S. Neogenin Interacts with Matriptase-2 to Facilitate Hemojuvelin Cleavage. J. Biol. Chem. 2012, 287, 35104–35117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gitlin-Domagalska, A.; Mangold, M.; Dębowski, D.; Ptaszyńska, N.; Anna, Ł.; Gütschow, M.; Rolka, K. Matriptase-2: Monitoring and inhibiting its proteolytic activity. Future Med. Chem. 2018, 10, 2745–2761. [Google Scholar] [CrossRef] [PubMed]
- Belot, A.; Gourbeyre, O.; Palin, A.; Rubio, A.; Largounez, A.; Besson-Fournier, C.; Latour, C.; Lorgouilloux, M.; Gallitz, I.; Montagner, A.; et al. Endoplasmic reticulum stress controls iron metabolism through TMPRSS6 repression and hepcidin mRNA stabilization by RNA-binding protein HuR. Haematologica 2020. [Google Scholar] [CrossRef]
- Ponka, P. Tissue-specific regulation of iron metabolism and heme synthesis: Distinct control mechanisms in erythroid cells. Blood 1997, 89, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.F.; Shi, L.; Wu, D.C. Stress erythropoiesis: New signals and new stress progenitor cells. Curr. Opin. Hematol. 2011, 18, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, L.; Pagani, A.; Camaschella, C. Furin-mediated release of soluble hemojuvelin: A new link between hypoxia and iron homeostasis. Blood 2008, 111, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.-H.; Li, C.; Xu, X.; Zheng, Y.; Xiao, C.; Zerfas, P.; Cooperman, S.; Eckhaus, M.; Rouault, T.; Mishra, L.; et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005, 2, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, G.; Samuels, M.E.; Ludwig, E.H.; MacDonald, M.L.E.; Franchini, P.L.; Dubé, M.-P.; Andres, L.; Macfarlane, J.; Sakellaropoulos, N.; Politou, M.; et al. Mutations in HFE2 cause iron overload in chromosome 1q–linked juvenile hemochromatosis. Nat. Genet. 2003, 36, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.W.; Pinkus, J.L.; Pinkus, G.S.; Fleming, M.D.; Andrews, N.C. A mouse model of juvenile hemochromatosis. J. Clin. Investig. 2005, 115, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-S.; Gao, J.; Koeberl, D.D.; Enns, C.A. The Role of Hepatocyte Hemojuvelin in the Regulation of Bone Morphogenic Protein-6 and Hepcidin Expression in Vivo. J. Biol. Chem. 2010, 285, 16416–16423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.-S.; Anderson, S.A.; Wang, J.; Yang, F.; DeMaster, K.; Ahmed, R.; Nizzi, C.P.; Eisenstein, R.S.; Tsukamoto, H.; Enns, C.A. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood 2011, 117, 1687–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nili, M.; Shinde, U.; Rotwein, P. Soluble Repulsive Guidance Molecule c/Hemojuvelin Is a Broad Spectrum Bone Morphogenetic Protein (BMP) Antagonist and Inhibits both BMP2- and BMP6-mediated Signaling and Gene Expression. J. Biol. Chem. 2010, 285, 24783–24792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, M.; Lopez, M.A.; Gabayan, V.; Ganz, T.; Rivera, S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood 2006, 108, 3730–3735. [Google Scholar] [CrossRef] [PubMed]
- Krijt, J.; Jonášová, A.; Neuwirtová, R.; Nečas, E. Effect of erythropoietin on hepcidin expression in hemojuvelin-mutant mice. Blood Cells Mol. Dis. 2010, 44, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Kempe, D.S.; Lang, P.A.; Duranton, C.; Akel, A.; Lang, K.S.; Huber, S.M.; Wieder, T.; Lang, F. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006, 20, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Mirciov, C.; Wilkins, S.J.; Hung, G.C.C.; Helman, S.L.; Anderson, G.J.; Frazer, D.M. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica 2018, 103, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Frýdlová, J.; Rogalsky, D.W.; Truksa, J.; Traeger, L.; Steinbicker, A.U.; Vokurka, M.; Krijt, J. Liver HFE protein content is posttranscriptionally decreased in iron-deficient mice and rats. Am. J. Physiol. Liver Physiol. 2018, 315, G560–G568. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Meynard, D.; Monnier, A.; Darnaud, V.; Bouvet, R.; Wang, R.H.; Deng, C.; Vaulont, S.; Mosser, J.; Coppin, H.; et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 2008, 112, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, J.; Heck, S.; Chasis, J.A.; An, X.; Mohandas, N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc. Natl. Acad. Sci. USA 2009, 106, 17413–17418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrance, J.D.; Bothwell, T.H. Tissue iron stores. In Methods in Hematology; Cook, J.D., Ed.; Churchill Livingstone: New York, NY, USA, 1981; pp. 90–115. [Google Scholar]







| Group | ProE % | Baso % | Poly % | Ortho + Reti % | RBC % |
|---|---|---|---|---|---|
| C57BL/6 | 0.16 ± 0.06 | 3.76 ± 1.96 | 9.21 ± 0.01 | 17.45 ± 6.93 | 69.42 ± 8.95 |
| mask | 0.17 ± 0.02 | 2.84 ± 0.91 | 4.91 ± 1.25 | 19.58 ± 2.37 | 72.51 ± 4.56 |
| mask ID | 0.37 ± 0.12 | 11.25 ± 1.68 | 15.98 ± 0.95 | 30.40 ± 0.30 | 42.01 ± 0.55 |
| Group | Spleen Fe, Males (µg/g) | Spleen Fe, Females (µg/g) | Plasma Fe, Males (µmol/L) | Plasma Fe, Females (µmol/L) |
|---|---|---|---|---|
| C57BL/6 | 706 ± 192 | 1356 ± 54 | 22.3 ± 3.1 | 25.7 ± 3.5 |
| C57BL/6 ID | 243 ± 66 * | 478 ± 495 | 26.3 ± 1.9 | 21.3 ± 3.5 |
| mask | 643 ± 174 | 814 ± 300 | 5.2 ± 3.0 # | 8.7 ± 2.1 # |
| mask ID | 514 ± 166 | 480 ± 214 | 4.0 ± 1.0 | 3.7 ± 0.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krijt, J.; Frýdlová, J.; Gurieva, I.; Přikryl, P.; Báječný, M.; Steinbicker, A.U.; Vokurka, M.; Truksa, J. Matriptase-2 and Hemojuvelin in Hepcidin Regulation: In Vivo Immunoblot Studies in Mask Mice. Int. J. Mol. Sci. 2021, 22, 2650. https://doi.org/10.3390/ijms22052650
Krijt J, Frýdlová J, Gurieva I, Přikryl P, Báječný M, Steinbicker AU, Vokurka M, Truksa J. Matriptase-2 and Hemojuvelin in Hepcidin Regulation: In Vivo Immunoblot Studies in Mask Mice. International Journal of Molecular Sciences. 2021; 22(5):2650. https://doi.org/10.3390/ijms22052650
Chicago/Turabian StyleKrijt, Jan, Jana Frýdlová, Iuliia Gurieva, Petr Přikryl, Martin Báječný, Andrea U. Steinbicker, Martin Vokurka, and Jaroslav Truksa. 2021. "Matriptase-2 and Hemojuvelin in Hepcidin Regulation: In Vivo Immunoblot Studies in Mask Mice" International Journal of Molecular Sciences 22, no. 5: 2650. https://doi.org/10.3390/ijms22052650