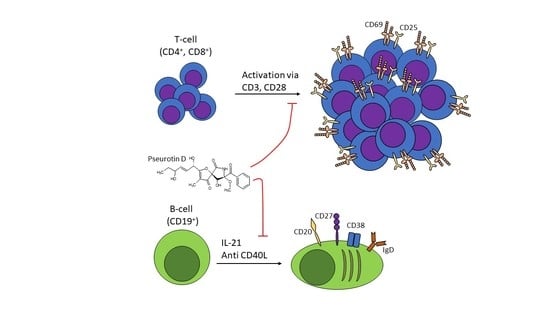
Pseurotin D Inhibits the Activation of Human Lymphocytes
Abstract
:1. Introduction
2. Results
2.1. Pseurotin Inhibits the Activation of Both CD4+ and CD8+ T Cells
2.2. Pseurotin Inhibits the Activation of STAT Signaling Pathways in T cells
2.3. Pseurotin Inhibits the Proliferation of T Cells
2.4. Pseurotin D Affects the Differentiation of B Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Isolation
4.3. Activation and Treatment
4.4. Cell Proliferation and Cytotoxicity Determination
4.5. Determination of Activation Markers
4.6. Apoptosis Detection
4.7. Production of Cytokines
4.8. Western Blot
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varga, J.; Frisvad, J.C.; Samson, R.A. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef]
- Maiya, S.; Grundmann, A.; Li, X.; Li, S.M.; Turner, G. Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus. Chembiochem 2007, 8, 1736–1743. [Google Scholar] [CrossRef]
- Tsunematsu, Y.; Fukutomi, M.; Saruwatari, T.; Noguchi, H.; Hotta, K.; Tang, Y.; Watanabe, K. Elucidation of pseurotin biosynthetic pathway points to trans-acting C-methyltransferase: Generation of chemical diversity. Angew. Chem. Int. Ed. Engl. 2014, 53, 8475–8479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, M.; Ninomiya, T. Chemical modification of pseurotin A: One-pot synthesis of synerazol and pseurotin E and determination of absolute stereochemistry of pseurotin E. J. Antibiot. 2008, 61, 692–695. [Google Scholar] [CrossRef] [Green Version]
- Bloch, P.; Tamm, C.; Bollinger, P.; Petcher, T.J.; Weber, H.P. Pseurotin, a new metabolite of Pseudeurotium ovalis Stolk having an unusual hetero-spirocyclic system. Helv. Chim. Acta. 1976, 59, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.; Han, S. Total syntheses of spirocyclic PKS-NRPS-based fungal metabolites. Chem. Commun. 2018, 54, 6750–6758. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.M. Biosynthesis of natural products by microbial iterative hybrid PKS-NRPS. RSC Adv. 2013, 3, 18228–18247. [Google Scholar] [CrossRef] [Green Version]
- Ando, O.; Satake, H.; Nakajima, M.; Sato, A.; Nakamura, T.; Kinoshita, T.; Furuya, K.; Haneishi, T. Synerazol, a New Antifungal Antibiotic. J. Antibiot. 1991, 44, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komagata, D.; Fujita, S.; Yamashita, N.; Saito, S.; Morino, T. Novel neuritogenic activities of pseurotin A and penicillic acid. J. Antibiot. 1996, 49, 958–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asami, Y.; Kakeya, H.; Komi, Y.; Kojima, S.; Nishikawa, K.; Beebe, K.; Neckers, L.; Osada, H. Azaspirene, a fungal product, inhibits angiogenesis by blocking Raf-1 activation. Cancer Sci. 2008, 99, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, Y.; Yabuta, Y.; Sekine, A.; Fujii, K.; Harada, K.; Oikawa, T.; Sato, M.; Furumai, T.; Oki, T. Directed biosynthesis of fluorinated pseurotin A, synerazol and gliotoxin. J. Antibiot. 2004, 57, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asami, Y.; Kakeya, H.; Onose, R.; Yoshida, A.; Matsuzaki, H.; Osada, H. Azaspirene: A novel angiogenesis inhibitor containing a 1-oxa-7-azaspiro[4.4]non-2-ene-4,6-dione skeleton produced by the fungus Neosartotya sp. Organ. Lett. 2002, 4, 2845–2848. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Bi, H.; Chai, W.; Lian, X.Y.; Zhang, Z. Antiglioma pseurotin A from marine Bacillus sp. FS8D regulating tumour metabolic enzymes. Nat. Prod. Res. 2017, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, O.; Rubanova, D.; Chytkova, B.; Kubala, L. Natural pseurotins inhibit proliferation and inflammatory responses through the inactivation of STAT signaling pathways in macrophages. Food Chem. Toxicol. 2020, 141, 111348. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, O.; Fedr, R.; Skoroplyas, S.; Chalupa, D.; Sklenar, M.; Tharra, P.R.; Svenda, J.; Kubala, L. Natural pseurotins and analogs thereof inhibit activation of B-cells and differentiation into the plasma cells. Phytomedicine 2020, 69, 153194. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Ninomiya, T.; Akabane, H.; Kushida, N.; Tsujiuchi, G.; Ohyama, M.; Gomi, S.; Shito, K.; Murata, T. Pseurotin A and its analogues as inhibitors of immunoglobulin E [correction of immunoglobuline E] production. Bioorg. Med. Chem. Lett. 2009, 19, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef]
- Bluml, S.; McKeever, K.; Ettinger, R.; Smolen, J.; Herbst, R. B-cell targeted therapeutics in clinical development. Arthritis Res. Ther. 2013, 15 (Suppl. 1), S4. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services Centers for Disease Control and Prevention. Summary Health Statistics for U.S. Adults: National Health Interview Survey; 2017. Available online: https://www.cdc.gov/nchs/nhis/shs.htm (accessed on 3 December 2020).
- Rabb, H. The T cell as a bridge between innate and adaptive immune systems: Implications for the kidney. Kidney Int. 2002, 61, 1935–1946. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yee, C.; Beavo, J.A. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science 1999, 283, 848–851. [Google Scholar] [CrossRef]
- Hasbold, J.; Hong, J.S.; Kehry, M.R.; Hodgkin, P.D. Integrating signals from IFN-gamma and IL-4 by B cells: Positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J. Immunol. 1999, 163, 4175–4181. [Google Scholar]
- Avery, D.T.; Ma, C.S.; Bryant, V.L.; Santner-Nanan, B.; Nanan, R.; Wong, M.; Fulcher, D.A.; Cook, M.C.; Tangye, S.G. STAT3 is required for IL-21-induced secretion of IgE from human naive B cells. Blood 2008, 112, 1784–1793. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.B.; Yu, J.J.; Yu, R.Y.; Mendez, L.M.; Shaknovich, R.; Zhang, Y.; Cattoretti, G.; Ye, B.H. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 2008, 111, 1515–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ul-Haq, Z.; Naz, S.; Mesaik, M.A. Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box. Cytokine Growth Factor. Rev. 2016, 32, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.J.; Hartley, J.A. The emerging role of anti-CD25 directed therapies as both immune modulators and targeted agents in cancer. Br. J. Haematol. 2017, 179, 20–35. [Google Scholar] [CrossRef] [Green Version]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Kimura, M.Y.; Hayashizaki, K.; Tokoyoda, K.; Takamura, S.; Motohashi, S.; Nakayama, T. Crucial role for CD69 in allergic inflammatory responses: CD69-Myl9 system in the pathogenesis of airway inflammation. Immunol. Rev. 2017, 278, 87–100. [Google Scholar] [CrossRef]
- Reddy, M.; Eirikis, E.; Davis, C.; Davis, H.M.; Prabhakar, U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: An in vitro model to monitor cellular immune function. J. Immunol. Methods 2004, 293, 127–142. [Google Scholar] [CrossRef]
- Marchingo, J.M.; Kan, A.; Sutherland, R.M.; Duffy, K.R.; Wellard, C.J.; Belz, G.T.; Lew, A.M.; Dowling, M.R.; Heinzel, S.; Hodgkin, P.D. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 2014, 346, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, B.; Lin, Y.; Pan, J.; Zhao, X.X.; Ren, S.X. Protective Effects of Prunasin A against the Differentiation of Osteoclasts and Destruction of Cartilage via the Receptor Activator of Nuclear Factor-Kappa-Beta Ligand/Mitogen-Activated Protein Kinase/Osteoprotegerin Pathway in a Rat Model of Arthritis. Pharmacology 2019, 104, 216–225. [Google Scholar] [CrossRef]
- Moriggl, R.; Topham, D.J.; Teglund, S.; Sexl, V.; McKay, C.; Wang, D.; Hoffmeyer, A.; van Deursen, J.; Sangster, M.Y.; Bunting, K.D.; et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 1999, 10, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Rajasingh, J.; Raikwar, H.P.; Muthian, G.; Johnson, C.; Bright, J.J. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem. Biophys. Res. Commun. 2006, 340, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Qiu, P.C.; Yuan, Y.; Zheng, L.; He, J.B.; Wang, C.; Guo, Q.; Kenny, J.; Liu, Q.; Zhao, J.M.; et al. Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species. Theranostics 2019, 9, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, A.K.; Rizzi, M.; Reith, M.; Nutt, S.L.; Eibel, H. Inhibition of human B-cell development into plasmablasts by histone deacetylase inhibitor valproic acid. J. Allergy Clin. Immunol. 2013, 131, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, K.S.; Siddique, A.; Mohyeldin, M.M.; Qusa, M.H.; Goda, A.A.; Singh, S.S.; Ayoub, N.M.; King, J.A.; Jois, S.D.; El Sayed, K.A. Pseurotin A as a novel suppressor of hormone dependent breast cancer progression and recurrence by inhibiting PCSK9 secretion and interaction with LDL receptor. Pharmacol. Res. 2020, 158, 104847. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F.; Denev, P.N.; Lojek, A.; Batsalova, T.G.; et al. Tilia tomentosa pectins exhibit dual mode of action on phagocytes as beta-glucuronic acid monomers are abundant in their rhamnogalacturonans I. Carbohydr. Polym. 2017, 175, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Vasicek, O.; Lojek, A.; Ciz, M. Serotonin and its metabolites reduce oxidative stress in murine RAW264.7 macrophages and prevent inflammation. J. Physiol. Biochem. 2020, 76, 49–60. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubanova, D.; Dadova, P.; Vasicek, O.; Kubala, L. Pseurotin D Inhibits the Activation of Human Lymphocytes. Int. J. Mol. Sci. 2021, 22, 1938. https://doi.org/10.3390/ijms22041938
Rubanova D, Dadova P, Vasicek O, Kubala L. Pseurotin D Inhibits the Activation of Human Lymphocytes. International Journal of Molecular Sciences. 2021; 22(4):1938. https://doi.org/10.3390/ijms22041938
Chicago/Turabian StyleRubanova, Daniela, Petra Dadova, Ondrej Vasicek, and Lukas Kubala. 2021. "Pseurotin D Inhibits the Activation of Human Lymphocytes" International Journal of Molecular Sciences 22, no. 4: 1938. https://doi.org/10.3390/ijms22041938