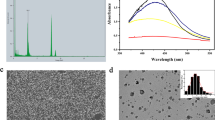
Abstract
Pathogens are a major threat of plant-based production. Expanding restrictions for the use of classical pesticides is increasing the need of alternative applications to control plant diseases. Nanoparticles have recently received increasing research interest as a potential means to protect plants from adverse conditions including pathogen attack. To assess the beneficial potential of silver nanoparticles to protect plants against the bacterial pathogen Pseudomonas syringae, of which numerous economically relevant pathovars are known, we evaluated the effect of silver nanoparticle pre-treatment in the model pathosystem Arabidopsis thaliana–P. syringae. For this purpose, A. thaliana leaves were treated with different silver nanoparticle concentrations prior to P. syringae infection and visible alterations of the leaf tissue in relation to the individual and combined treatments were scored. While treatment with silver nanoparticles in the concentration range between 0.5 and 10 ppm suppressed P. syringae symptom development, concentrations above 5 ppm caused necroses and chloroses in a dose-dependent manner. This indicates that silver nanoparticles affect plant physiological processes related to cell and tissue integrity that are also associated with the development of infection symptoms caused by P. syringae. Therefore, silver nanoparticle treatments in a suitable concentration range support the maintenance of tissue integrity during pathogen infection in combination with their antimicrobial activity, thus preventing loss of biomass. This makes silver nanoparticles a promising tool for integrative crop protection strategies in commercial production.


Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- dpi:
-
days post infection
- NPs:
-
Nanoparticles
- PAR:
-
Photosynthetically Active Radiation
- Pto :
-
Pseudomonas syringae pv. tomato
References
Vert, M., Doi, Y., Hellwich, K.-H., Hess, M., Hodge, P., Kubisa, P., Rinaudo, M., & Schué, F. (2012). Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Applied Chemistry, 84, 377–410.
Sanzari, I., Leone, A., & Ambrosone, A. (2019). Nanotechnology in plant science: To make a long story short. Front Bioeng Biotechnol, 7, 120.
Fincheira, P., Tortella, G., Duran, N., Seabra, A. B., & Rubilar, O. (2020). Current applications of nanotechnology to develop plant growth inducer agents as an innovation strategy. Critical Reviews in Biotechnology, 40, 15–30.
Rubilar, O., Diez, M. C., Tortella, G. R., Briceño, G., Marcato, P. D., & Durán, N. (2014). New strategies and challenges for nanobiotechnology in agriculture. Journal of Biobased Materials and Bioenergy, 8, 1–12.
Sekhon, B. S. (2014). Nanotechnology in agri-food production: An overview. Nanotechnology, Science and Applications, 7, 31–53.
Sharon, M., Choudhary, A. K., & Kumar, R. (2010). Nanotechnology in agricultural diseases and food safety. J Phytol, 2, 83–92.
Ahmad, S. A., Das, S. S., Khatoon, A., Ansari, M. T., Afzal, M., Hasnain, M. S., & Nayak, A. K. (2020). Bactericidal activity of silver nanoparticles A mechanistic review. Mater Sci Energy Technol, 3, 756–769.
Jasim, B., Thomas, R., Mathew, J., & Radhakrishnan, E. K. (2017). Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm J, 25, 443–447.
Sharma, P., Bhatt, D., Zaidi, M. G. H., Saradhi, P. P., Khanna, P. K., & Arora, S. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Applied Biochemistry and Biotechnology, 167, 2225–2233.
Mehta Pallavi, C. M., Srivastava, R., Arora, S., & Sharma, A. K. (2016). Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech, 6, 254.
Yan, A., & Chen, Z. (2019). Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. International Journal of Molecular Sciences, 20, 1003.
Vannini, C., Domingo, G., Onelli, E., De Mattia, F., Bruni, I., Marsoni, M., & Bracale, M. (2014). Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. Journal of Plant Physiology, 171, 1142–1148.
Nair, P. M. G., & Chung, I. M. (2014). Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere, 112, 105–113.
Homaee, M. B., & Ehsanpour, A. A. (2016). Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L) grown in vitro. Horticulture, Environment, and Biotechnology, 57, 544–553.
Qian, H., Peng, X., Han, X., Ren, J., Sun, L., & Fu, Z. (2013). Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. Journal of Environmental Sciences, 25, 1947–1956.
Syu, Y., Hung, J.-H., Chen, J.-C., & Chuang, H. (2014). Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiology and Biochemistry, 83, 57–64.
Franci, G., Falanga, A., Galdiero, S., Palomba, L., Rai, M., Morelli, G., & Galdiero, M. (2015). Silver nanoparticles as potential antibacterial agents. Molecules, 20, 8856–8874e.
Wu, D., Fan, W., Kishen, A., Gutmann, J. L., & Fan, B. (2014). Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. Journal of Endodontia, 40, 285–290.
Sharma, T. K., Sapra, M., Chopra, A., Sharma, R., Patil, S. D., Malik, R. K., Pathania, R., & Navani, N. K. (2012). Interaction of bacteriocin-capped silver nanoparticles with food pathogens and their antibacterial effect. Int J Green Nanotechnol, 4, 93–110.
Kim, S. W., Jung, J. H., Lamasal, K., Kim, Y. S., Min, J. S., & Lee, Y. S. (2012). Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology, 40, 53–58.
Lamasal, K., Kim, S. W., Jung, J. H., Kim, Y. S., & Lee, Y. S. (2011). Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology, 39(2), 6–32.
Katagiri, F., Thilmony, R., & He, S. Y. (2002). The Arabidopsis thaliana-Pseudomonas syringae interaction. Arabidopsis Book, 1, e0039.
Velásquez, A. C., Oney, M., Huot, B., Xu, S., & He, S. Y. (2017). Diverse mechanisms of resistance to Pseudomonas syringae in a thousand natural accessions of Arabidopsis thaliana. New Phytologist, 214, 1673–1687.
Block, A., & Alfano, J. R. (2011). Plant targets for Pseudomonas syringae type III effectors Virulence targets or guarded decoys? Current Opinion in Microbiology, 14, 39–46.
Großkinsky, D. K., Tafner, R., Moreno, M. V., Stenglein, S. A., García de Salamone, I. E., Nelson, L. M., Novák, O., Strnad, M., van der Graaff, E., & Roitsch, T. (2016). Cytokinin production by Pseudomonas fluorescens G20–18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Science and Reports, 6, 23310.
Großkinsky, D. K., Koffler, B. E., Roitsch, T., Maier, R., & Zechmann, B. (2012). Compartment-specific antioxidative defense in Arabidopsis against virulent and avirulent Pseudomonas syringae. Phytopathology, 102, 662–673.
Vass, I. Z., Deák, Z., Paul, K., Kovács, S., & Vass, I. (2015). Interaction of nanoparticles with biological systems. Acta Biol Szeged, 59(Suppl. 2), 225–245.
Gliga, A. R., Skoglund, S., Wallinder, I. O., Fadeel, B., & Karlsson, H. L. (2014). Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Particle and Fibre Toxicology, 11, 11. https://doi.org/10.1186/1743-8977-11-11
Stoehr, L. C., Gonzalez, E., Stampfl, A., Casals, E., Duschl, A., & Puntes, V. (2011). Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells. Particle and Fibre Toxicology, 8, 425–430.
Akter, M., Sikder, M. T., Rahman, M. M., Ullah, A. K. M. A., Hossain, K. F. B., Banik, S., Hosokawa, T., Saito, T., & Kurasaki, M. (2018). A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives, Journal of Advanced Research, 9, 1-16. https://doi.org/10.1016/j.jare.2017.10.008
AshaRani, P. V., Low Kah Mun, G., Hande, M. P., & Valiyaveettil, S. (2009). Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano, 3, 279–290. https://doi.org/10.1021/nn800596w
Abdel-Megeed, A. (2013). Controlling of Pseudomonas syringae by nanoparticles produced by Streptomyces bikiniensis. J Pure Appl Microbiol, 7, 1121–1129.
Jo, Y.-K., Kim, B. H., & Jung, G. (2009). Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Disease, 93, 1037–1043. https://doi.org/10.1094/pdis-93-10-1037
Kaveh, R., Li, Y.-S., Ranjbar, S., Tehrani, R., Brueck, C. L., & Aken, B. V. (2013). Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environmental Science and Technology, 47(18), 10637–10644. https://doi.org/10.1021/es402209w
Wang, J., Koo, Y., Alexander, A., Yang, Y., Westerhof, S., Zhang, Q., Schnoor, J. L., Colvin, V. L., Braam, J., & Alvarez, P. J. J. (2013). Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environmental Science and Technology, 47, 5442–5449.
Ismail GA, El-Sheekh MM, Samy RM, Gheda SF (2021) Antimicrobial, antioxidant, and antiviral activities of biosynthesized silver nanoparticles by phycobiliprotein crude extract of the cyanobacteria Spirulina platensis and Nostoc linckia. BioNanoScience 11(4). https://doi.org/10.1007/s12668-021-00828-3
Shahryari, F., Rabiei, Z., & Sdighian, S. (2020). Antibacterial activity of synthesized silver nanoparticles by sumac aqueous extract and silver-chitosan nanocomposite against Pseudomonas syringae pv. syringae. J Plant Pathol, 102, 469–475.
Reddy, M. C., Murthy, K. S., Srilakshmi, A., Sambasiva Rao, K. R. S., & Pullaiah, T. (2015). Phytosynthesis of eco-friendly silver nanoparticles and biological applications–A novel concept in nanobiotechnology. African Journal of Biotechnology, 14, 222–247.
Khan, M., Khan, A. U., Hasan, M. A., Yadav, K. K., Pinto, M. M. C., Malik, N., Yadav, V. K., Khan, A. H., Islam, S., & Sharma, G. K. (2021). Agro-nanotechnology as an emerging field: A novel sustainable approach for improving plant growth by reducing biotic stress. Applied Sciences, 11(5), 2282. https://doi.org/10.3390/app11052282
Acknowledgements
The authors thank prof. Christian Andreasen, Department of Plant and Environmental Sciences, University of Copenhagen, for providing greenhouse facilities.
Funding
KP was part of the EU COST ACTION FA1306 grant for Short-Term Scientific Missions (STSM) to Department of Plant and Environmental Sciences, University of Copenhagen. Silver nanoparticles purchase was supported by the Hungarian grant no. TÁMOP-4.1.1.C-13/1/KONV-2014–0001. TR would like to acknowledge funding by the Ministry of Education, Youth and Sports of CR within the National Sustainability Program I (NPU I), grant number LO1415.
Author information
Authors and Affiliations
Contributions
KP and DKG designed and carried out the experiments, analyzed the data, and drafted the manuscript. All authors contributed to interpreting the data, writing the manuscript, and approving its final version for publication.
Corresponding author
Ethics declarations
Conflict of Interest
No conflicts of interest declared.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, K., Großkinsky, D.K., Vass, I. et al. Silver Nanoparticles Affect Arabidopsis thaliana Leaf Tissue Integrity and Suppress Pseudomonas syringae Infection Symptoms in a Dose-Dependent Manner. BioNanoSci. 12, 332–338 (2022). https://doi.org/10.1007/s12668-022-00957-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00957-3