Abstract
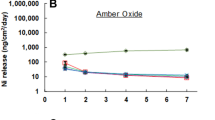
Nickel-titanium alloys have been used in medical applications for several years; however, biocompatibility of the material remains controversial. In the present study, the human umbilical vein endothelial cells (HUVEC) were cultured in contact with the nitinol used in two different heat treatment surface modifications—helium and hydrogen. The amount of Ni ions released from these alloys in contact with HUVEC was measured in media and in the cells by ICP-MS. An increased release of Ni ions was detected in He alloy compared with H2 alloy modification with an elevation with the metal exposition duration (24 h vs. 72 h). The cells contained the Ni ions in both selected alloy modifications with the lower levels in H2 alloys. To evaluate the potential of multiple metal applications, similar values were observed in media and in cell suspension for all surface modification combinations. The model analysis of effect of metal ion release on distant cells in the body showed that the concentration is interestingly similar to concentrations in cells in direct contact with the metal alloy. The cells are able to regulate the concentration of Ni ions within the cell. According to our best knowledge, the study for the first time describes the presence of Ni ions released from nitinol directly in the cells. In the case of the H2 modification, the lowest levels of Ni ions were detected both in medium and in the cells, which likely increases the biocompatibility of the nitinol alloy.




Similar content being viewed by others
References
Balla VK, Banerjee S, Bose S, Bandyopadhyay A (2010) Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta Biomater 6(6):2329–2334. https://doi.org/10.1016/j.actbio.2009.11.021
Bogdanski D, Köller M, Müller D, Muhr G, Bram M, Buchkremer HP, Stöver D, Choi J, Epple M (2002) Easy assessment of the biocompatibility of Ni–Ti alloys by in vitro cell culture experiments on a functionally graded Ni–NiTi–Ti material. Biomaterials 23(23):4549–4555
Carroll WM, Kelly MJ (2003) Corrosion behavior of nitinol wires in body fluid environments. J Biomed Mater Res A 67(4):1123–1130
Chrzanowski W, Abou Neel EA, Armitage DA, Knowles JC (2008) Surface preparation of bioactive Ni–Ti alloy using alkali, thermal treatments and spark oxidation. J Mater Sci Mater Med 19(4):1553–1557
Clarke B, Carroll W, Rochev Y, Hynes M, Bradley D, Plumley D (2006) Influence of nitinol wire surface treatment on oxide thickness and composition and its subsequent effect on corrosion resistance and nickel ion release. J Biomed Mater Res A 79A(1):61–70. https://doi.org/10.1002/jbm.a.30720
Das K, Bose S, Bandyopadhyay A (2007) Surface modifications and cell-materials interactions with anodized Ti. Acta Biomater 3(4):573–585. https://doi.org/10.1016/j.actbio.2006.12.003
Duerig TW, Pelton A, Stöckel D (1999) An overview of nitinol medical applications. Mater Sci Eng A 273:149–160
Es-Souni M, Es-Souni M, Brandies HF (2001) On the transformation behaviour, mechanical properties and biocompatibility of two NiTi-based shape memory alloys:: NiTi42 and NiTi42Cu7. Biomaterials 22(15):2153–2161. https://doi.org/10.1016/S0142-9612(00)00406-3
Gendre D, Czernic P, Conéjéro G, Pianelli K, Briat J-F, Lebrun M, Mari S (2007) TcYSL3, a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. Plant J 49(1):1–15
Haider W, Munroe N, Tek V, Gill PKS, Tang Y, McGoron AJ (2011) Cytotoxicity of metal ions released from nitinol alloys on endothelial cells. J Mater Eng Perform 20(4–5):816–818. https://doi.org/10.1007/s11665-011-9884-5
Haider, Waseem. 2010. “Enhanced biocompatibility of NiTi (nitinol) via surface treatment and alloying”
Hanawa T (2004) Metal ion release from metal implants. Mater Sci Eng C 24(6):745–752
Jenko M, Godec M, Kocijan A, Rudolf R, Dolinar D, Ovsenik M, Gorenšek M, Mozetic M (2019) A new route to biocompatible nitinol based on a rapid treatment with H2/O2 gaseous plasma. Appl Surf Sci 473:976–984
Kobayashi S, Ohgoe Y, Ozeki K, Sato K, Sumiya T, Hirakuri KK, Aoki H (2005) Diamond-like carbon coatings on orthodontic archwires. Diam Relat Mater 14(3):1094–1097
Lešková, Alexandra, Milan Zvarík, Takao Araya, and Ricardo FH Giehl. 2019. “Nickel toxicity targets cell wall-related processes and PIN2-mediated auxin transport to inhibit root elongation and gravitropic responses in Arabidopsis.” Plant Cell Physiol
L’H, Y, F. Rayes, and A. O. Warrak. (2009). “Regulation, orthopedic, dental, endovascular and other applications of Ti–Ni shape memory alloys.” In Shape memory alloys for biomedical applications, 306–326. Elsevier
Lifeng Z, Yan H, Yang D, Xiaoying L, Tingfei X, Deyuan Z, Ying H, Jinfeng Y (2011) The underlying biological mechanisms of biocompatibility differences between bare and TiN-coated NiTi alloys. Biomed Mater (Bristol, England) 6(2):25012. https://doi.org/10.1088/1748-6041/6/2/025012
Lü X, Bao X, Huang Y, Yinghua Q, Lu H, Zuhong L (2009) Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials 30(2):141–148. https://doi.org/10.1016/j.biomaterials.2008.09.011
Monteilh-Zoller MK, Hermosura MC, Nadler MJS, Scharenberg AM, Penner R, Fleig A (2003) TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol 121(1):49–60
Muñoz A, Costa M (2012) Elucidating the mechanisms of nickel compound uptake: a review of particulate and nano-nickel endocytosis and toxicity. Toxicol Appl Pharmacol 260(1):1–16
Nematzadeh F, Sadrnezhaad SK (2012) Effects of material properties on mechanical performance of nitinol stent designed for femoral artery: finite element analysis. Scientia Iranica 19(6):1564–1571
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51(6):730–750
Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T (2011) AtIRT1, the primary Iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol 52(8):1433–1442
Okazaki Y, Gotoh E (2008) Metal release from stainless steel, co–Cr–Mo–Ni–Fe and Ni–Ti alloys in vascular implants. Corros Sci 50(12):3429–3438
Pelton, A. R., D. Stöckel, and T. W. Duerig. 2000. “Medical uses of nitinol.” In Materials science forum, 327:63–70. Trans Tech Publ
Pelton, Alan R. 2004. SMST 2003: proceedings of the international conference on shape memory and superelastic technologies. ASM International
Plant SD, Grant DM, Leach L (2005) Behaviour of human endothelial cells on surface modified NiTi alloy. Biomaterials 26(26):5359–5367. https://doi.org/10.1016/j.biomaterials.2005.01.067
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials science: an introduction to materials in medicine. Academic press
Rokicki R. (2013). “Method for surface inclusions detection in nitinol which are primary corrosion and fatigue initiation sites and indicators of overall quality of nitinol material.” https://www.google.com/patents/US8377237. Accessed 13 May 2016
Russell SM 2001. SMST-2000: proceedings of the international conference on shape memory and superelastic technologies. ASM International
Ryhänen J, Niemi E, Serlo W, Niemelä E, Sandvik P, Pernu H, Salo T (1997) Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J Biomed Mater Res 35(4):451–457. https://doi.org/10.1002/(SICI)1097-4636(19970615)35:4<451::AID-JBM5>3.0.CO;2-G
Ryhänen J (1999) Biocompatibility evaluation of nickel-titanium shape memory metal alloy. University of Oulu, Oulu
Schaaf G, Erenoglu BE, von Wirén N (2004) Physiological and biochemical characterization of metal-phytosiderophore transport in graminaceous species. Soil Sci Plant Nutr 50(7):989–995
Ševčíková J, Bártková D, Goldbergová M, Kuběnová M, Čermák J, Frenzel J, Weiser A, Dlouhý A (2018) On the Ni-ion release rate from surfaces of binary NiTi shape memory alloys. Appl Surf Sci 427(January):434–443. https://doi.org/10.1016/j.apsusc.2017.08.235
Sevcikova J, Goldbergova MP (2017) Biocompatibility of NiTi alloys in the cell behaviour. BioMetals 30(2):163–169
Shabalovskaya S, Anderegg J, Van Humbeeck J (2008) Critical overview of nitinol surfaces and their modifications for medical applications. Acta Biomater 4(3):447–467. https://doi.org/10.1016/j.actbio.2008.01.013
Shabalovskaya S, Rondelli G, James A, Xiong JP, Ming W (2004) Comparative corrosion performance of black oxide, sandblasted, and fine-drawn nitinol wires in potentiodynamic and potentiostatic tests: effects of chemical etching and electropolishing. J Biomed Mater Res B Appl Biomater 69B(2):223–231. https://doi.org/10.1002/jbm.b.30006
Sheriff, J., A. R. Pelton, and L. A. Pruitt. 2006. “Hydrogen effects on nitinol fatigue.” In SMST-2004: proceedings of the international conference on shape memory and superelastic technologies, 111–6. ASM International
Shih C-C, Lin S-J, Chen Y-L, Yea-Yang S, Lai S-T, Wu GJ, Kwok C-F, Chung K-H (2000) The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J Biomed Mater Res 52(2):395–403. https://doi.org/10.1002/1097-4636(200011)52:2<395::AID-JBM21>3.0.CO;2-B
Stoeckel D, Pelton A, Duerig T (2004) Self-expanding nitinol stents: material and design considerations. Eur Radiol 14(2):292–301
Sui JH, Cai W (2006) Effect of diamond-like carbon (DLC) on the properties of the NiTi alloys. Diam Relat Mater 15(10):1720–1726
Válková L, Ševčíková J, Goldbergová MP, Weiser A, Dlouhý A (2018) Osteoarthritic process modifies expression response to NiTi alloy presence. J Mater Sci Mater Med 29(9):146
Wataha JC, O’Dell NL, Singh BB, Ghazi M, Whitford GM, Lockwood PE (2001) Relating nickel-induced tissue inflammation to nickel release in vivo. J Biomed Mater Res 58(5):537–544
Wen X, Wang X, Zhang N (1996) Microrough surface of metallic biomaterials: a literature review. Biomed Mater Eng 6(3):173–189
Wever DJ, Veldhuizen AG, Sanders MM, Schakenraad JM, van Horn JR (1997) Cytotoxic, allergic and genotoxic activity of a nickel-titanium alloy. Biomaterials 18(16):1115–1120
Winn B, Derrick Quarles C, Kenneth Marcus R, LaBerge M (2011) Nickel ions inhibit alpha-actin expression and decrease aspect ratio of rat vascular smooth muscle cells in vitro. Metallomics 3(9):934–940. https://doi.org/10.1039/c1mt00035g
Yang D, Lü X, Hong Y, Xi T, Zhang D (2014) The molecular mechanism for effects of TiN coating on NiTi alloy on endothelial cell function. Biomaterials 35(24):6195–6205. https://doi.org/10.1016/j.biomaterials.2014.04.069
Yokoyama K’i, Hamada K, Asaoka K (2001) Fracture analysis of hydrogen-charged nickel-titanium superelastic alloy. Mater Trans 42(1):141–144
Zhang YM, Bataillon-Linez P, Huang P, Zhao YM, Han Y, Traisnel M, Xu KW, Hildebrand HF (2004) Surface analyses of micro-arc oxidized and hydrothermally treated titanium and effect on osteoblast behavior. J Biomed Mater Res Part A 68(2):383–391. https://doi.org/10.1002/jbm.a.20063
Funding
This study received financial support from the Czech Science Foundation under the contract no. 15-16336S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. The Ni ion release is affected by heat treatment surface modification of NiTi alloy (He vs. H2).
2. Ni ions released from NiTi alloy enter the cells in both surface modifications.
3. Similar level of nickel ions in the cells was detected as effect of medium with metal ions.
4. Cells are able to release and regulate absorbed Ni ions.
Rights and permissions
About this article
Cite this article
Veverkova, J., Bartkova, D., Weiser, A. et al. Effect of Ni ion release on the cells in contact with NiTi alloys. Environ Sci Pollut Res 27, 7934–7942 (2020). https://doi.org/10.1007/s11356-019-07506-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07506-8