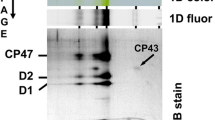
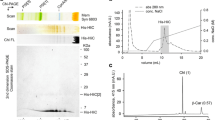
Abstract
High-light-inducible proteins (Hlips) are single-helix transmembrane proteins that are essential for the survival of cyanobacteria under stress conditions. The model cyanobacterium Synechocystis sp. PCC 6803 contains four Hlip isoforms (HliA-D) that associate with Photosystem II (PSII) during its assembly. HliC and HliD are known to form pigmented (hetero)dimers that associate with the newly synthesized PSII reaction center protein D1 in a configuration that allows thermal dissipation of excitation energy. Thus, it is expected that they photoprotect the early steps of PSII biogenesis. HliA and HliB, on the other hand, bind the PSII inner antenna protein CP47, but the mode of interaction and pigment binding have not been resolved. Here, we isolated His-tagged HliA and HliB from Synechocystis and show that these two very similar Hlips do not interact with each other as anticipated, rather they form HliAC and HliBC heterodimers. Both dimers bind Chl and β-carotene in a quenching conformation and associate with the CP47 assembly module as well as later PSII assembly intermediates containing CP47. In the absence of HliC, the cellular levels of HliA and HliB were reduced, and both bound atypically to HliD. We postulate a model in which HliAC-, HliBC-, and HliDC-dimers are the functional Hlip units in Synechocystis. The smallest Hlip, HliC, acts as a ‘generalist’ that prevents unspecific dimerization of PSII assembly intermediates, while the N-termini of ‘specialists’ (HliA, B or D) dictate interactions with proteins other than Hlips.








Similar content being viewed by others
Data Availabilty
The raw MS data has been submitted to the MassIVE repository (CCMS, University of California, San Diego) and can be accessed via the link ftp://massive.ucsd.edu/MSV000088753/.
Change history
23 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11120-023-01016-y
References
Arkowitz RA, Wickner W (1994) SecD and SecF are required for the proton electrochemical gradient stimulation of preprotein translocation. EMBO J 13:954–963. https://doi.org/10.1002/j.1460-2075.1994.tb06340.x
Bassi R, Croce R, Cugini D, Sandona D (1999) Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc Natl Acad Sci 96:10056–10061. https://doi.org/10.1073/pnas.96.18.10056
Becker K, Cormann KU, Nowaczyk MM (2011) Assembly of the water-oxidizing complex in photosystem II. J Photochem Photobiol B 104:204–211. https://doi.org/10.1016/j.jphotobiol.2011.02.005
Ben-Shem A, Frolow F, Nelson N (2003) Crystal structure of plant photosystem I. Nature 426:630–635. https://doi.org/10.1038/nature02200
Boehm M, Romero E, Reisinger V, Yu J, Komenda J, Eichacker LA, Dekker JP, Nixon PJ (2011) Investigating the early stages of photosystem II assembly in Synechocystis sp. PCC 6803. J Biol Chem 286:14812–14819. https://doi.org/10.1074/jbc.M110.207944
Boehm M, Yu J, Reisinger V, Bečková M, Eichacker LA, Schlodder E, Komenda J, Nixon PJ (2012) Subunit composition of CP43-less photosystem II complexes of Synechocystis sp. PCC 6803: implications for the assembly and repair of photosystem II. Philos Trans R Soc B 367:3444–3454. https://doi.org/10.1098/rstb.2012.0066
Botte M, Zaccai NR, Lycklama à J, Nijeholt JL, Martin R, Knoops K, Papai G, Zou J, Deniaud A, Karuppasamy M, Jiang Q, Roy AS, Schulten K, Schultz P, Rappsilber J, Zaccai G, Berger I, Collinson I, Schaffitzel C (2016) A central cavity within the holo-translocon suggests a mechanism for membrane protein insertion. Sci Rep 6:38399. https://doi.org/10.1038/srep38399
Chae PS, Rasmussen SGF, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK, Gellman SH (2012) A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chem Eur J 18:9485–9490. https://doi.org/10.1002/chem.201200069
Chidgey J, Linhartová M, Komenda J, Jackson PJ, Dickman MJ, Canniffe DP, Koník P, Pilný J, Hunter CN, Sobotka R (2014) A cyanobacterial chlorophyll synthase-HliD complex associates with the Ycf39 protein and the YidC/Alb3 insertase. Plant Cell 26:1267–1279. https://doi.org/10.1105/tpc.114.124495
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26(12):1367–1372. https://doi.org/10.1038/NBT.1511
Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 10:1794–1805. https://doi.org/10.1021/pr101065j
Dobáková M, Sobotka R, Tichý M, Komenda J (2009) Psb28 protein is involved in the biogenesis of the photosystem II inner antenna CP47 (PsbB) in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol 149:1076–1086. https://doi.org/10.1104/pp.108.130039
Dolganov NA, Bhayat D, Grossman AR (1995) Cyanobacterial protein with similarity to the chlorophyll a/b binding proteins of higher plants: Evolution and regulation. Proc Natl Acad Sci USA 92:636–640. https://doi.org/10.1073/pnas.92.2.636
Engelken J, Brinkmann H, Adamska I (2010) Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein superfamily. BMC Evol Biol 10:233. https://doi.org/10.1186/1471-2148-10-233
Gardel C, Johnson K, Jacq A, Beckwith J (1990) The secD locus of E. coli codes for two membrane proteins required for protein export. EMBO J 9:3209–3216. https://doi.org/10.1002/j.1460-2075.1990.tb07519.x
Havaux M, Guedeney G, He Q, Grossman AR (2003) Elimination of high-light-inducible polypeptides related to eukaryotic chlorophyll a/b-binding proteins results in aberrant photoacclimation in Synechocystis PCC6803. Biochim Biophys Acta Bioenerget 1557:21–33. https://doi.org/10.1016/S0005-2728(02)00391-2
He Q, Dolganov N, Björkman O, Grossman AR (2001) The high light-inducible polypeptides in Synechocystis PCC6803. Expression and function in high light. J Biol Chem 276:306–314. https://doi.org/10.1074/jbc.M008686200
Hey D, Grimm B (2018) ONE-HELIX PROTEIN2 (OHP2) is required for the stability of OHP1 and assembly factor HCF244 and is functionally linked to PSII biogenesis. Plant Physiol 177:1453–1472. https://doi.org/10.1104/pp.18.00540
Hey D, Grimm B (2020) ONE-HELIX PROTEIN1 and 2 form heterodimers to bind chlorophyll in photosystem II biogenesis. Plant Physiol 183:179–193. https://doi.org/10.1104/pp.19.01304
Hollingshead S, Kopečná J, Jackson PJ, Canniffe DP, Davison PA, Dickman MJ, Sobotka R, Hunter CN (2012) Conserved chloroplast open-reading frame ycf54 is required for activity of the magnesium protoporphyrin monomethylester oxidative cyclase in Synechocystis PCC 6803. J Biol Chem 287:27823–27833. https://doi.org/10.1074/jbc.M112.352526
Hontani Y, Kloz M, Polívka T, Shukla MK, Sobotka R, Kennis JTM (2018) Molecular origin of photoprotection in cyanobacteria probed by watermarked femtosecond stimulated Raman spectroscopy. J Phys Chem Lett 9:1788–1792. https://doi.org/10.1021/acs.jpclett.8b00663
Järvi S, Suorsa M, Aro EM (2015) Photosystem II repair in plant chloroplasts—regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim Biophys Acta (BBA) - Bioenerg 1847:900–909. https://doi.org/10.1016/J.BBABIO.2015.01.006
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauß N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411:909–917. https://doi.org/10.1038/35082000
Kameo S, Aso M, Furukawa R, Matsumae R, Yokono M, Fujita T, Tanaka A, Tanaka R, Takabayashi A (2021) Substitution of deoxycholate with the amphiphilic polymer amphipol A8–35 improves the stability of large protein complexes during native electrophoresis. Plant Cell Physiol 62:348–355. https://doi.org/10.1093/pcp/pcaa165
Knoppová J, Sobotka R, Tichý M, Yu J, Konik P, Halada P, Nixon PJ, Komenda J (2014) Discovery of a chlorophyll binding protein complex involved in the early steps of photosystem II assembly in Synechocystis. Plant Cell 26:1200–1212. https://doi.org/10.1105/tpc.114.123919
Knoppová J, Yu J, Konik P, Nixon PJ, Komenda J (2016) CyanoP is involved in the early steps of photosystem II assembly in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 57:1921–1931. https://doi.org/10.1093/pcp/pcw115
Komar J, Alvira S, Schulze RJ, Martin R, Nijeholt JL, Lee SC, Dafforn TR, Deckers-Hebestreit G, Berger I, Schaffitzel C, Collinson I (2016) Membrane protein insertion and assembly by the bacterial holo-translocon SecYEG–SecDF–YajC–YidC. Biochem J 473:3341–3354. https://doi.org/10.1042/BCJ20160545
Komenda J, Sobotka R (2016) Cyanobacterial high-light-inducible proteins - Protectors of chlorophyll-protein synthesis and assembly. Biochim Biophys Acta - Bioenerg 1857:288–295. https://doi.org/10.1016/j.bbabio.2015.08.011
Komenda J, Nickelsen J, Tichý M, Prášil O, Eichacker LA, Nixon PJ (2008) The cyanobacterial homologue of HCF136/YCF48 is a component of an early photosystem II assembly complex and is important for both the efficient assembly and repair of photosystem II in Synechocystis sp. PCC 6803. J Biol Chem 283:22390–22399. https://doi.org/10.1074/jbc.M801917200
Komenda J, Knoppová J, Kopečná J, Sobotka R, Halada P, Yu J, Nickelsen J, Boehm M, Nixon PJ (2012a) The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol 158:476–486. https://doi.org/10.1104/pp.111.184184
Komenda J, Sobotka R, Nixon PJ (2012b) Assembling and maintaining the photosystem II complex in chloroplasts and cyanobacteria. Curr Opin Plant Biol 15:245–251. https://doi.org/10.1016/j.pbi.2012.01.017
Komenda J, Krynická V, Zakar T (2019) Isolation of thylakoid membranes from the cyanobacterium Synechocystis sp. PCC 6803 and analysis of their photosynthetic pigment-protein complexes by clear native-PAGE. Bio-Protoc 9:3126. https://doi.org/10.21769/BIOPROTOC.3126
Kühlbrandt W, Wang DN, Fujiyoshi Y (1994) Atomic model of plant light-harvesting complex by electron crystallography. Nature 367:614–621. https://doi.org/10.1038/367614a0
Lee J, Lee HJ, Shin MK, Ryu WS (2004) Versatile PCR-mediated insertion or deletion mutagenesis. Biotechniques 36:398–400. https://doi.org/10.2144/04363BM04
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Liu Z, Yan H, Wang K, Kuang T, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292. https://doi.org/10.1038/nature02373
Li Y, Liu B, Zhang J, Kong F, Meng H, Li W, Rochaix JD, Li D, Peng L (2019) OHP1, OHP2, and HCF244 form a transient functional complex with the photosystem II reaction center. Plant Physiol 179:195–208. https://doi.org/10.1104/pp.18.01231
Llansola-Portoles MJ, Sobotka R, Kish E, Shukla MK, Pascal AA, Polívka T, Robert B (2017) Twisting a β-carotene, an adaptive trick from nature for dissipating energy during photoprotection. J Biol Chem 292:1396. https://doi.org/10.1074/JBC.M116.753723
Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R (2019) The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. https://doi.org/10.1093/nar/gkz268
Malavath T, Caspy I, Netzer-El SY, Klaiman D, Nelson N (2018) Structure and function of wild-type and subunit-depleted photosystem I in Synechocystis. Biochim Biophys Acta - Bioenerg 1859:645–654. https://doi.org/10.1016/j.bbabio.2018.02.002
Mazor Y, Borovikova A, Caspy I, Nelson N (2017) Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat Plants 3:17014. https://doi.org/10.1038/nplants.2017.14
Myouga F, Takahashi K, Tanaka R, Nagata N, Kiss AS, Funk C, Nomura Y, Nakagami H, Jansson S, Shinozaki K (2018) Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol 176:2277–2291. https://doi.org/10.1104/pp.17.01782
Niedzwiedzki DM, Tronina T, Liu H, Staleva H, Komenda J, Sobotka R, Blankenship RE, Polívka T (2016) Carotenoid-induced non-photochemical quenching in the cyanobacterial chlorophyll synthase-HliC/D complex. Biochim Biophys Acta - Bioenerg 1857:1430–1439. https://doi.org/10.1016/j.bbabio.2016.04.280
Pascual-Aznar G, Konert G, Bečková M, Kotabová E, Gardian Z, Knoppová J, Bučinská L, Kaňa T, Sobotka R, Komenda J (2021) Psb35 protein stabilizes the CP47 assembly module and associated high-light inducible proteins during the biogenesis of photosystem ii in the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol 62:178–190. https://doi.org/10.1093/pcp/pcaa148
Pazderník M, Mareš J, Pilný J, Sobotka R (2019) The antenna-like domain of the cyanobacterial ferrochelatase can bind chlorophyll and carotenoids in an energy-dissipative configuration. J Biol Chem 294:11131–11143. https://doi.org/10.1074/jbc.ra119.008434
Pogliano JA, Beckwith J (1994) SecD and SecF facilitate protein export in Escherichia coli. EMBO J 13:554–561. https://doi.org/10.1002/j.1460-2075.1994.tb06293.x
Proctor MS, Pazderník M, Jackson PJ, Pilný J, Martin EC, Dickman MJ, Canniffe DP, Johnson MP, Hunter CN, Sobotka R, Hitckcock A (2020) Xanthophyll carotenoids stabilise the association of cyanobacterial chlorophyll synthase with the LHC-like protein HliD. Biochem J 477:4021–4036. https://doi.org/10.1042/BCJ20200561
Promnares K, Komenda J, Bumba L, Nebesarova J, Vácha F, Tichý M (2006) Cyanobacterial small chlorophyll-binding protein ScpD (HliB) is located on the periphery of photosystem II in the vicinity of PsbH and CP47 subunits. J Biol Chem 281:32705–32713. https://doi.org/10.1074/jbc.M606360200
Rahimzadeh Karvansara P, Pascual Aznar G, Bečková M, Komenda J (2022) The Psb34 protein modulates binding of high-light-inducible proteins to CP47 containing photosystem II assembly intermediates in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res, accepted
Rappsilber J, Mann M, Ishihama Y (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2(8):1896–1906. https://doi.org/10.1038/NPROT.2007.261
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:e47. https://doi.org/10.1093/nar/gkv007
Rudolph JD, Cox J (2019) A network module for the Perseus software for computational proteomics facilitates proteome interaction graph analysis. J Proteome Res 18:2052–2064. https://doi.org/10.1021/acs.jproteome.8b00927
Shukla MK, Llansola-Portoles MJ, Tichý M, Pascal AA, Robert B, Sobotka R (2018) Binding of pigments to the cyanobacterial high-light-inducible protein HliC. Photosynth Res 137:29–39. https://doi.org/10.1007/s11120-017-0475-7
Skotnicová P, Staleva H, Kuznetsova V, Bína D, Konert M, Lu S, Polivka T, Sobotka R (2021) Plant LHC-like proteins show robust folding and static non-photochemical quenching. Nat Commun 12:6890. https://doi.org/10.1038/s41467-021-27155-1
Staleva H, Komenda J, Shukla MK, Šlouf V, Kaňa R, Polívka T, Sobotka R (2015) Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat Chem Biol 11:287–291. https://doi.org/10.1038/nchembio.1755
Tichý M, Bečková M, Kopečná J, Noda J, Sobotka R, Komenda J (2016) Strain of Synechocystis PCC 6803 with aberrant assembly of photosystem II contains tandem duplication of a large chromosomal region. Front Plant Sci 7:648. https://doi.org/10.3389/FPLS.2016.00648
Tribet C, Audebert R, Popot J-L (1996) Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci 93:15047–15050. https://doi.org/10.1073/pnas.93.26.15047
Tyanova S, Temu T, Cox J (2016a) The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11:2301–2319. https://doi.org/10.1038/NPROT.2016.136
Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J (2016b) The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 13:731–740. https://doi.org/10.1038/nmeth.3901
Umena Y, Kawakami K, Shen JR, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9Å. Nature 473:55–60. https://doi.org/10.1038/nature09913
Vass I (2012) Molecular mechanisms of photodamage in the photosystem II complex. Biochim Biophys Acta (BBA) - Bioenerg 1817:209–217. https://doi.org/10.1016/J.BBABIO.2011.04.014
Wei X, Su X, Cao P, Liu X, Chang W, Li M, Zhang X, Liu Z (2016) Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 534:69–74. https://doi.org/10.1038/nature18020
Xiao Y, Huang G, You X, Wang W, Kuang T, Han G, Sui SF, Shen JR (2021) Structural insights into cyanobacterial photosystem II intermediates associated with Psb28 and Tsl0063. Nat Plants 7:1132–1142. https://doi.org/10.1038/s41477-021-00961-7
Xu H, Vavilin D, Funk C, Vermaas WFJ (2002) Small Cab-like proteins regulating tetrapyrrole biosynthesis in the cyanobacterium Synechocystis sp. PCC 6803 Plant Mol Biol 49:149–160. https://doi.org/10.1023/A:1014900806905
Yao D, Kieselbach T, Komenda J, Promnares K, Hernández Prieto MA, Tichy M, Vermaas W, Funk C (2007) Localization of the small CAB-like proteins in photosystem II. J Biol Chem 282:267–276. https://doi.org/10.1074/jbc.M605463200
Zabret J, Bohn S, Schuller S, Arnolds O, Möller M, Meier-Credo J, Liauw P, Chan A, Tajkhorshid E, Langer J, Stoll R, Krieger-Liszkay A, Engel B, Rudack T, Schuller J, Nowaczyk M (2021) Structural insights into photosystem II assembly. Nat Plants 7:524–538. https://doi.org/10.1038/s41477-021-00895-0
Acknowledgements
European Research Council Synergy Award 854126 and the Czech Science Foundation, grant No. 19-29225X funded this work. RS also acknowledges institutional support RVO 61388971. PK acknowledges support from the Czech Ministry of Education, Youth and Sports project ‘Mechanisms and dynamics of macromolecular complexes: from single molecules to cells’, CZ.02.1.01/0.0/0.0/15_003/0000441. Jan Pilný is thanked for HPLC analysis of pigments.
Author information
Authors and Affiliations
Contributions
MMK and RS designed the study; AW, MMK, and PK performed the experiments; MMK, PK, and RS analyzed the data; MMK and RS wrote the paper. All authors read and accepted the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konert, M.M., Wysocka, A., Koník, P. et al. High-light-inducible proteins HliA and HliB: pigment binding and protein–protein interactions. Photosynth Res 152, 317–332 (2022). https://doi.org/10.1007/s11120-022-00904-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00904-z