Abstract
The population dynamics of shrews (Soricidae) are not well known even though they form an important part of forest ecosystems and represent suitable bioindicators of ecosystem quality. The aim of this study was to evaluate the population dynamics of shrews in mountain and upland forest clearings in four study areas within the Czech Republic and to reveal how climatic factors influenced fluctuations in their abundance for a decade (2007–2017). In total, we trapped 7,538 individuals of 18 small mammal species. From 760 individuals of seven shrew species, the common shrew (Sorex araneus) was significantly dominated in all study areas. We did not observe any significant, regular multi-annual cycles of the common shrew. However, a cross-correlation in density fluctuation of this species was detected in all mountain areas indicating the influence of environmental factors acting on a larger geographical scale. The autumn abundance of shrews was dependent on the subset of climatic variables, together explaining 56% of the variance in the linear regression model. Except for the length of the snow cover of ≥ 5 cm, all other significant variables were associated with North Atlantic Oscillation (NAO). Longer duration of snow cover during the winter before trapping, higher average NAO value during months before trapping, and NAO value in September influenced negatively the autumn abundance of shrews, contrary, higher value of NAO in May and October increased the abundance. Our results demonstrate the sensitivity of shrews to winters with a longer period of snow cover and to climatic oscillations associated with the NAO, whose effect is monthly dependent and probably indirectly influencing shrews through their prey.
Similar content being viewed by others
Introduction
The climate change is a global phenomenon with major impacts on ecosystems and biodiversity. It is the main driver of changes in the phenology, and it affects geographical distribution, phenotypic traits, and population dynamics of both plant and animal species (Walther et al. 2002; Parmesan 2006; Feehan et al. 2009; Chen et al. 2011; Gardner et al. 2011; Hu et al. 2022; Taylor et al. 2022). Predictions of the climate development in the Central European region are not uniform. We do not know whether the influence of the greenhouse effect will prevail and the warming will intensify (Jacob et al. 2014) or whether the Gulf Stream will weaken and cool down (Boers 2021; Caesar et al. 2021); however, there is an agreement on the expectation of more frequent occurrence of extreme events (torrential rains, long dry periods, and extreme temperatures) (Coppola et al. 2021).
The course of the main climatic factors (i.e., precipitation, wind, and temperature) at the continental level is often closely related to changes in the air pressure difference between some places; this phenomenon allows us to use a single value to express the variability of the main climatic factors with relatively high accuracy. An important system affecting Europe and eastern North America is the North Atlantic Oscillation (NAO). The NAO value is derived from the difference of atmospheric air pressure between the Azores and Iceland (Hurrell 1995). The impact of the NAO phase on climatic conditions is very complicated, resulted from the complexity of the phenomenon itself (Hurrell 2011). Its fluctuations induce considerable variations in the mean direction and speed of the wind patterns over the North Atlantic (Hurrell et al. 2003), the heat and moisture transport to the North America and Europe (Christoudias et al. 2012), and the intensity and number of storms (Trigo 2006). Manifestations of the NAO vary significantly regionally in Europe (Ottersen et al. 2001; Cleary et al. 2017; Rousi et al. 2020). In addition, the influence of the NAO on the climate of Central Europe has been studied mainly seasonally (during winter or summer, e.g., Bojariu and Giorgi 2005), but the effect can vary even in individual months (López-Moreno and Vicente-Serrano 2008).
Small terrestrial mammals (Rodentia, Eulipotyphla) are widespread, abundant, and important in the food webs of forest ecosystems. Due to their ubiquity, small size, short life, and relatively high and variable abundance, they respond relatively quickly to environmental changes and habitat disturbance (Bertolino et al. 2015). Therefore, they are considered as bioindicators of sustainable forest management (Carey and Harrington 2001; Pearce and Venier 2005). However, with long-term monitoring, they have a potential to indicate changes associated with climate (Hope et al. 2017).
Shrews represent one of the smallest mammals in the world with unique physiological and ecological adaptations. Due to their characteristic traits (small predators of invertebrates with a high metabolic rate and a need for a high-energy diet, which do not hibernate and are active both during the day and night), they are sensitive to changes in environmental conditions (Brown et al. 1997; Taylor 1998; Churchfield 2002; Ochocińska and Taylor 2005; Schaeffer et al. 2020). Shrews also have relatively small home ranges and do not migrate over long distances, so they are highly dependent on their environment, making them ideal model organisms for studying species-environment interactions, including those related to climate change (Hope et al. 2016; Hu et al. 2022; Taylor et al. 2022).
The population dynamics of shrews (Soricidae) is variable and not thoroughly explored, especially for less abundant species. Population density time series are often too short (Churchfield et al. 1995; Berryman 2002) and those lasting at least 10 years are rare. However, such studies are essential for describing temporal variability and assessing the influence of environmental factors affecting their abundance (Marcström et al. 1990; Matlack et al. 2002). Although some species such as the common shrew (Sorex araneus) are relatively widespread, studying their population dynamics can be challenging since their abundance is consistently lower than that of other small mammals (especially rodents).
Both cyclic (Sheftel 1989; Tast et al. 2005; Zub et al. 2012; Bobretsov et al. 2018; Erdakov et al. 2019) and non-cyclic (Pankakoski 1985; Henttonen et al. 1989; Churchfield et al. 1995; Popov 2005; Tomášková et al. 2005; Zakharov et al. 2020) populations of shrews have been recognized. Intrinsic (density-dependent) and extrinsic (density-independent) factors may play an important role in the formation of this temporal population variation (Stenseth et al. 2002). Shrews are very sensitive to severe environmental conditions (especially during winter) due to their fast metabolism, small body size, and limited ability to store more energy in the form of fat tissue (Churchfield 1981; Brown et al. 1997; Schaeffer et al. 2020). During the winter, they can consume more than 50% of their body fat in just 2.5 h (Lázaro and Dechmann 2021). Cold temperature increases their metabolic costs, but their food is often less available during winter (Churchfield 2002). The postnatal seasonal and reversible reduction in the body size (Dehnel’s phenomenon) of soricine shrews including the braincase were detected in northern temperate winters and hypothesized to be an energy-saving overwintering strategy, an alternative to hibernation or migration (Lázaro et al. 2017; Schaeffer et al. 2020). The negative impact of climatic conditions on different shrews’ populations during winter was frequently identified as direct (Pankakoski 1985; Churchfield 2002; Zakharov et al. 2020) or indirect through food availability (Churchfield 1982; Kaikusalo and Hanski 1985; Pankakoski 1985; Henttonen et al. 1989; Jędrzejewska et al. 1998). In addition, population variations may be strongly influenced by intraspecific (Moraleva 1989; Sheftel 1989) and interspecific competition (Huitu et al. 2004; Zub et al. 2012), predation (Henttonen 1985; Korpimäki 1986; Henttonen et al. 1989), variation in reproduction intensity (Kaikusalo and Hanski 1985; Zakharov et al. 1991), forest management (Bogdziewicz and Zwolak 2014), local succession of vegetation (Mažeikyte 2009), or habitat structure (Zárybnická et al. 2017; Bobretsov et al. 2018).
Forest clearings are important habitats for small mammals as they provide favorable conditions for reproduction, hibernation, and dispersal (Anikanova et al. 2009). For common shrews, young and overgrowing clearings and young forests may be the preferred habitat types (Guseva and Korosov 2015). Many studies have showed that this species often increases in abundance after clear-cutting (Mažeikyte 2009; Bogdziewicz and Zwolak 2014; Ivanter and Kurhinen 2015). Due to the high abundance and diversity of small mammal communities (including shrews), small-sized forest clearings can be important for maintaining forest biodiversity in managed Central European forests (Krojerová-Prokešová et al. 2016). Even though small mammals can colonize quickly and exploit new resources in secondary forests, new forest plantations should not be regarded as a sufficient replacement for higher quality habitats such as ancient forests (Fuentes-Montemayor et al. 2020). Old-growth forests are ecologically unique in comparison to managed forests, many of which are species-impoverished (Saitoh and Nakatsu 1997; Carey 2003). However, the structure and diversity of small mammal communities in managed forests do not differ from the natural forests if the scale of individual stands and clearings is relatively small (clearings < 2 ha; Bryja et al. 2002; Suchomel et al. 2014).
Based on our previous studies that examined the effect of elevation and age of forest stands on the abundance and species composition of shrews (Dokulilová and Suchomel 2016, 2017), we concluded that small forest clearings (especially in higher elevations) are suitable habitats for common shrews as well as important refuges for other shrew species. In this study, we analyzed data on autumn abundances of small mammals from small forest clearings in upland and mountain areas to ascertain: (1) regularity and fluctuations of the population density of common shrew, (2) interspecific synchrony in the population dynamics of common shrew and other abundant small mammal species (Apodemus spp., bank vole Clethrionomys glareolus, and Microtus spp.), and (3) influence of selected climatic factors at the local (air temperature, precipitation, and snow cover) and continental scale (indices of the North Atlantic Oscillation NAO) on the population dynamics of common shrew during one decade of monitoring. This study represents the most comprehensive study of the population dynamics of common shrew in managed Central European forests.
Materials and methods
Study areas
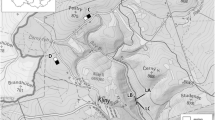
The small mammal communities (rodents and eulipotyphlans) were monitored in four areas (Fig. 1): Beskydy Mountains (north-eastern Moravia; 49° 30′ N, 18° 20′ E), Drahanská Uplands (central Moravia; 49°16′ N, 16° 50′ E), Jeseníky Mountains (northern Moravia; 50° 10′ N, 17° 16′ E), and Ore Mountains (north-western Bohemia; 50° 39′ N, 13° 35′ E). These areas differ mainly in terms of climate (temperature and precipitation) and the natural species composition of the forest. About 80% of the forests in the Beskydy Mts. were composed of European beech (Fagus sylvatica) and white fir (Abies alba). Beech, fir, and oaks (Quercus spp.) had the same proportion even in the warmest Drahanská Uplands. In the Jeseníky and the Ore Mts., almost 90% of the forests consisted of Norway spruce (Picea abies), beech, and white fir. As a result of centuries of intensive forest management, the composition of the forests in all four areas is very similar today: the proportion of spruce has increased to 50–80%, while the percentage of broadleaved trees has decreased to approx. 20% (ÚHÚL 2023). It should also be noted that the Ore Mts. were severely damaged by industrial emissions at the end of the twentieth century.
The small mammal communities were monitored at small forest clearings (≤ 2 ha) for 8 (Ore Mts.) or 11 years (Beskydy Mts., Jeseníky Mts., and Drahanská Upl.) from 2007 to 2017. Basic characteristics of the study areas are given in Table 1.
Monitored clearings
Clear-cuts with a well-developed herb layer were selected randomly in each study area (Table 2). The total number slightly changed during monitoring due to the age of the stand (for research purposes, it was necessary to limit the monitoring of clearings to 10 years after their establishment so older clearings were later replaced with newer young ones). In the Ore Mts., monitoring was interrupted in 2009 due to early snowfall; the total length of data collection in this locality was shortened due to limited financial resources. A total of 190 monitoring plots, differing in habitat structure and age, were monitored during the study.
All monitored plots were small, artificially reforested clearings (up to 2 hectares), which represented the early successional forest stages from three to ten years old. These stages have the least-developed canopy and most-developed herbaceous layer (average 94%). Saplings of beech (Fagus sylvatica, 72.5%), spruce (Picea spp., 14.8%), or oak (Quercus spp., 12.7%) predominated. There was only marginal occurrence of saplings from other tree species, e.g., fir (Abies alba), pine (Pinus sylvestris), maple (Acer spp.), and linden (Tilia spp.).
Trapping of small mammals
Trapping of small mammals was carried out annually during autumn (September–November) in order from higher to lower elevations to allow data comparison between study areas. The trapping techniques have been described in detail and justified by Krojerová-Prokešová et al. (2016, 2018). The chosen capture method was not originally intended to target shrews but instead aimed at pest rodent species that cause damage to forest plantations. Shrews incidentally fell into traps as non-target species. We are aware that the trapping method of snap-traps may not provide reliable results for some species of shrews, especially those of smaller size (Henttonen et al. 1989; Anděra 2010; Čepelka et al. 2019). However, it can be useful in assessing population dynamics and the distribution of these species (Popov 2005). Additionally, there are studies focused on small mammal communities using snap-traps which also presented significant results regarding shrews (Bryja and Zukal 2000; Suchomel et al. 2014; Krojerová-Prokešová et al. 2016). Moreover, the same methodology was used in all study areas. The population density of Soricidae and the common shrew strongly positively correlated (Spearman R = 0.954, p < 0.001), thus only data about the most common species of Soricidae, the common shrew, were used for evaluation. The relatively large number of trapped common shrews (678 individuals) enabled the evaluation of population dynamics of this species.
The research for this study was conducted in managed forest plantations, where small mammals that endanger commercially important seedlings are labeled as pest species and can be legally poisoned. While poisoning was not allowed at the study sites during the research period, snap–trapping was permitted by Ethics Commission of the Academy of Sciences of the CR (No. 150/2006). All aspects of trapping complied with EU Council Directive 86/609/EEC on the experimental use of animals.
The cumulative trapping effort was 73,848 trap-nights across 190 clearings. Trapped individuals were identified by species, sexed, weighed, measured (body length, tail length, and hind foot length), and assessed for reproductive condition. The animals were also used for other research that required necropsy including analysis of the reproduction stage of the population and monitoring the presence of Hantavirus and other pathogens in the small mammal community. Publications described two new species of Hantavirus in the family Soricidae (Schlegel et al. 2012; Radosa et al. 2013).
Climate variables
All local climate variables (air temperature, precipitation, snow cover height, and duration) were taken from data recorded by the Czech Hydrometeorological Institute (www.chmi.cz) based on fifth-day interval measures (pentads) collected at the meteorological stations in the surrounding of the study areas, in relation to the elevations of monitored clearings. The monthly values of North Atlantic Oscillation (NAO) based on the surface sea-level pressure difference between the Subtropical (Azores) High and the Subpolar Low data were used also as climatic variables. The values were downloaded from the National Centers for Environmental Information National Oceanic and Atmospheric Administration (NCEI NOAA; available from https://www.ncdc.noaa.gov/teleconnections/nao/). A list of all climatic variables is provided in Table S1 (Online Resource 1).
Data processing
The dominance (D) of the shrew species was calculated according to the formula \(D=ni/N\times 100 \left(\%\right)\), where “ni” is the number of individuals of the given species and “N” is the sum of individuals of all species captured during all monitored years at all clearings within a specific area (Tischler 1949). The relative abundance (rA) of small mammals (rodents and shrews) was calculated per 100 trap-nights according to the formula \(rA=100\times n/P\), where “n” is the number of captured animals and “P” is number of trap-nights. This approach was used to make rA values comparable between study areas/clearings with different trapping effort.
The relative abundance of common shrew (the most abundant eulipotyphlans) was compared to dominant rodent species, which were pooled into three ecological groups according to their feeding strategy: Apodemus spp. (granivorous Apodemus agrarius, A. flavicollis, and A. sylvaticus), bank vole (omnivorous Clethrionomys glareolus), and Microtus spp. (herbivorous Microtus arvalis and M. agrestis). The average relative abundance values detected for these groups for each study area annually in autumn (hereafter autumn abundance) were log-transformed according to the formula log10(rA + 1) and checked for the assumption of data normality by Kolmogorov–Smirnov (K-S) test and Shapiro–Wilk test. The autumn abundance values of all groups fulfilled the assumption (see Appendix 1 in Online Resource 1).
For the time series analysis, we calculated the autumn abundance at annual intervals of all groups (Sorex araneus, Apodemus spp., Microtus spp., and bank vole) for particular areas. Separate series were calculated for all areas for each group and for common shrew within each area. The aim was to characterize the degree of synchrony in abundance fluctuation between small mammals’ groups within study area and for common shrew among study areas using cross-correlation analyses (Berryman and Turchin 2001).
The influence of selected climatic variables as predictors (explanatory variables) on the log-transformed autumn abundance of common shrew (response = dependent variable) was tested using stepwise linear regression. To reduce the influence of multicollinearity of variables, we used the Pearson correlation coefficient (r) to screen for correlated climatic variables (see Online Resource 2). We retained only variables with r < 0.7 (Bosso et al. 2018; Hu et al. 2022). All statistical analyses were done in IBM SPSS Statistics 26.0.
Results
Diversity of small mammals in the study areas
A total of 7,538 individuals of 18 small mammal species were trapped during the years 2007–2017 (Table 3). A total of 760 individuals belonged to seven species of shrews (10.08% of the total number of small mammals captured). The highest number of shrew species was recorded in the Drahanská Upl. (Table 4). Total abundance was also the highest there (rA = 1.519) compared to the Beskydy Mts. (rA = 0.704), the Jeseníky Mts. (rA = 0.870), and the Ore Mts. (rA = 0.974) (Table 4). A total of 41% of all 760 shrews were captured in the Drahanská Upl.
The common shrew was the most abundant shrew species (rA = 0.918; D = 90.17%; Table 4). It significantly dominated over other shrews in all studied areas and occurred in 40.6% of all monitored clearings. It was followed by the Eurasian pygmy shrew, Sorex minutus (rA = 0.083; D = 7.50%; Table 4), which also occurred in all four studied areas but with significantly lower abundance and regularity. Other species of shrews were found only rarely.
Population dynamics of the common shrew; comparison with dominant rodent species
The autumn abundance of common shrew among study areas showed strong positive cross-correlation at a zero time-lag (Fig. 2) for all three mountain areas with cross-correlation coefficients (CCFs) varied from 0.641 to 0.834 among pairs of study areas (Table S2 in Online Resource 1). This means that autumn abundance of common shrew fluctuates with the same pattern among all mountain areas even the geographic distance of the Ore Mts. is approximately 350 km far away from the two Moravian mountain ranges: Beskydy and Jeseníky Mts. This pattern is also evident when the log-transformed autumn abundance values are graphically displayed, although the range of values differ among study areas (Fig. 3). The pattern detected for Drahanská Upl. situated in lower elevation was slightly different, especially due to high autumn abundance detected in 2010 (n = 12; rA = 0.62 in 2009 compared to n = 72; rA = 3.72 in 2010) and during the next year but for the part of the period, first three (2007–2009), and last four years (2014–2017), also copied the pattern detected for mountain areas (Fig. 3). The CCF value for the pair with the closest Beskydy Mts. was only slightly lower (CCF = 0.572, Table S2 in Online Resource 1). Besides this synchronization, the results did not reveal any significant regular multi-annual cyclicity of the common shrew in study areas.
Further, time series cross-correlations between common shrew and other small mammals were also the highest at a zero time-lag, with the highest cross-correlation coefficient (CCF) of 0.450 ± SE 0.150 detected for the pair with Microtus spp. (Table S3 in Online Resource 1). Detected results indicate that there is a similarity in autumn abundance fluctuation among different species with different feeding strategies, even though the pattern is not clear (Fig. 4). The similarity is the most evident in Ore Mts. (Fig. 4d).
The influence of climatic factors on the abundance of the common shrew
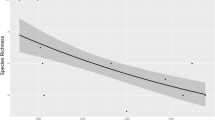
Year-to-year fluctuations in common shrew autumn abundance between different study areas showed a similar pattern as mentioned above (Figs. 2 and 3). Therefore, we evaluated the influence of various climatic factors on the abundance of the common shrew. Stepwise linear regression identified as significant five of a total 19 predictors included in the analysis. All five variables explained together 56% of the variance in the autumn abundance of the common shrew as dependent variable (R2 = 0.560; Table 5). The significance of predictors is given in Appendix 2 (Online Resource 1). Partial regression showed that longer duration of the snow cover of ≥ 5 cm during the winter before trapping influenced negatively the autumn abundance of shrews (Fig. 5a). Further, the higher values of NAO in May and October increased the abundance of common shrew detected in autumn (Fig. 5c and e); on the other hand, higher values of NAO in September and average value of NAO calculated for the month of the year before trapping since December decreased the number of common shrews detected in autumn (Fig. 5b and d).
The partial regression plots between log-transformed autumn abundance of common shrew and climatic factors identify as significant in the stepwise linear regression: a the duration of snow cover ≥ 5 cm in days, b the value of NAO in September, c the value of NAO in May, d average value of NAO in the year of trapping from December to September/October in dependence to study area, and e NAO value in October
Discussion
The common shrew was the most abundant species from the Soricidae family, which confirms earlier findings (Anděra 2000, 2010). Other species of shrews were recorded much rarely. Although the snap-trapping underestimates lighter individuals (Čepelka et al. 2019), the usual weights of the eulipotyphlans detected are comparable with rodents except for the Eurasian pygmy shrew. Therefore, we assume that only the population density of this species could have been underestimated due to the methodology used. We found certain differences in the structure of the small mammal community between study areas. Both the widest species spectrum (15 species; see Table 3) and the highest relative abundance of ubiquitous species of small mammals were found in the Drahanská Upl. The same was true for shrews (Table 4). We assume the observed differences were caused by different climate conditions. The Drahanská Upl. lie the most southerly and has the lowest elevation; it is therefore the warmest and the driest of the monitored areas and with the mildest winters (Table 1). These conditions result in a longer growing season, faster development, more diverse communities of plants and animals, and generally higher productivity. Even most shrew species probably found more suitable and stable conditions there. This was reflected in the different relative abundance and asynchronous fluctuations of populations compared to mountain areas. Bicolored white-toothed shrew (Crocidura leucodon) and lesser shrew (C. suaveolens) (Anděra 2000, 2010; Anděra and Gaisler 2012) were more abundant in the Drahanská Upl. Conversely, the typically mountain species alpine shrew (Sorex alpinus) (Anděra 2000, 2010; Anděra and Gaisler 2012) was found only in the Beskydy Mts. Eurasian water shrew (Neomys fodiens) and Miller’s water shrew (N. milleri) are mostly found in mid-elevation areas and near watercourses (Anděra 2000, 2010; Anděra and Gaisler 2012). Their rare occurrence in forest clearings was therefore expectable.
The studied small forest clearings appear to be most suitable for the common shrew and Eurasian pygmy shrew. For shrews, the selection of habitats is mainly determined by food supply, which also affects abundance of both species (Lukyanova et al. 2021). In addition, their presence and abundance may also be influenced by competition and predation (Huitu et al. 2004) related to the nature and density of the vegetation, which affects both food availability and shelter options (Jędrzejewska and Jędrzejewski 1990; Longland and Price 1991).
Common shrew was significantly more abundant than Eurasian pygmy shrew in all areas. Although competitive exclusion can limit the number of species and individuals (Väli and Tõnisalu 2021), it is known that some shrew species can coexist in one habitat despite their great similarity in morphology and ecology (Churchfield and Rychlik 2006). Differences in body size, which are associated with differences in food composition and acquisition, contribute to their ability to coexist in one habitat (Churchfield et al. 1999; Brannon 2000; Churchfield 2002), with Eurasian pygmy shrew clearly preferring the epigeic and common shrew the hypogeic invertebrates (Anděra 2000).
Population dynamics of the common shrew
In this study, the cyclicity in population dynamics was only evaluated for the common shrew as the most abundant species of Soricidae. We detected a partial synchronization in density fluctuations between all mountain areas (Fig. 3; see below) However, our analyses did not reveal any significant regular multi-annual population cycles of the common shrew. Similar results have been observed in Western and Central Europe (Churchfield et al. 1995; Tomášková et al. 2005), and regular cyclicity was not observed even in central European populations of rodents (Čepelka et al. 2020). Population cycle has been commonly observed in northern temperate regions, especially in Fennoscandia, with the stable climate, in which overpopulation and related intra and interspecific competition has been assumed as a limiting factor for further population growth (Henttonen et al. 1977; Myllymäki 1977; Viitala 1977, 1984; Hansson and Henttonen 1985; Zub et al. 2012). Further, it has been mentioned that this cyclicity decreases southwards (Hansson and Henttonen 1985). Moreover, collapses of cyclic dynamics were registered in many regions recently and the relation to mild winters and unstable snow cover due to climate change has been discussed (Ims et al. 2008; Cornulier et al. 2013; Korpela 2014; Zakharov et al. 2020). In Central Europe, except for the likely influence of the climate change, the shrew populations are influenced by more continental climate with distinct seasonality (Tkadlec and Zejda 1998; Tomášková et al. 2005). Population cycles are less common here (Pupila and Bergmanis 2006), although rarely they have also been recorded (Zub et al. 2012).
The synchronicity of the common shrew population abundance with those of abundant rodents
The population densities of syntopic rodents and shrews do not fluctuate synchronously in the Czech forests (Tomášková et al. 2005; Čepelka et al. 2017). We detected weak synchronicity between all ecological groups of small mammals, especially of common shrew with herbivores (i.e., with the genus Microtus). The strongest synchronicity was detected in the Ore Mts., which may be influenced by higher predation pressure of boreal owl (Aegolius funereus), especially in young plantations on this mountain plateau (Zárybnická et al. 2015, 2017). Similar environmental conditions (large-scale spruce monocultures, bark beetle clear-cuts, and clearings with plantations) have also arisen in the Jeseníky and the Beskydy Mts. in recent years. Unfortunately, data on the rate of predation are missing there up to date. Interspecies synchronism in the population dynamics of voles and shrews associated with predation has been most frequently found in northern Europe (Hansson 1984; Henttonen 1985; Korpimäki 1986; Korpimäki and Norrdahl 1989; Korpimäki et al. 2005). The observed synchronicities may be related to the predominance of mountainous areas in our study; the climatic conditions of the Central European mountains are very similar to the temperate regions of Fennoscandia.
The influence of climatic factors on the abundance of common shrew
Climatic conditions are some of the extrinsic factors that significantly affect the population dynamics of small mammals (Chen et al. 2015; Hlôška et al. 2016). In seasonal environments, winter climatic conditions can drive the population dynamics of small mammals, especially rodents, beyond the winter season (Formozov 1946, 1961; Hlôška et al. 2016; Scott et al. 2022). Especially, the abundance of subnivium-adapted rodents increased after long lasting and snow rich winters with stable winter conditions (Hlôška et al. 2016; Scott et al. 2022). However, the shrews as an insectivorous species may react differently.
The shrews’ high sensitivity to extremes can be explained by their small size, high metabolic rate (Taylor 1998), year-round activity, and lack of fat reserves (Churchfield 1990). According to Ivanter (2020), the most important climatic factors that cause changes in the abundance of the common shrew are the height of the snow cover, the depth of soil freezing in winter, temperatures in spring, and precipitation in the winter-spring period. Previous studies in Central European temperate forests detected a connection between climatic factors and abundance of the common shrew (Zub et al. 2012; Hlôška et al. 2016; Zárybnická et al. 2017). Especially, a higher abundance of the common shrew was recorded after winters with low snow cover in spruce plantations in the Czech Ore Mts. (Zárybnická et al. 2017). In our study, the number of shrews the following autumn was negatively affected by the snow cover duration. Since several freeze–thaw cycles were present during each winter in all study areas and our results did not confirm the effect of snow cover height or lower winter temperatures, the length of snow cover cannot be simply associated with stronger winter conditions. Nevertheless, shrews reduce most activities (e.g., breeding and movement) in favor of foraging to meet higher energy and nutritional needs due to increased fat turnover rates in winter (Schaeffer et al. 2020). The longer duration of the snow cover can further increase the energy demands of the winter period, delays the start of the growing season and the development of invertebrates (Churchfield et al. 2012; Ivanter 2020), and thus probably negatively affects the survival of shrews till spring. As a result, fewer individuals are involved in breeding, which may ultimately affect the number of shrews caught in the autumn.
The regression model explained together 56% of the variance in the autumn abundance of common shrew as dependent variable. Except for mentioned length of the snow period, all other significant variables were associated with NAO index. More important than winter NAO were values of this index in May, September, and in October and the average NAO value during months before the trapping since December of the previous year. This indicate that the shrew’s abundance is influenced also by climatic conditions during vegetation season. However, winter may not be the only energy-demanding season for shrews. Both sexes face extremely high energetic demands especially during the breeding season (usually April–September; Churchfield 1990). The food intake of lactating females is up to 2.5 times higher than their own winter intake (Taylor 1998). It was also detected that spring adults spent more time at rest than winter individuals to save energy (Schaeffer et al. 2020).
Most studies describing the influence of the NAO in the Czech Republic indicate a much closer connection of the phenomenon with air temperature than with precipitation (Doležalová 2007). However, according to other opinions, the NAO also significantly affects precipitation in a large area of Central Europe, including the Czech Republic (López-Moreno and Vicente-Serrano 2008). Higher NAO values in the winter, summer, and autumn months are mainly associated with above-average temperatures and below-average precipitation in Central Europe; in spring, with above-average temperatures and precipitation (Pokorná et al. 2007; López-Moreno and Vicente-Serrano 2008; Chronis et al. 2011).
We detected the influence of positive phase of NAO in May on the increasing autumn shrew abundance. A warmer and wetter May increases plant production (Ivanter 2020) and increases the metabolic rate and reproductive success of invertebrates (Frazier et al. 2006). Shrews therefore may have their main food sources (like, e.g., arthropods or mollusks) available earlier and in greater quantities (Churchfield 1982) during an important mating season.
Further, the positive NAO values in September and the positive average NAO value during all months before trapping since beginning of winter were also important in the regression model and, contrary, negatively affected the density of the common shrew in autumn. A drier and warmer year can mean lower water availability. This can cause a lower production of plant biomass and a lower abundance of those species that require more moisture, e.g., mollusks (Churchfield 1982; Martin and Sommer 2004). Lower numbers of shrews can be a consequence of the limiting amount of water and especially the lower amount of some prey species (Matlack et al. 2002; Ivanter 2020).
Contrary, the opposite effect of positive phase of NAO in October probably had only the short-term effect. Since the trapping was realized mostly during October, the effect is probably caused by higher shrew activity associated with warmer and drier weather. However, a detailed understanding of the influence of the NAO on climatic conditions, vegetation, shrew populations, and their food needs further research.
Conclusion
Our study confirmed that the common shrew was the most common soricine species at the forest clearings in all monitored areas. Although no significant regular multi-annual cycles in shrew’s abundance were recorded, the study revealed that interactions among selected climatic variables influenced the observed population fluctuation of this species. This approach did not allow determining the main driver of this fluctuation, but the subset of climatic variables explained 56% of the variance in the autumn abundance of the common shrew. The time-series cross-correlation among mountain areas, even far away to each other, highlighted the influence of climatic conditions acting on a larger geographical scale. The effect of positive phase of NAO, especially during winter, on rodent survival is known in Central Europe (e.g. Šipoš et al. 2017), but our results revealed that insectivorous shrews can response differently than rodents. Due to their unique physiological and ecological adaptations, the duration of the winter season and probably the prey abundance affected by climatic conditions during the vegetation season plays a significant role. This indicates the importance of monitoring the impact of ongoing climate change on population of shrews and their main prey species.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Anděra M (2000) Atlas rozšíření savců v České republice. Předběžná verze. III. Hmyzožravci (Insectivora). Národní muzeum, Praha
Anděra M (2010) Current distributional status of insectivores in the Czech Republic (Eulipotyphla). Lynx (Praha) n.s. 41:15–63
Anděra M, Gaisler J (2012) Savci České republiky: popis, rozšíření, ekologie, ochrana. Academia, Praha
Anikanova VS, Ieshko EP, Bugmyrin SV (2009) Dynamics of the helminth fauna in the common shrew (Sorex araneus L.) from cut-over lands of different age in Karelia (in Russian: ДИHAMИКA ГEЛЬMИHTOФAУHЫ OБЫКHOBEHHOЙ БУPOЗУБКИ (SOREX ARANEUS L.) PAЗHOBOЗPACTHЫX BЫPУБOК КAPEЛИИ). Parasitol 43:79–89
Berryman A (2002) Population cycles: the case for trophic interactions. Oxford University Press, Oxford
Berryman A, Turchin P (2001) Identifying the density-dependent structure underlying ecological time series. Oikos 92:265–270. https://doi.org/10.1034/j.1600-0706.2001.920208.x
Bertolino S, Colangelo P, Mori E, Capizzi D (2015) Good for management, not for conservation: an overview of research, conservation and management of Italian small mammals. Hystrix It J Mamm 26:25–35. https://doi.org/10.4404/hystrix-26.1-10263
Bobretsov AV, Lukyanova LE, Maklakov KV (2018) Effects of landscape heterogeneity on common shrew (Sorex araneus) population dynamics. Russ J Ecol 49:543–547. https://doi.org/10.1134/S1067413618060048
Boers N (2021) Observation-based early-warning signals for a collapse of the Atlantic Meridional Overturning Circulation. Nat Clim Chang 11:680–688. https://doi.org/10.1038/s41558-021-01097-4
Bogdziewicz M, Zwolak R (2014) Responses of small mammals to clear-cutting in temperate and boreal forests of Europe: a meta-analysis and review. Eur J For Res 133:1–11. https://doi.org/10.1007/s10342-013-0726-x
Bojariu R, Giorgi F (2005) The North Atlantic Oscillation signal in a regional climate simulation for the European region. Tellus A Dyn Meteorol Oceanogr 57:641–653. https://doi.org/10.3402/tellusa.v57i4.14709
Bosso L, Smeraldo S, Rapuzzi P et al (2018) Nature protection areas of Europe are insufficient to preserve the threatened beetle Rosalia alpina (Coleoptera: Cerambycidae): Evidence from species distribution models and conservation gap analysis. Ecol Entomol 43:192–203. https://doi.org/10.1111/een.12485
Brannon MP (2000) Niche relationships of two syntopic species of shrews, Sorex fumeus and S. cinereus, in the Southern Appalachian mountains. J Mammal 81:1053–1061. https://doi.org/10.1644/1545-1542(2000)081%3c1053:NROTSS%3e2.0.CO;2
Brown CR, Hunter EM, Baxter RM (1997) Metabolism and thermoregulation in the forest shrew Myosorex varius (Soricidae: Crocidurinae). Comp Biochem Physiol - A Physiol 118:1285–1290. https://doi.org/10.1016/S0300-9629(97)00223-5
Bryja J, Heroldová M, Zejda J (2002) Effects of deforestation on structure and diversity of small mammal communities in the Moravskoslezské Beskydy Mts (Czech Republic). Acta Theriol 47:295–306
Bryja J, Zukal J (2000) Small mammal communities in newly planted biocorridors and their surroundings in southern Moravia (Czech Republic). Folia Zool 49:191–197
Caesar L, McCarthy GD, Thornalley DJR et al (2021) Current Atlantic Meridional Overturning Circulation weakest in last millennium. Nat Geosci 14:118–120. https://doi.org/10.1038/s41561-021-00699-z
Carey AB (2003) Restoration of landscape function: reserves or active management? Forestry 76:221–230. https://doi.org/10.1093/forestry/76.2.221
Carey AB, Harrington CA (2001) Small mammals in young forests: implications for management for sustainability. For Ecol Manage 154:289–309. https://doi.org/10.1016/S0378-1127(00)00638-1
Čepelka L, Heroldová M, Homolka M et al (2017) Diverzita a početnost drobných savců v lesních výsadbách na podzim 2015. Zprávy Lesn Výzkumu 62:189–196
Čepelka L, Michalko R, Kula E (2019) Efficiency of pitfall traps and snap traps in small terrestrial mammals depends on their diet composition. Turk J Zool 43:297–304. https://doi.org/10.3906/zoo-1810-4
Čepelka L, Šipoš J, Suchomel J, Heroldová M (2020) Can we detect response differences among dominant rodent species to climate and acorn crop in a Central European forest environment? Eur J For Res 139:539–548. https://doi.org/10.1007/s10342-020-01267-7
Chen IC, Hill JK, Ohlemüller R et al (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026. https://doi.org/10.1126/science.1206432
Chen L, Wang G, Wan X, Liu W (2015) Complex and nonlinear effects of weather and density on the demography of small herbivorous mammals. Basic Appl Ecol 16:172–179. https://doi.org/10.1016/j.baae.2014.12.002
Christoudias T, Pozzer A, Lelieveld J (2012) Influence of the North Atlantic Oscillation on air pollution transport. Atmos Chem Phys 12:869–877. https://doi.org/10.5194/acp-12-869-2012
Chronis T, Raitsos DE, Kassis D, Sarantopoulos A (2011) The summer North Atlantic Oscillation influence on the eastern Mediterranean. J Clim 24:5584–5596. https://doi.org/10.1175/2011JCLI3839.1
Churchfield S (1981) Water and fat contents of British shrews and their role in the seasonal changes in body weight. J Zool 194:165–173. https://doi.org/10.1111/j.1469-7998.1981.tb05767.x
Churchfield S (1982) Food availability and the diet of the common shrew, Sorex araneus, in Britain. J Anim Ecol 51:15–28
Churchfield S (1990) The natural history of shrews. Christopher Helm, London
Churchfield S (2002) Why are shrews so small? The costs and benefits of small size in northern temperate Sorex species in the context of foraging habits and prey supply. Acta Theriol 47:169–184. https://doi.org/10.1007/bf03192486
Churchfield S, Hollier J, Brown VK (1995) Population dynamics and survivorship patterns in the common shrew Sorex araneus in southern England. Acta Theriol 40:53–68
Churchfield S, Nesterenko VA, Shvarts EA (1999) Food niche overlap and ecological separation amongst six species of coexisting forest shrews (Insectivora: Soricidae) in the Russian Far East. J Zool 248:349–359. https://doi.org/10.1017/S0952836999007074
Churchfield S, Rychlik L (2006) Diets and coexistence in Neomys and Sorex shrews in Bialowieza forest, eastern Poland. J Zool 269:381–390. https://doi.org/10.1111/j.1469-7998.2006.00115.x
Churchfield S, Rychlik L, Taylor JRE (2012) Food resources and foraging habits of the common shrew, Sorex araneus: Does winter food shortage explain Dehnel’s phenomenon? Oikos 121:1593–1602. https://doi.org/10.1111/j.1600-0706.2011.20462.x
Cleary DM, Wynn JG, Ionita M et al (2017) Evidence of long-term NAO influence on East-Central Europe winter precipitation from a guano-derived δ15N record. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-14488-5
Coppola E, Nogherotto R, Ciarlo’ JM et al (2021) Assessment of the European climate projections as simulated by the large EURO-CORDEX regional and global climate model ensemble. J Geophys Res Atmos 126:1–20. https://doi.org/10.1029/2019JD032356
Cornulier T, Yoccoz NG, Bretagnolle V et al (2013) Europe-wide dampening of population cycles in keystone herbivores. Science 340:63–66. https://doi.org/10.1126/science.1228992
Dokulilová M, Suchomel J (2016) Influence of habitat conditions on abundance and diversity of shrews (Eulipotyphla, Soricidae) in Moravia. Proc Int Phd Students Conf (MendelNet 2016). Mendel Univ Brno, Fac AgriSciences, Brno, pp 390–394
Dokulilová M, Suchomel J (2017) Abundance of common shrew (Sorex araneus) in selected forest habitats of Moravia (Czech Republic). Acta Univ Agric Silvic Mendelianae Brun 65:401–409
Doležalová M (2007) Projevy Severoatlantské oscilace v časové variabilitě teploty vzduchu a srážek na území České republiky. Meteorol Časopis 10:91–99
Erdakov LN, Panov VV, Litvinov YN (2019) The cyclicity in the dynamics of different populations of the common shrew. Russ J Ecol 50:551–559. https://doi.org/10.1134/S1067413619060043
Feehan J, Harley M, Van Minnen J (2009) Climate change in Europe. 1. Impact on terrestrial ecosystems and biodiversity. A Review Agron Sustain Dev 29:409–421. https://doi.org/10.1051/agro:2008066
Formozov AN (1946) Snow cover as an integral factor of the environment and its importance in the ecology of mammals and birds. In: Materials for Fauna and Flora of USSR. New Ser Zool 5:1–152
Formozov AN (1961) The significance of snow cover in the ecology and geographical distribution of mammals and birds. Role of snow cover in natural processes. Institute of Geography of the Academy of Sciences of USSR, Moscow, pp 166–210
Frazier MR, Huey RB, Berrigan D (2006) Thermodynamics constrains the evolution of insect population growth rates: “warmer is better.” Am Nat 168:512–520. https://doi.org/10.1086/506977
Fuentes-Montemayor E, Ferryman M, Watts K et al (2020) Small mammal responses to long-term large-scale woodland creation: the influence of local and landscape-level attributes. Ecol Appl 30:e02028. https://doi.org/10.1002/eap.2028
Gardner JL, Peters A, Kearney MR et al (2011) Declining body size: a third universal response to warming? Trends Ecol Evol 26:285–291. https://doi.org/10.1016/j.tree.2011.03.005
Guseva TL, Korosov A V (2015) Common shrew (Sorex araneus (Linnaeus, 1758)) distribution across mosaic landscapes of Southern Karelia. Proc Karelian Res Cent Russ Acad Sci 4:100–107. https://doi.org/10.17076/eco210
Hansson L (1984) Predation as the factor causing extended low densities in microtine cycles. Oikos 43:255–256
Hansson L, Henttonen H (1985) Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia 67:394–402. https://doi.org/10.1007/BF00384946
Henttonen H (1985) Predation causing extended low densities in microtine cycles : further evidence from shrew dynamics. Oikos 45:156–157
Henttonen H, Haukisalmi V, Kaikusalo A et al (1989) Long-term population dymanics of common shew Sorex araneus in Finland. Ann Zool Fenn 26:349–355
Henttonen H, Kaikusalo A, Tast J, Viitala J (1977) Interspecific competition between small rodents in subarctic and boreal ecosystems. Oikos 29:581–590. https://doi.org/10.2307/3543596
Hlôška L, Chovancová B, Chovancová G, Fleischer P (2016) Influence of climatic factors on the population dynamics of small mammals (Rodentia, Soricomorpha) on the sites affected by windthrow in the High Tatra Mts. Folia Oecologica 43:12–20
Hope AG, Greiman SE, Tkach VV et al (2016) Shrews and their parasites: small species indicate big changes. NOAA Artic, Artic Report Card 2016. Available at: https://arctic.noaa.gov/Report-Card/Report-Card-2016/ArtMID/5022/ArticleID/268/Shrews-and-Their-Parasites-Small-Species-Indicate-Big-Changes
Hope AG, Waltari E, Morse NR et al (2017) Small mammals as indicators of climate, biodiversity, and ecosystem change. Alaska Park Sci 16:71–76
Hu W, Onditi KO, Jiang X et al (2022) Modeling the potential distribution of two species of shrews (Chodsigoa hypsibia and Anourosorex squamipes) under climate change in China. Diversity 14:87. https://doi.org/10.3390/d14020087
Huitu O, Norrdahl K, Korpimäki E (2004) Competition, predation and interspecific synchrony in cyclic small mammal communities. Ecography 27:197–206
Hurrell JW (1995) Decadal trends in the North Atlantic Oscillation: regional temperatures and precipitation. Science 269:676–679
Hurrell JW (2011) North Atlantic Oscillation. Encyclopaedia of Ocean Sciences 4:1904–1911
Hurrell JW, Kushnir Y, Ottersen G, Visbeck M (2003) An overview of the North Atlantic Oscillation. Geophys Monogr Ser 134:1–35. https://doi.org/10.1029/134GM01
Ims RA, Henden JA, Killengreen ST (2008) Collapsing population cycles. Trends Ecol Evol 23:79–86. https://doi.org/10.1016/j.tree.2007.10.010
Ivanter EV (2020) Population dynamics of the common shrew (Sorex araneus) (experience in an analytical review of the state of the problem). Biol Bull 47:844–853. https://doi.org/10.1134/S1062359020070055
Ivanter EV, Kurhinen JP (2015) Effect of anthropogenic transformation of forest landscapes on populations of small insectivores in eastern Fennoscandia. Russ J Ecol 46:252–259. https://doi.org/10.1134/S1067413615020046
Jacob D, Petersen J, Eggert B et al (2014) EURO-CORDEX: new high-resolution climate change projections for European impact research. Reg Environ Chang 14:563–578. https://doi.org/10.1007/s10113-013-0499-2
Jędrzejewska B, Jędrzejewski W (1990) Antipredatory behaviour of bank voles and prey choice of weasels — enclosure experiments. Ann Zool Fenn 27:321–328
Jędrzejewska B, Jędrzejewski W (1998) Predation in vertebrate communities: the Białowieża Primeval Forest as a case study. Ecological Studies 135, Springer, Berlin
Kaikusalo A, Hanski I (1985) Population dynamics of Sorex araneus and S. caecutiens in Finnish Lapland. Acta Zool Fenn 173:283–285
Korpela K (2014) Biological interactions in the boreal ecosystem under climate change - are the vole and predator cycles disappearing? University of Jyväskylä, Jyväskylä, pp 57
Korpimäki E (1986) Predation causing synchronous decline phases in microtine and shrew populations in western Finland. Oikos 46:124–127
Korpimäki E, Norrdahl K (1989) Avian and mammalian predators of shrews in Europe: Regional differences, between year and seasonal variation, and mortality due to predation. Ann Zool Fenn 26:389–400
Korpimäki E, Norrdahl K, Huitu O, Klemola T (2005) Predator-induced synchrony in population oscillations of coexisting small mammal species. Proc Biol Sci 272:193–202. https://doi.org/10.1098/rspb.2004.2860
Krojerová-Prokešová J, Homolka M, Barančeková M et al (2016) Structure of small mammal communities on clearings in managed Central European forests. For Ecol Manage 367:41–51. https://doi.org/10.1016/j.foreco.2016.02.024
Krojerová-Prokešová J, Homolka M, Heroldová M et al (2018) Patterns of vole gnawing on saplings in managed clearings in Central European forests. For Ecol Manage 408:137–147. https://doi.org/10.1016/j.foreco.2017.10.047
Lázaro J, Dechmann DKN (2021) Dehnel’s phenomenon. Curr Biol 31:R463–R465. https://doi.org/10.1016/j.cub.2021.04.006
Lázaro J, Dechmann DKN, LaPoint S et al (2017) Profound reversible seasonal changes of individual skull size in a mammal. Curr Biol 27:R1106–R1107. https://doi.org/10.1016/j.cub.2017.08.055
Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: Can predation risk explain microhabitat use? Ecology 72:2261–2273
López-Moreno JI, Vicente-Serrano SM (2008) Positive and negative phases of the wintertime North Atlantic Oscillation and drought occurrence over Europe: a multitemporal-scale approach. J Clim 21:1220–1243. https://doi.org/10.1175/2007JCLI1739.1
Lukyanova LE, Ukhova NL, Ukhova OV, Gorodilova YV (2021) Common shrew (Sorex araneus, Eulipotyphla) population and the food supply of its habitats in ecologically contrasting environments. Russ J Ecol 52:316–328. https://doi.org/10.1134/S106741362104007X
Marcström V, Höglund N, Krebs CJ (1990) Periodic fluctuations in small mammals at Boda, Sweden from 1961 to 1988. J Anim Ecol 59:753–761
Martin K, Sommer M (2004) Relationships between land snail assemblage patterns and soil properties in temperate-humid forest ecosystems. J Biogeogr 31:531–545. https://doi.org/10.1046/j.1365-2699.2003.01005.x
Matlack RS, Kaufman DW, Kaufman GA, McMillan BR (2002) Long-term variation in abundance of Elliot’s short-tailed shrew (Blarina hylophaga) in tallgrass prairie. J Mammal 83:280–289. https://doi.org/10.1644/1545-1542(2002)083%3c0280:LTVIAO%3e2.0.CO;2
Mažeikyte JR (2009) Population dynamics of the common shrew and pygmy shrew (Soricomorpha: Soricidae) in a clear-cut of a mixed forest in eastern Lithuania. Est J Ecol 58:205–215. https://doi.org/10.3176/eco.2009.3.05
Moraleva NV (1989) Intraspecific interactions in the common shrew Sorex araneus in Central Siberia. Ann Zool Fenn 26:425–432
Myllymäki A (1977) Intraspecific competition and home range dynamics in the field vole Microtus agrestis. Oikos 29:553–569. https://doi.org/10.2307/3543594
Ochocińska D, Taylor JRE (2005) Living at the physiological limits: field and maximum metabolic rates of the common shrew (Sorex araneus). Physiol Biochem Zool 78:808–818. https://doi.org/10.1086/431190
Ottersen G, Planque B, Belgrano A et al (2001) Ecological effects of the North Atlantic Oscillation. Oecologia 128:1–14. https://doi.org/10.1007/s004420100655
Pankakoski E (1985) Relationship between some meteorological factors and population dynamics of Sorex araneus in southern Finland. Acta Zool Fenn 173:287–289
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Pearce J, Venier L (2005) Small mammals as bioindicators of sustainable boreal forest management. For Ecol Manage 208:153–175. https://doi.org/10.1016/j.foreco.2004.11.024
Pokorná L, Beranová R, Huth R (2007) The Relationships between circulation modes and climatic elements in the Czech Republic and their time variations. Meteorol Bull 60:65–76
Popov IY (2005) Structure and dynamics of shrews on permanent plots in the European southern taiga. In: Merritt JF, Churchfield S, Hutterer R, Sheftel BI (eds) Advances in the Biology of Shrews II. Special Publication of the International Society of Shrew Biologists, New York, pp 291–301
Pupila A, Bergmanis U (2006) Species diversity, abundance and dynamics of small mammals in the Eastern Latvia. Acta Univ Latv 710:93–101
Radosa L, Schlegel M, Gebauer P et al (2013) Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect Genet Evol 19:403–410. https://doi.org/10.1016/j.meegid.2013.04.008
Rousi E, Rust HW, Ulbrich U, Anagnostopoulou C (2020) Implications of winter NAO flavors on present and future European climate. Climate 8:13. https://doi.org/10.3390/cli8010013
Saitoh T, Nakatsu A (1997) The impact of forestry on the small rodent community of Hokkaido, Japan. Mammal Study 22:27–38
Schaeffer PJ, O’Mara MT, Breiholz J et al (2020) Metabolic rate in common shrews is unaffected by temperature, leading to lower energetic costs through seasonal size reduction. R Soc Open Sci 7:191989. https://doi.org/10.1098/rsos.191989
Schlegel M, Radosa L, Rosenfeld UM et al (2012) Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Genes 45:48–55. https://doi.org/10.1007/s11262-012-0736-7
Scott AM, Gilbert JH, Pauli JN (2022) Small mammal dynamics in snow-covered forests. J Mammal 103:680–692. https://doi.org/10.1093/jmammal/gyac004
Sheftel BI (1989) Long-term and seasonal dynamics of shrews in Central Siberia. Ann Zool Fenn 26:357–369
Šipoš J, Suchomel J, Purchart L, Kindlmann P (2017) Main determinants of rodent population fluctuations in managed Central European temperate lowland forests. Mammal Res 62:283–295. https://doi.org/10.1007/s13364-017-0316-2
Stenseth NC, Mysterud A, Ottersen G et al (2002) Ecological effects of climate fluctuations. Science 297:1292–1296
Suchomel J, Purchart L, Čepelka L, Heroldová M (2014) Structure and diversity of small mammal communities of mountain forests in Western Carpathians. Eur J For Res 133:481–490. https://doi.org/10.1007/s10342-013-0778-y
Tast J, Kaikusalo A, Järvinen A (2005) Population fluctuations of Sorex araneus at Kilpisjärvi, Finnish Lapland, as compared with rodent cycles. In: Merritt JF, Churchfield S, Hutterer R, Sheftel BI (eds) Advances in the Biology of Shrews II. Special Publication of the International Society of Shrew Biologists, New York, pp 215–228
Taylor JRE (1998) Evolution of energetic strategies in shrews. In: Wójcik JM, Wolsan M (eds) Evolution of shrews. Mammal Research Institute, Pol Acad Sci, Białowieża, pp 309–346
Taylor JRE, Muturi M, Lázaro J et al (2022) Fifty years of data show the effects of climate on overall skull size and the extent of seasonal reversible skull size changes (Dehnel’s phenomenon) in the common shrew. Ecol Evol 12:1–13. https://doi.org/10.1002/ece3.9447
Tischler W (1949) Grundzüge der terrestrischen Tierökologie (in German). Friedrich Vieweg und Sohn, Braunschweig
Tkadlec E, Zejda J (1998) Small rodent population fluctuations: the effects of age structure and seasonality. Evol Ecol 12:191–210. https://doi.org/10.1023/A:1006583713042
Tomášková L, Bejček V, Sedláček F et al (2005) Population biology of shrews (Sorex araneus and S. minutus) from a polluted area in central Europe. In: Merritt JF, Churchfield S, Hutterer R, Sheftel BI (eds) Advances in the Biology of Shrews II. Special Publication of the International Society of Shrew Biologists, New York, pp 189–197
Trigo IF (2006) Climatology and interannual variability of storm-tracks in the Euro-Atlantic sector: a comparison between ERA-40 and NCEP/NCAR reanalyses. Clim Dyn 26:127–143. https://doi.org/10.1007/s00382-005-0065-9
ÚHÚL (2023) The Forest Management Institute (FMI). www.uhul.cz. Retrieved 30 March 2023
Väli Ü, Tõnisalu G (2021) Community-and species-level habitat associations of small mammals in a hemiboreal forest-farmland landscape. Ann Zool Fenn 58:1–11. https://doi.org/10.5735/086.058.0101
Viitala J (1977) Social organization in cyclic subarctic populations of the voles Clethrionomys rufocanus (Sund.) and Microtus agrestis (L.). Acta Zool Fenn 14:53–93
Viitala J (1984) Stability of overwintering populations of Clethrionomys and Microtus at Kilpisjärvi, Finnish Lappland. Spec Publ Carnegie Museum Nat Hist 10:109–112
Walther G-R, Post E, Convey P et al (2002) Ecological response to recent climate change. Nature 416:389–395
Zakharov VM, Pankakoski E, Sheftel BI et al (1991) Developmental stability and population dynamics in the common shrew, Sorex araneus. Am Nat 138:797–810
Zakharov VM, Trofimov IE, Sheftel BI (2020) Fluctuating asymmetry and population dynamics of the common shrew, Sorex araneus, in central Siberia under climate change conditions. Symmetry 12:1–7. https://doi.org/10.3390/sym12121960
Zárybnická M, Bejček V, Šťastný K et al (2017) Long-term changes of small mammal communities in heterogenous landscapes of Central Europe. Eur J Wildl Res 63:89. https://doi.org/10.1007/s10344-017-1147-9
Zárybnická M, Sedláček O, Salo P et al (2015) Reproductive responses of temperate and boreal Tengmalm’s Owl Aegolius funereus populations to spatial and temporal variation in prey availability. Ibis 157:369–383. https://doi.org/10.1111/ibi.12244
Zub K, Jędrzejewska B, Jędrzejewski W, Bartoń K (2012) Cyclic voles and shrews and non-cyclic mice in a marginal grassland within European temperate forest. Acta Theriol 57:205–216. https://doi.org/10.1007/s13364-012-0072-2
Acknowledgements
We would like to thank Alisa Royer Selivanova for English revision of the manuscript.
Funding
Open access publishing supported by the National Technical Library in Prague. The study was supported with grants NAZV QH72075 and NAZV QK1820091 by the Ministry of Agriculture of the Czech Republic, and by the Institutional Research Plan (RVO: 68081766).
Author information
Authors and Affiliations
Contributions
MD prepared the data, contributed to data analyses, and compiled the first draft of the manuscript. JKP statistically analyzed the data, prepared the figures, and significantly contributed to writing. MH and JS designed the study. All authors collected data about small mammals in field and reviewed previous versions of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All aspects of trapping complied with EU Council Directive 86/609/EEC on the experimental use of animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dokulilová, M., Krojerová-Prokešová, J., Heroldová, M. et al. Population dynamics of the common shrew (Sorex araneus) in Central European forest clearings. Eur J Wildl Res 69, 54 (2023). https://doi.org/10.1007/s10344-023-01682-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01682-2