Abstract
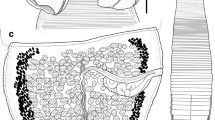
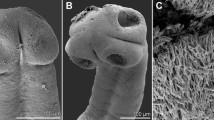
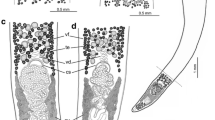
A new genus, Megancestus n. gen., is proposed to accommodate the caryophyllidean tapeworm Biacetabulum carpiodi Mackiewicz, 1969 from carpsuckers and quillback (Carpiodes spp.) in North America. This species is not closely related to other species of Biacetabulum Hunter, 1927 and is transferred to a newly erected genus. This new genus is typified by the possession of a small body (total length of 3.1–7.5 mm) with a scolex that bears a pair of large acetabulum-like loculi, two pairs of shallow lateral loculi, and a slightly convex apical disc, testes arranged in one or two layers, oval, thick-walled cirrus-sac, well-developed external seminal vesicle, separate gonopores, H-shaped ovary, few median vitelline follicles, and the uterus extending by a single loop anterior to the cirrus-sac. Megancestus differs from all Nearctic caryophyllidean genera (family Capingentidae), including Biacetabulum, by vitelline follicles dorsal to the ovary that connect the preovarian and postovarian vitelline fields. The most closely related Hunterella Mackiewicz et McCrae, 1962 differs by shape of the scolex (tholate, i.e., devoid of any loculi), dumbbell-shaped ovary and the uterus not extending anterior to the cirrus-sac. Megancestus carpiodi (Mackiewicz, 1969) n. comb. is the only species of the genus and it is a stenoxenous parasite, which has been found only in the river carpsucker (Carpiodes carpio—type host), quillback (Carpiodes cyprinus) and highfin carpsucker (Carpiodes velifer) (Catostomidae: Ictiobinae) in the lower and middle Mississippi basin.




Similar content being viewed by others
References
Bagley JC, Mayden RL, Harris PM (2018) Phylogeny and divergence times of suckers (Cypriniformes: Catostomidae) inferred from Bayesian total-evidence analyses of molecules, morphology, and fossils. Peer J 6:e5168. https://doi.org/10.7717/peerj.5168
Baylis HA (1928) Some parasitic worms, mainly from fishes, from Lake Tanganyika. Ann Mag Nat Hist 1:552–562. https://doi.org/10.1080/00222932808672818
Chervy L (2009) Unified terminology for cestode microtriches: a proposal from the International Workshops on Cestode Systematics in 2002–2008. Folia Parasitol 56:199–230. https://doi.org/10.14411/fp.2009.025
Choudhury A, Scholz T (2020) Ex uno plures: morphotype and lineage diversity of Bothriocephalus (Cestoda: Bothriocephalidea) in North American freshwater fishes. J Parasitol 106:589–602. https://doi.org/10.1645/17-98
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Etnier DA, Starnes WC (1993) The fishes of Tennessee. Knoxville: The University of Tennessee Press
Froese R, Pauly D (Eds.) (2020) Fishbase. World Wide Web electronic publication. http://www.fishbase.org
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Hoffman GL (1999) Parasites of North American freshwater fishes, 2nd edn. Ithaca: Cornell University Press
Hunter GW (1927) Notes on the Caryophyllaeidae of North America. J Parasitol 14:16–26. https://doi.org/10.2307/3271398
Kuchta R, Řehulková E, Francová K, Scholz T, Šimková A (2020) What do we know about parasites of cypriniform fishes? Diversity of monogeneans and tapeworms across two continents. Int J Parasitol 50:771–786. https://doi.org/10.1016/j.ijpara.2020.06.005
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Mackiewicz JS (1969) Penarchigetes oklensis gen. et sp. n. and Biacetabulum carpiodi sp. n. (Cestoidea: Caryophyllaeidae) from catostomid fish in North America. Proc Helminthol Soc Wash 36:119–126
Mackiewicz JS (1994) Order Caryophyllidea van Beneden in Carus, 1863. In: Khalil LF, Jones A, Bray RA (eds) Keys to the cestode parasites of vertebrates. Wallingford: CAB International, pp 21–43
Mackiewicz JS, McCrae R (1962) Hunterella nodulosa gen. n, sp. n. (Cestoidea: Caryophyllaeidae) from Catostomus commersoni (Lacépède) (Pisces: Catostomidae) in North America. J Parasitol 48:798–806. https://doi.org/10.2307/3275098
Mackiewicz JS, McCrae RC (1965) Biacetabulum biloculoides n. sp. (Cestoidea: Caryophyllaeidae) from Catostomus commersoni (Lacépède) in North America. Proc Helminthol Soc Wash 32:225–228
Margolis L, Arthur JR (1979) Synopsis of the parasites of fishes of Canada. Bull Fish Res Bd Can 199:1–269
McDonald TE, Margolis L (1995) Synopsis of the parasites of fishes of Canada: supplement (1978–1993). Can Spec Publ Fish Aquat Sci 122:1–265
Oros M, Scholz T, Hanzelová V, Mackiewicz JS (2010) Scolex morphology of monozoic cestodes (Caryophyllidea) from the Palaearctic Region: a useful tool for species identification. Folia Parasitol 57:37–46. https://doi.org/10.14411/fp.2010.006
Oros M, Brabec J, Kuchta R, Choudhury A, Scholz T (2016) A synoptic review of Promonobothrium Mackiewicz, 1968 (Cestoda: Caryophyllidea), parasites of suckers (Catostomidae) in North America, with description of two new species. Folia Parasitol 63:008. https://doi.org/10.14411/fp.2016.008
Oros M, Uhrovič D, Scholz T (2018) A new classification of Glaridacris Cooper, 1920 (Cestoda: Caryophyllidea), parasites of suckers (Catostomidae) in North America, including erection of Pseudoglaridacris n. gen. J Parasitol 104:60–69. https://doi.org/10.1645/17-58
Oros M, Uhrovič D, Choudhury A, Mackiewicz JS, Scholz T (2020) Scolex morphology of monozoic tapeworms (Caryophyllidea) from the Nearctic Region: taxonomic and evolutionary implications. Folia Parasitol 67:1–11. https://doi.org/10.14411/fp.2020.003
Page LM, Burr BM (2011) A field guide to freshwater fishes of North America north of Mexico. Boston: Houghton Mifflin Harcourt
Pleijel F, Jondelius U, Norlinder E, Nygren A, Oxelman B, Schander C, Sundberg P, Thollesson M (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol Phylogenet Evol 48:369–371. https://doi.org/10.1016/j.ympev.2008.03.024
Rambaut A (2012) FigTree v1. 4. Molecular evolution, phylogenetics and epidemiology. Edinburgh: University of Edinburgh, Institute of Evolutionary Biology. Retrieved from http://tree.bio.ed.ac.uk/software/figtree
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Scholz T, Choudhury A (2014) Parasites of freshwater fishes in North America: why so neglected? J Parasitol 100:26–45. https://doi.org/10.1645/13-394.1
Scholz T, Oros M (2017) Caryophyllidea van Beneden in Carus, 1863. In: Caira JN, Jensen K (ed), Planetary biodiversity inventory (2008–2016): tapeworms from vertebrate bowels of the Earth. Lawrence: The University of Kansas, Natural History Museum, 25:47–64
Scholz T, Oros M, Choudhury A, Brabec J, Waeschenbach A (2015) New circumscription of freshwater fish parasites Monobothrium Diesing, 1863 and Promonobothrium Mackiewicz, 1968 (Cestoda: Caryophyllidea) using morphological and molecular evidence. J Parasitol 101:29–36. https://doi.org/10.1645/14-610.1
Scholz T, Choudhury A, Uhrová L, Brabec J (2019) The Proteocephalus species-aggregate in freshwater centrarchid and percid fishes of the Nearctic region (North America). J Parasitol 105:798–812. https://doi.org/10.14411/fp.2020.021
Scholz T, Waeschenbach A, Oros M, Brabec J, Littlewood D (2021) Phylogenetic reconstruction of early diverging tapeworms (Cestoda: Caryophyllidea) reveals ancient radiations in vertebrate hosts and biogeographic regions. Int J Parasitol 51:263–277. https://doi.org/10.1016/j.ijpara.2020.09.009
Uhrovič D, Oros M, Kudlai O, Choudhury A, Scholz T (2021) Molecular evidence of three closely related species of Biacetabulum Hunter, 1927 (Cestoda: Caryophyllidea): A case of recent speciation in different fish hosts (Catostomidae)? Parasitology (in press). https://doi.org/10.1017/S0031182021000743
Waeschenbach A, Webster BL, Littlewood DTJ (2012) Adding resolution to ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with large fragments of mtDNA. Mol Phylogenet Evol 63:834–847. https://doi.org/10.1016/j.ympev.2012.02.020
Williams DD, Ulmer MJ (1970) Caryophyllaeid cestodes from four species of Carpiodes (Teleostei: Catostomidae). Proc Iowa Acad Sci 77:185–195
Acknowledgements
We thank Steve Curran, Florian Reyda (both USA) and Roman Kuchta (Czech Republic) for help with collecting fishes in the USA, and two anonymous reviewers for their helpful suggestions. This study was partly supported by the Grant Agency VEGA (No. 2/0126/20), Ministry of Education, Sports and Youth (project No. LTAUSA18010) and the Institute of Parasitology (RVO: 60077344). Stays of M.O. and T.S. in North America in 2013 and 2017, respectively, were enabled by the Fulbright Commission. Technical help of Martina Borovková, Institute of Parasitology, BC CAS, České Budějovice, is much appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Guillermo Salgado-Maldonado
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uhrovič, D., Scholz, T., Kudlai, O. et al. Disentangling taxonomy of Biacetabulum (Cestoda, Caryophyllidea), parasites of catostomid fishes in North America: proposal of Megancestus gen. n. to accommodate B. carpiodi. Parasitol Res 120, 1993–2001 (2021). https://doi.org/10.1007/s00436-021-07188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07188-7