Abstract
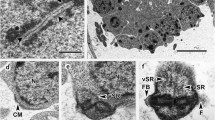
The spermatozoon ultrastructure of the progenetic cestode Diplocotyle olrikii (Spathebothriidea) has been examined using transmission electron microscopy and cytochemical staining with periodic acid-thiosemicarbazide-silver proteinate (PA-TSC-SP) for glycogen. The spermatozoon is a filiform cell, tapered at both extremities. Its moderately electron-dense cytoplasm possesses two parallel axonemes of unequal lengths. New for the Cestoda is a finding of three types of the mature spermatozoa with respect to different axonemal structure. The first type has both axonemes with standard 9 + ‘1’ trepaxonematan pattern. The second type is represented by a spermatozoon having one axoneme with 9 + ‘1’ structure and the second one with 9 + 0 pattern. The third type includes the two axonemes with 9 + 0 pattern. Microtubule doublets of the 9 + 0 axonemes contain either inner dynein arms or no dynein arms. In addition to the two axonemes, all three types of the mature sperm cells contain parallel nucleus, parallel cortical microtubules, four electron-dense plaques/attachment zones, and glycogen. The anterior extremity of the gamete exhibits a centriole surrounded by a semiarc of up to five electron-dense tubular structures. The distal end of the first type spermatozoa exhibits two morphological variants, represented either by (i) nucleus or (ii) remnants of the disorganized axoneme. Distal extremity of the spermatozoa of the second and third types contains doublets and singlets of disorganized axoneme. The ultrastructural characters of the spermatozoon of progenetic D. olrikii support the basal position of the Spathebothriidea within the Eucestoda.






Similar content being viewed by others
References
Bruňanská M (2009) Spermatological characters of the caryophyllidean cestode Khawia sinensis Hsü, 1935, a carp parasite. Parasitol Res 105:1603–1610
Bruňanská M (2010) Recent insights into spermatozoa development and ultrastructure in the Eucestoda. In: Lejeune T, Delvaux P (eds) Human spermatozoa: maturation, capacitation and abnormalities. Nova Science Publishers, Inc., New York, pp 327–354
Bruňanská M, Kostič B (2012) Revisiting caryophyllidean type of spermiogenesis in the Eucestoda based on spermatozoon differentiation and ultrastructure of Caryophyllaeus laticeps (Pallas, 1781). Parasitol Res 110:141–149
Bruňanská M, Poddubnaya LG (2010) Spermatological characters of the spathebothriidean tapeworm Didymobothrium rudolphii (Monticelli, 1890). Parasitol Res 106:1435–1442
Bruňanská M, Scholz T, Nebesářová J (2003a) Reinvestigation of the spermatozoon ultrastructure of the cestode Proteocephalus longicollis (Zeder, 1800), a parasite of salmonid fish. Parasitol Res 91:357–362. https://doi.org/10.1017/s00436-003-0977-4
Bruňanská M, Nebesářová J, Scholz T (2003b) Ultrastructure of the spermatozoon of the proteocephalidean cestode Proteocephalus torulosus (Batsch 1786). Parasitol Res 89:345–351
Bruňanská M, Scholz T, Ibraheem MH (2004a) Ultrastructural particularities of the spermatozoon of the cestode Electrotaenia malopteruri (Fritsch, 1886) (Proteocephalidae: Gangesiinae), a parasite of Malapterurus electricus (Siluriformes: Malapteruridae) from the Nile, Egypt. Parasitol Res 93:114–120
Bruňanská M, Scholz T, Ibraheem MH (2004b) Ultrastructural characteristics of the spermatozoon of the cestode Corallobothrium solidum Fritsch, 1886 (Proteocephalidae: Corallobothriinae), a parasite of Malapterurus electricus (Siluriformes: Malapteruridae) from the Nile, Egypt. Parasitol Res 94:421–426
Bruňanská M, Poddubnaya LG, Dezfuli B (2005) Vitellogenesis in two spathebothriidean cestodes. Parasitol Res 96:390–397
Bruňanská M, Scholz T, Dezfuli B, Poddubnaya LG (2006) Spermiogenesis and sperm ultrastructure of Cyathocephalus truncatus (Pallas, 1781) Kessler, 1868 (Cestoda: Spathebothriidea). J Parasitol 92:884–892
Bruňanská M, Fagerholm HP, Nebesářová J, Kostič B (2010) Ultrastructure of the mature spermatozoon of Eubothrium rugosum (Batsch, 1779) with a re-assessment of the spermatozoon ultrastructure of Eubothrium crassum (Bloch, 1779) (Cestoda: Bothriocephalidea). Helminthologia 47:257–263
Bruňanská M, Poddubnaya LG, Xylander WER (2012) Spermatozoon cytoarchitecture of Amphilina foliacea (Platyhelminthes, Amphilinidea). Parasitol Res 111:2063–2069
Bruňanská M, Bílý T, Nebesářová J (2015) Nippotaenia mogurndae Yamaguti et Myiata, 1940 (Cestoda, Nippotaeniidea): first data on spermiogenesis and sperm ultrastructure. Parasitol Res 114:1443–1453
Bruňanská M, Matoušková M, Nebesářová J, Mackiewicz JS, Poddubnaya LG (2019) First ultrastructural and cytochemical data on the spermatozoon and its differentiation in progenetic and adult Archigetes sieboldi Leuckart, 1878 (Cestoda, Caryophyllidea, Caryophyllaeidae). Parasitol Res 118:1205–1214
Chervy L (2002) The terminology of larval cestodes or metacestodes. Syst Parasitol 52:1–33
Cielocha JJ, Yoneva A, Cantino ME, Daniels S, Jensen K (2013) Ultrastructure of the sperm of Adelobothrium sp. (Cestoda: Lecanicephalidea). Invertebr Biol 132:315–325. https://doi.org/10.1111/ivb.12036
Ehlers U (1984) Phylogenetisches System der Plathelminthes. Verh des Naturwiss Ver Hamb 27:291–294
Gamil IS (2008) Ultrastructural studies of the spermatogenesis and spermiogenesis of the caryophyllidean cestode Wenyonia virilis (Woodland, 1923). Parasitol Res 103:777–785
Gibson DI (1994) Order Spathebothriidea Wardle & McLeod, 1952. In: Khalil LF, Jones A, Bray RA (eds) Keys to the cestode parasites of vertebrates. CAB International, Wallingford, pp 15–19
Hoberg EP, Mariaux J, Justine JL, Brooks DR, Weekes PJ (1997) Phylogeny of the orders of the Eucestoda (Cercomeromorphae) based on comparative morphology: historical perspectives and a new working hypothesis. J Parasitol 83:1128–1147
Hoberg EP, Mariaux J, Brooks DR (2001) Phylogeny among the orders of the Eucestoda (Cercomeromorphae): integrating morphology, molecules and total evidence. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 112–126
Justine JL (2001) Spermatozoa as phylogenetic characters for the Platyhelminthes. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 231–238
Justine JL, Mattei X (1983) A spermatozoon with two 9 + 0 axonemes in a parasitic flatworm, Didymozoon (Digenea: Didymozoidae). J Submicrosc Cytol 15:1101–1105
Justine JL, Mattei X (1984) Atypical spermiogenesis in a parasitic flatworm, Didymozoon (Trematoda: Digenea: Didymozoidae). J Ultrastruct Res 87:106–111
Kuchta R, Pearson R, Scholz T, Ditrich O, Olson PD (2014) Spathebothriidea: survey of species, scolex and egg morphology, and interrelationships of a non-segmented, relictual tapeworm group (Platyhelminthes: Cestoda). Folia Parasitol 61:331–346
Levron C, Miquel J, Oros M, Scholz T (2010) Spermatozoa of tapeworms (Platyhelminthes, Eucestoda): advances in ultrastructural and phylogenetic studies. Biol Rev 85:523–543
Mackiewicz JS (2003) Caryophyllidea (Cestoidea): Molecules, morphology and evolution. Acta Parasitol 48:143–154
MacKinnon BM, Burt MDB (1984) The comparative ultrastructure of spermatozoa from Bothrimonus sturionis Duv. 1842 (Pseudophyllidea), Pseudanthobothrium hanseni Baer, 1956 (Tetraphyllidea), and Monoecocestus americanus Stiles, 1895 (Cyclophyllidea). Can J Zool 62:1059–1066
Mariaux J (1998) A molecular phylogeny of the Eucestoda. J Parasitol 84:114–124
Mariaux J, Olson PD (2001) Cestode systematics in the molecular era. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 127–134
Marigo AM, Bâ CT, Miquel J (2011) Spermiogenesis and spermatozoon ultrastructure of the dilepidid cestode Molluscotaenia crassiscolex (von Linstow, 1890), an intestinal parasite of the common shrew Sorex araneus. Acta Zool (Stockholm) 92:116–125
Marques JF, Santos MJ, Gibson DJ, Cabral HN, Olson PD (2007) Cryptic species of Didymobothrium rudolphii (Cestoda: Spathebothriidea) from the sand sole, Solea lascaris, off the Portuguese coast, with an analysis of their molecules, morphology, ultrastructure and phylogeny. Parasitology 134:1057–1072
Matoušková M, Bílý T, Bruňanská M, Mackiewicz JS, Nebesářová J (2018) Ultrastructure, cytochemistry and electron tomography analysis of Caryophyllaeides fennica (Schneider, 1902) (Cestoda: Lytocestidae) reveals novel spermatology characteristics in the Eucestoda. Parasitol Res 117:3091–3102
Matoušková M, Bílý T, Bruňanská M, Oros M, Kostič B, Nebesářová J (2019) New data on spermiogenesis and trepaxonematan axoneme in basal tapeworms (Cestoda, Caryophyllidea, Lytocestidae) parasitizing cyprinid fishes. Sci Rep 12881 9. https://doi.org/10.1038/s41598-019-49312-9
Miquel J, Feliu C, Marchand B (1999) Ultrastructure of spermiogenesis and the spermatozoon of Mesocestoides litteratus (Cestoda, Mesocestoididae). Int J Parasitol 29:499–510
Miquel J, Eira C, Świderski Z, Conn DB (2007a) Mesocestoides lineatus (Goeze, 1782) (Mesocestoididae): new data on sperm ultrastructure. J Parasitol 93:545–552
Miquel J, Świderski Z, Neifar L, Eira C (2007b) Ultrastructure of the spermatozoon of Parachristianella trygonis Dollfus, 1946 (Trypanorhyncha, Eutetrarhynchidae). J Parasitol 93:1296–1302
Miquel J, Torres J, Foronda P, Feliu C (2010) Spermiogenesis and spermatozoon ultrastructure of the davaineid cestode Raillietina micracantha (Fuhrmann, 1909). Acta Zool (Stockholm) 91:212–221
Okaka CE (2000) Maturity of the procercoid of Cyathocephalus truncatus (Eucestoda: Spathebothriidae) in Gammarus pulex (Crustacea: Amphipoda) and the tapeworms life cycle using the amphipod as the sole host. Helminthologia 37:153–157
Olson PD, Littlewood DTJ, Bray RA, Mariaux J (2001) Interrelationships and evolution of the tapeworms (Platyhelminthes: Cestoda). Mol Phylogenet Evol 19:443–467
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Dezfuli BS (2005a) Fine structure of the male reproductive ducts, vagina and seminal receptacle of Cyathocephalus truncatus (Pallas 1781) (Cestoda: Spathebothriidea). Folia Parasitol 52:241–250
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Scholz T (2005b) Fine structure of the female reproductive ducts of Cyathocephalus truncatus (Cestoda: Spathebothriidea), from salmonid fish. Folia Parasitol 52:323–338
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Scholz T (2005c) Ultrastructural studies on the reproductive system of progenetic Diplocotyle olrikii (Cestoda: Spathebothriidea): ovarian tissue. Acta Parasitol 50:199–207
Poddubnaya LG, Mackiewicz JS, Swiderski Z, Bruňanská M, Scholz T (2005d) Fine structure of egg-forming complex ducts, eggshell formation and supporting neuronal plexus in progenetic Diplocotyle olrikii (Cestoda: Spathebothriidea). Acta Parasitol 50:292–304
Poddubnaya LG, Gibson DI, Swiderski Z, Olson PD (2006) Vitellocyte ultrastructure in the cestode Didymobothrium rudolphii (Monticelli, 1890): possible evidence for the recognition of divergent taxa within the Spathebothriidea. Acta Parasitol 51:255–263
Poddubnaya LG, Gibson DI, Olson PD (2007) Ultrastructure of the ovary, ovicapt and oviduct of the spathebothriidean tapeworm Didymobothrium rudolphii (Monticelli, 1890). Acta Parasitol 52:127–134
Protasova EN, Roitman VA (1995) [Cyathocephalates, tapeworm helminths of marine and freshwater fishes (Cestoda: Pseudophyllidea: Cyathocephalata).] Essentials of Cestodology. Vol. 12. Nauka, Moscow, 134 pp (In Russian)
Roberts LS, Janovy J (2005) Gerald D Schmidt & Larry S. Roberts’ foundations of parasitology, Seventh edn. Colin H Wheatley, McGraw-Hill, New York 702 p
Sandeman IM, Burt MDB (1972) Biology of Bothrimonus (=Diplocotyle) (Pseudophyllidea: Cestoda): ecology, life cycle, and evolution: a review and synthesis. J Fish Res Board Can 29:1381–1395
Shapiro JE, Hershenov BR, Tulloch GS (1961) The fine structure of Haematoloechus spermatozoon tail. J Biophys Biochem Cytol 9:211–217
Thiéry J-P (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc 6:987–1018
Yoneva A, Levron C, Oros M, Orosová M, Scholz T (2012) Spermiogenesis and spermatozoon ultrastructure of Hunterella nodulosa (Cestoda: Caryophyllidea), a monozoic parasite of suckers (Catostomidae) in North America. Folia Parasitol 59:179–186
Acknowledgements
This study was supported by the Grant Agency of the Slovak Republic VEGA (project no. 2/0104/16 to MB). We acknowledge the core facility of the Institute of Parasitology, BC ASCR in České Budějovice, Czech Republic, supported by the MEYS CR (LM2015062 Czech-BioImaging).This research was undertaken within the framework of a joint research projects Nos. AV ČR-16-08 and SAV-18-21 supported by a bilateral agreement on scientific exchange and cooperation signed by the Czech and Slovak Academies of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: David Bruce Conn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bruňanská, M., Matoušková, M., Jasinská, R. et al. Heteromorphism of sperm axonemes in a parasitic flatworm, progenetic Diplocotyle olrikii Krabbe, 1874 (Cestoda, Spathebothriidea). Parasitol Res 119, 177–187 (2020). https://doi.org/10.1007/s00436-019-06524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06524-2