Abstract
Poly-β-hydroxybutyrate (PHB) is a potential source of biodegradable plastics that are environmentally friendly due to their complete degradation to water and carbon dioxide. This study aimed to investigate PHB production in the cyanobacterium Synechocystis sp. PCC6714 MT_a24 in an outdoor bioreactor using urban wastewater as a sole nutrient source. The culture was grown in a thin-layer raceway pond with a working volume of 100 L, reaching a biomass density of up to 3.5 g L−1 of cell dry weight (CDW). The maximum PHB content was found under nutrient-limiting conditions in the late stationary phase, reaching 23.7 ± 2.2% PHB per CDW. These data are one of the highest reported for photosynthetic production of PHB by cyanobacteria, moreover using urban wastewater in pilot-scale cultivation which multiplies the potential of sustainable cultivation approaches. Contamination by grazers (Poterioochromonas malhamensis) was managed by culturing Synechocystis in a highly alkaline environment (pH about 10.5) which did not significantly affect the culture growth. Furthermore, the strain MT_a24 showed significant wastewater nutrient remediation removing about 72% of nitrogen and 67% of phosphorus. These trials demonstrate that the photosynthetic production of PHB by Synechocystis sp. PCC6714 MT_a24 in the outdoor thin-layer bioreactor using urban wastewater and ambient carbon dioxide. It shows a promising approach for the cost-effective and sustainable production of biodegradable carbon-negative plastics.
Key points
• High PHB production by cyanobacteria in outdoor raceway pond
• Urban wastewater used as a sole source of nutrients for phototrophic growth
• Potential for cost-effective and sustainable production of biodegradable plastics
Graphical abstract

Similar content being viewed by others
Introduction
The recent times can be referred to as the “plastic age” due to the massive use of these materials which replace natural substances in many aspects of human life. Nevertheless, petroleum-based plastics cause serious environmental problems due to their non-degradable nature (Getachew and Woldesenbet 2016). Many strategies were suggested to control and reduce plastic waste, especially microplastics and nanoplastics; yet, the disposal of durable plastics still causes enormous contamination which affects terrestrial as well as aquatic environments (Rai et al. 2021). Despite the negative environmental impact, the global plastic demand is still increasing (McAdam et al. 2020). This alarming situation leads to a search for alternatives. Biodegradable plastics or bioplastics have been considered as a potential solution, which can gradually substitute petroleum-based plastics (Geyer et al. 2017). Yet, bioplastics represent only a small fraction of the overall used plastics, only 1% of the whole market (http://www.european-bioplastics.org/). To increase bioplastic availability, a sustainable cultivation technology utilizing a cheap source of input material needs to be found. One possibility is culturing cyanobacteria using nutrient-rich wastewater to obtain valuable biomass while reducing environmental pollutants (Zerrouki and Henni 2019; Grivalský et al. 2022).
Resources for biodegradable plastics made by microorganisms such as polyhydroxyalkanoates (PHA) show to be the suitable solution to replace conventional petroleum-based products and protect the environment. Among them, polyhydroxybutyrates (PHBs) are fully biodegradable (Getachew and Woldesenbet 2016) and some of them have properties similar to petroleum-based polymers such as polypropylene and polyethylene (McAdam et al. 2020). These features make PHB potentially sustainable for eco-friendly production with the advantage of waste-to-value technology. The PHB can be used as packing material, e.g., for the food industry where biodegradability is a strong benefit. Although PHB is commercially produced by heterotrophic bacteria, at present the phototrophic production of PHB by cyanobacteria attracts increasing attention (Kamravamanesh et al. 2018b; Koch et al. 2019; Rueda et al. 2020). However, its production process has to be improved (Kamravamanesh et al. 2019).
In cyanobacteria, PHB is synthesized as a biopolymer under various stress conditions. Its biosynthesis can be enhanced, e.g., by cultivation conditions that involve nitrogen or phosphorus limitation as well as the addition of sugars or organic acids. Especially, nitrogen starvation induces chlorosis, a detrimental process in which cyanobacteria cells degrade the photosynthetic machinery and accumulate biopolymers such as glycogen, PHB, and polyphosphates (Forchhammer and Schwarz 2019; Lapointe et al. 2022). Particularly, during the later stages of chlorosis, glycogen is converted to PHB (Koch et al. 2019). The compound forms inclusion bodies in the cells which are accumulated as intracellular reserves of energy and carbon (Getachew and Woldesenbet 2016; Troschl et al. 2018).
In mass cultivation, the supplement of organic carbon source (i) might increase the risk of contamination and deploy the photosynthetic potential of cyanobacteria and (ii) might be a non-feasible cost approach regarding industrial application (Troschl et al. 2017; Kamravamanesh et al. 2018b). PHB content in nutrient-deprived cyanobacterial cells is usually less than 20% of cell dry weight (CDW) which is still rather low compared to 70–80% found in heterotrophic bacterial biomass (Drosg et al. 2015).
However, the main ecological, as well as economical aspect, is the direct production of PHB from ambient CO2 as the major greenhouse gas (Troschl et al. 2017; Kamravamanesh et al. 2018b). Several studies have been carried out to increase the low level of PHB in cyanobacterial cells by looking for appropriate strains, suitable substrates, genetic manipulations, or their combinations (Nishioka et al. 2001; Bhati and Mallick 2015; Kamravamanesh et al. 2018a; Koch et al. 2020b). An engineered cyanobacterial strain was constructed in which a PHB content was about 80% per CDW when cultivated under nitrogen and phosphorus limitations with acetate as a carbon source (Koch et al. 2020b). This strain was comparable to heterotrophic bacteria as a PHB producer (Drosg et al. 2015; Troschl et al. 2018). However, the main limitation of genetically improved strains is strict safety requirements allowing the use of only closed cultivation units to protect the environment.
Here, we present a pioneering study that is aimed at PHB production by cyanobacteria using urban wastewater as a growth medium. The pilot-scale production of PHB was realized in the culture of the cyanobacterial strain Synechocystis sp. PCC6714 MT_a24 (generated by UV mutagenesis) in a thin-layer raceway pond using wastewater as a sole source of nutrients. The culture grew in an alkaline environment (pH 9–10) to control contamination by grazers, which is one of the main biological obstacles in mass microalgae production (Grivalský et al. 2021).
Materials and methods
Strain and culture preparation
The cyanobacterium Synechocystis sp. PCC 6714 MT_a24 (further abbreviated as Synechocystis) used in these trials was obtained from the Technical University of Vienna, Austria. This strain was generated by UV mutagenesis and recognized as a promising PHB producer (Kamravamanesh et al. 2018a; Mittermair et al. 2021). In the laboratory, the seed cultures were grown in 10-L glass bottles mixed by bubbling with air +1% CO2 (v/v) using an inorganic BG-11 medium (Allen and Stanier 1968) at 28–30 °C, pH 8. The cultures for the pilot scale experiment were grown under about 100 μmol photons m−2 s−1 of photosynthetically active radiation (PAR) provided continuously by a panel of dimmable warm-white lamps (55 W, Dulux L, Osram, Germany) which was placed behind the flasks till they reached the late exponential phase.
Pre-treatment of WW for growth tests
The centrate used in the cultivation was prepared from wastewater collected directly from the local wastewater treatment plant (WWTP) in Třeboň (Czech Republic) as described previously (Carneiro et al. 2021). To avoid cell aggregation, due to the automatic addition of flocculant in the process, the activated sludge was taken from WWTP just after secondary aerobic digestion (secondary-treated wastewater) and centrifuged at 4000 × g for 5 min to separate liquid centrate from solid sludge (similar procedure as in the WWTP). Liquid fraction–centrate (further abbreviated as WW) of brownish color was used (non-diluted) as a cultivation medium. The optical density of WW was about OD750 = 0.31. The total nitrogen and total phosphorus contents were 230–260 mg L−1 and 150–170 mg L−1, respectively. The WW was pre-treated as follows: (i) heat treatment for one hour at 100 °C; (ii) UV light (254 nm) treatment for 30 min; (iii) 50% dilution with distilled water; and (iv) untreated wastewater, processed in WWTP.
Laboratory growth tests
To determine the biotic and abiotic inhibitors before the pilot-scale cultivation, several pre-treatment methods were tested. The Synechocystis cultures were inoculated in 400-mL glass tubes using WW media pre-treated as described above (i–iv). The pH was maintained in the range of 7.5–8.0, and the cultures were grown at 28–30 °C under continuous illumination of 100 μmol photons m−2 s−1; BG-11 medium was used as a control. All trials were tested in triplicates. Chemical analysis of treated wastewater (described in the “Chemical analysis of wastewater” section; Table 1) and colony-forming units of bacterial contamination on LB plates were determined. The growth characteristics (specific growth rates and cell numbers) were assessed in a 1-week trial to determine the most suitable pre-treatment method for outdoor trial to obtain a cultivation medium with the lowest content of inhibitors.
Outdoor trials
The seed culture grown in two 10-L flasks was mixed with pre-treated WW to the final volume of 100 L. The resulting Synechocystis culture had an optical density of about OD750 = 0.41. The pilot-scale bioreactor, thin-layer raceway pond (TL-RWP) was placed in a polycarbonate greenhouse as described previously (Grivalský et al. 2019). The bioreactor had a surface area of 5 m2, a culture depth between 15 and 25 mm, and a total volume of 100 L. The trials were carried out under a diurnal regime in June 2022 in Třeboň using a flow speed of 0.2 m s−1. The maximum light intensity in the greenhouse during the day was in the range of 100–800 μmol photons m−2 s−1 and the temperature of the cultures ranged between 18 and 22 °C in the morning while at midday the values varied between 27 and 39 °C. The cultures were grown in an undiluted urban WW which was pre-treated by UV sterilization. At the beginning of the cultivation the WW was supplemented with 100 mM NaHCO3, as a carbon source as no gaseous CO2 was supplied during the trial. The cultures were grown at a pH value of 7.5 to 8.0 for three days, but once the grazers appeared (day 3), the pH was increased by 0.5 M NaOH and maintained at 10 (Supplementary Fig. S1). The evaporation was daily compensated by the addition of tap water which slightly contributed to major nutrients (20 μg L−1 of NO3− and 28 μg L−1 of PO43− at maximum (Carneiro et al. 2021; Ranglová et al. 2021). The biomass samples were harvested by centrifugation at 17,000 × g for 7 min, frozen in liquid nitrogen, and lyophilized. For analytical measurements, biomass samples were taken daily. Samples for PHB assay were taken on days 10 and 15, and afterward daily in the late stationary phase. The total period of cultivation trials took a total of 31 days.
Analytical methods
Biochemical analysis
For cell dry weight (CDW) determination, 3 mL of the culture was filtered to pre-weighed glass filters (GC-50), washed with deionized water, and these were dried at 105 °C overnight. After weighing (balance precision of ± 0.01 mg), the specific growth rate was calculated as follows:
where CDW2 and CDW1 are the final and initial cell biomass densities [g CDW L−1] at times t2 and t1 [day], respectively, measured during the exponential growth phase.
Biomass concentration was also estimated by measuring the optical density (OD750). The pigment analysis of total chlorophyll (Chl) and total carotenoid (Car) content was carried out according to Wellburn (1994). A 1-mL culture sample was centrifuged, and the pellet was dissolved in methanol to a total volume of 2 mL. The cells were mechanically disintegrated using a small amount of sea sand and measured by a high-resolution spectrophotometer (UV 2600 UV–VIS, Shimadzu, Japan). The samples were taken daily at 10:00 h in triplicate after compensation for evaporation. The pH, temperature of the culture, and ambient data (temperature and sunlight intensity) were recorded automatically using pH and dissolved oxygen sensors and a weather station (IP Warioweather, model ME 13).
Microscopy
The culture was observed daily under a microscope (Olympus BX51, Japan) with 1000 times magnification. Intracellular PHB granules were analyzed from 100 μl of Synechocystis culture stained with Nile red (5 μl). The mixture was centrifuged (10,000 × g; 1 min) and the pellet was dropped onto agar-coated microscope slides. Images were taken with a laser scanning confocal microscope (Zeiss LSM 880; Carl Zeiss Microscopy GmbH) equipped with a Plan-Apochromatic 63 × /1.4 Oil DIC M27 objective. The signal was detected by a GaAsP photomultiplier in 8-bit mode. An argon laser with a wavelength of 488 nm was used to excite the samples and their emission was detected at 517–695 nm with the detector gain set to 800.
Chemical analysis of wastewater
The chemical analysis of WWs and the culture were performed by a commercial company (Povodí Vltavy Ltd. České Budějovice, Czech Republic) using certified methods for water management. Analyses of BOD (biological oxygen demand), COD (chemical oxygen demand), TOC (total organic carbon), ammonium nitrogen (NH4-N), nitrate concentration (NO3-N), nitrite concentration (NO2-N), and orthophosphate-phosphorus (PO4-P) were performed after centrifugation (12,000 × g; 7 min) and filtering (pore size of 0.45 μm) of the supernatant. Total phosphorus (TP) and total nitrogen content (TN) were analyzed from non-processed samples. All samples were stored at − 20 °C before analysis.
PHB determination
The determination of PHB content in the biomass was performed after acidophilic hydrolysis using a modified method of Karr et al. (1983). Biomass samples were centrifuged (9000 × g; 7 min), frozen, and lyophilized. The dried pellet was weighed and dissolved in 1 mL of concentrated sulfuric acid. The suspension was incubated for 1 h at 100 °C. During this step, the PHB is converted to trans-crotonic acid. The samples were cooled down and diluted 20 times using 0.014 M of sulfuric acid. The supernatant obtained after centrifugation (12,000 × g; 15 min) was measured by HPLC (Agilent 1100, column; Watrex 300 × 8 mm Polymer IEX H form). Commercially available PHB (Sigma-Aldrich) was used as a standard.
Photosynthesis measurements
Photosynthesis measurements can be used as a fast and reliable indicator of culture performance and can indicate growth constraints conditions (Masojídek et al. 2021). The photosynthetic activity of the culture was monitored ex situ in culture samples using a chlorophyll fluorimeter (PAM 101–103, H.Walz, Germany). The maximum photochemical efficiency of PSII (Fv/Fm) was measured as previously described (Ranglová et al. 2019; Masojídek et al. 2021; Grivalský et al. 2022) in the presence of 10−5 M 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) that promotes PSII centers closure. The samples for photosynthesis measurements were dark adapted for 15 min before the measurements. Dissolved oxygen (DO) concentrations were recorded automatically by oximeter and the values were estimated in % of saturation (%sat).
Detection of contamination
The sample of infected culture was centrifuged (14,000 × g, 1 min), and washed with distilled water. The flagellated predator microorganism was identified by the amplification of 18S rRNA using the universal eukaryotic primers: the forward primer 18S euk Fw (GTCAGAGGTGAAATTCTTGGATTTA) and the reverse primer 18S euk Rv (AGGGCAGGGACGTAATCAACG) (Rasoul-Amini et al. 2009). The genomic DNA was extracted by commercial kit NucleoSpin Plant II (Macherey-Nagel, Germany) following the instructions of the manufacturer. The PCR reaction mix included 0.4 μM of each primer, 200 μM dNTP, 1.25 U DreamTaq DNA Polymerase (Thermo Fisher Scientific, USA), 1 × PCR buffer, and 100 ng of template DNA in a total reaction volume of 25 μL. The temperature program consisted of an initial denaturation at 95 °C for 3 min, 30 cycles (95 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min), and a final polymerization at 72 °C for 10 min. The PCR product was purified by NucleoSpin Gel and PCR Clean-up (Macherey-Nagel, Germany) according to the enclosed protocol. The sample was sequenced by a commercial facility (Eurofins Genomics, Germany), and the resulting sequence was directly compared with GenBank using BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The obtained sequence was deposited in GenBank under the accession number OQ569478.
Statistical analysis
All measurements were carried out in triplicates. Data are shown as an average of triplicates, and error bars represent analytical standard deviation. GraphPad Prism 5 was used to determine significant differences.
Results
Laboratory experiments
During 9-day laboratory experiments the cultures of Synechocystis were grown in urban WW pre-treated in several ways—heat treatment, UV treatment, 50% dilution with distilled water in comparison with untreated wastewater and the WW fully processed in WWTP to estimate biotic and abiotic effects of urban wastewater. According to the analysis (Table 1), ammonium (NH4-N) was the main source of nitrogen in WW (170–180 mg L−1), while nitrate (NO−3) was the main source of nitrogen in the BG-11 medium (250 mg L−1). The concentration of phosphate in the WW was much higher (150 ± 10 mg L−1) compared to the BG-11 medium (7 mg L−1). Two pre-treatment methods, heat and UV exposures, showed no significant changes in the chemical composition of WW.
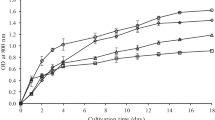
The biomass production in WW regularly processed in WWTP (abbreviated as CWW) was very low (Fig. 1A) due to low nutrient (N and P) content. The biomass density in the culture grown in twice diluted WW increased in the first 3 days of the trial and then dropped down. Thus, the cultivation in CWW and twice diluted WW was considered as unsuitable for further experiments. Cultures treated by heat, UV, and those in untreated WW grew well (Fig. 1). The specific growth rate measured for heat treated (μ = 0.42 ± 0.08 day−1), UV-treated (μ = 0.41 ± 0.07 day−1), and untreated (μ = 0.46 ± 0.01 day−1) WW as well as for BG-11 medium (μ = 0.44 ± 0.07 day−1) were rather similar during the first 3 days. Then, the cultures grown in WW regardless of pre-treatment reached the stationary phase but in the culture grown in the BG-11 medium it happened on day 5. The longer time of growth phase was caused by the optimal content of nutrients in the BG-11 medium. At the end of the experiment, the biomass content in UV treated (CDW = 1.41 g L−1) and untreated (CDW = 1.46 g L−1) WW was similar; while in heat-treated samples was 20–22% lower (CDW = 1.14 g L−1). The final biomass content in the BG-11 medium was about twice higher (CDW = 3.17 g L−1) as compared to the WW-grown cultures. The measurements of optical density and cell number showed similar trends (Fig. 1B and C).
Growth characteristics of Synechocystis sp. in urban wastewater (WW) after various pre-treatments: untreated WW; heat treated (HWW); UV-treated (UV WW); twice diluted WW; processed WW from WWTP (CWW) in comparison with control – BG-11 medium. A Biomass density calculated as an increase of cell dry weight (CDW); B optical density measured at OD750; C cell numbers. Each point represents a mean of three independent biological replicates and error bars represent the analytical standard deviation
The variously treated WWs differ in the presence of bacteria (Table 2). The number of bacteria measured in untreated WW was 18,000 ± 1000 CFU per mL. UV treatment was the most efficient to eliminate bacteria as no colony-forming units were found in tests on LB agar plates. Heat treatment reduced the bacterial CFU significantly to 230 ± 30 CFU per mL.
Outdoor trials
Growth characteristics and PHB accumulation
Based on the laboratory tests the Synechocystis was cultured on a pilot scale to test the possibility of PHB production. The cultures were grown in an outdoor thin-layer raceway pond (TL-RWP) filled with undiluted urban WW which was pre-treated by UV sterilization (Fig. 2). During the first 2 days, an acclimation phase to the outdoor conditions was observed. This was caused by a usual response of the culture when it is transferred from the laboratory to outdoor high-light conditions and a shift to the diurnal cycle (Ranglová et al. 2021; Grivalský et al. 2022). To characterize the growth of Synechocystis in the urban WW, physiological variables of the culture were monitored (temperature, irradiance, pH) (Supplementary Fig. S1). In the outdoor trial, the growth rate during the exponential phase was μ = 0.49 ± 0.06 day−1. On day 3, the grazers were observed (Supplementary Fig. S2), and the pH was increased to about 10 until the end of the trial. It is important to note that biomass productivity was not significantly affected by the pH increase (Fig. 3).
In the late stationary phase, the chlorosis phenomenon was accompanied by specific color changes (Fig. 2B) and odor. After 2 weeks of cultivation, the outdoor culture reached the stationary phase with the biomass concentration of 3.37 g L−1 (Fig. 3). To evaluate the PHB production in the stationary phase, the PHB content was measured on days 10 and 15, and afterward daily in the late stationary phase, when chlorosis occurred. The PHB content was 4.8 ± 0.99% PHB per CDW on day 10 and it was doubled (10.2 ± 1.4% PHB per CDW) on day 15. In the late stationary phase, the PHB content per CDW increased corresponding to 0.62 g L−1. On the last day 31 when the culture was harvested, the content of PHB was 22.7 ± 2.5% PHB per CDW. Semi-quantitative analysis of PHB granules was conducted in the cells during the late stationary phase on day 29, following Nile red staining, as illustrated in Supplementary Figure S3, in which the cell heterogeneity of PHB producers is also visible.
Pigment analysis
The pigment composition in the biomass was determined throughout the experiment. Chlorophyll (Chl) content was about 0.5% per CDW during the first 10 days of the trial except days 3 and 4 when the presence of grazers probably caused pigment degradation. During the first 10 days of the trial, the carotenoid content doubled from 0.15 to 0.32% per CDW (Fig. 4). In the latter phase, the chlorophyll and carotenoid content decreased; however, the ratio of Car:Chl increased. This stage, also called chlorosis, was characterized by turning the culture to orange-brown (Fig. 2B). On the day 30 before harvesting, the contents of both chlorophyll and carotenoids were low, at about 0.2% per CDW.
Nutrient removal
The total nitrogen content (TN) (mainly in the form of ammonium) of 220 mg L−1 present in urban WW was comparable to nitrogen content in the BG-11 medium (Table 1). The removal rates of TN and TP achieved in the trial were 71.8% and 67.4%, respectively (Fig. 5). At the end of the trial, the content of residual ammonia (N-NH4) in filtered supernatant was 45 mg L−1, and the content of orthophosphate (PO4-P) was 19 mg L−1.
Photosynthesis performance
Photosynthetic performance was assessed from daily measurements to follow the physiological state of the culture as the maximum photochemical yield of photosystem II, Fv/Fm. The samples were measured daily at 11:00 h (Fig. 6A). The low initial value (0.11) was probably caused by the exposure of the laboratory culture in the outdoor TL-RWP with high solar irradiance. The Fv/Fm values rose during the growth up to day 9 (the maximum value was 0.55), with small perturbations that appeared during the presence of grazers on days 3 and 4. Starting on day 10, the Fv/Fm values started to decrease as nutrients were consumed which was accompanied by steadily increased PHB content. In the late stationary phase, Fv/Fm was decreased as low as 0.06 on day 28 while the basal fluorescence F0 increased during the cultivation reaching its maximum on day 21 (Fig. 6B).
The dissolved oxygen (DO) build-up via photosynthetic activity reached a maximum value on day 6 (245%sat) in the exponential growth phase (Supplementary Fig. S4). Even with a high level of saturation, this value still does not substantially affect the photochemical efficiency as the gas exchange in open systems is rather efficient (Masojídek et al. 2021). Low values of saturation obtained during days 3 and 4 were caused by the presence of grazers. The DO concentration also depends on solar irradiance (Ranglová et al. 2021). Maximum DO concentration during the stationary phase was around 150%sat during sunny days. In the late stationary phase, DO decreased due to low photosynthetic activity during the nutrient limitation and PHB production process, when CO2 is hardly fixed (Troschl et al. 2018).
Contamination monitoring
The presence of contaminants was monitored by microscopical observation, and the species which negatively affected biomass production were identified by 18S rRNA sequencing analysis based on the highest similarity score (100% identity). The presence of grazers was observed on day 3 during the outdoor cultivation trial as shown in Supplementary Fig. S2. The major invasive species was identified by sequencing of 18 s rRNA based on the highest similarity score (100% identity) as the flagellate Poterioochromonas malhamensis which is a common predator of microalgae cultures (Touloupakis et al. 2016; Ma et al. 2018). The increase of the pH value to 10 initiated its rapid loss of motility and subsequent cell lysis within hours which caused a slight and acceptable loss in cyanobacterial biomass productivity (Touloupakis et al. 2016).
Discussion
Our previous pilot-scale studies showed that urban wastewater is a suitable source of nutrients for the cultivation of microalgae (Carneiro et al. 2021; Ranglová et al. 2021; Grivalský et al. 2022). Since the main disadvantage of PHB production by microorganisms is the cost, we tested whether microalgae cultures can also produce PHB in an outdoor culture unit using urban wastewater. Outdoor cultivation in open units which are directly exposed to the environment can be invaded by other microorganisms. The elimination of contaminants is thus rather difficult. The selection of a suitable microalgae strain is desirable for outdoor cultivations that can grow fast or under specific conditions (high pH, salinity) may reduce and resist contamination. It is crucial to know the appropriate cultivation conditions and ways how to eliminate invaders such as other microalgae, grazers, or other microorganisms that may compete for nutrients or destroy the culture (Carney and Lane 2014; Di Caprio 2020). The other crucial point is whether the selected strain can produce PHB.
In these trials, the randomly mutated Synechocystis strain MT_a24 is shown to produce 23% PHB per CDW during nutrient-limited conditions on a pilot scale in open ponds which suggests its potential for industrial production. Recently, it was also shown that the randomly mutated MT_a24 strain produced 37% PHB per CDW in a nutrient-limited growth medium (Kamravamanesh et al. 2018a). Laboratory experiments also showed no negative effect of untreated WW as the bacterial microbiome did not significantly influence growth (Di Caprio 2020). The UV pre-treatment of wastewater was used to estimate pathogen-related problems (Lee et al. 2022).
Bacterial microflora may exert both positive and negative influences on PHB content within the biomass due to the capacity of certain bacterial species to accumulate PHB under conditions characterized by an excess of carbon sources and limited levels of nitrogen and phosphorus, which aligns with the conditions prevailing during our trials (Verlinden et al. 2007). However, the microbial analyses were not conducted during the outdoor trial, and therefore, PHB content was calculated based on the total dry biomass. The outdoor trials in this study showed that the UV-treatment is effective to eliminate the presence of bacteria even in open, pilot-scale bioreactors. This pre-treatment was evaluated as a cheap, fast, and reliable way to reduce microbial contaminations in outdoor bioreactors which obstruct growth. It helps the success of the cultivation of the selected strains although it cannot prevent the invasion of microorganisms during growth (Carney and Lane 2014). Especially at the beginning of the cultivation when the cells require a certain time to adapt to outdoor conditions. During the acclimation period, the cultures are more sensitive to constraints, such as high sunlight (Lakatos et al. 2021) which was also observed during the first 2 days in the presented outdoor trial (Fig. 3).
Furthermore, the secondary treatment process—the high, alkaline pH—was used in the presented outdoor trials as an effective strategy for controlling contamination of Synechocystis cultures as described previously (Touloupakis et al. 2016). It is important to note that the high pH treatment had no significant effect on growth and the outdoor cultures revealed a higher growth rate (μ = 0.49 ± 0.06 day−1) compared to the laboratory trials (μ = 0.42 ± 0.008 day−1). The photosynthetic activity estimated as the photochemical yield Fv/Fm was also unaffected by the increased pH on day 4 as revealed by the present experiments (Fig. 4). This result correlates with earlier studies as no changes in PSII abundance were observed between neutral and alkaline pH of the culture media (Summerfield and Sherman 2008; Touloupakis et al. 2016). In the dormant stage when the cells show only residual photosynthetic activity, the main light-harvesting pigments are usually degraded and protective carotenoids are strongly accumulated (Klotz et al. 2016; Valev et al. 2020).
The correlation can be found between the decline of the photosynthetic activity (Fv/Fm) and nutrient content which indicated/induced the PHB production (Fig. 3), (Kaewbai-ngam et al. 2016; Koch et al. 2020b; Troschl et al. 2017). The phenomenon of PHB production as an alternative source of carbon storage during nutrient deficiency is also accompanied by several changes in cell physiology such as the increase in carotenoid content which was probably induced by higher outdoor irradiance in the greenhouse as these act as photoprotective pigments (Takaichi and Mochimaru 2007). As reported in several studies, the Synechocystis cells adapt to high light (HL) conditions by the enhanced accumulation of carotenoids (Masamoto and Furukawa 1997; Schagerl and Müller 2006; Wang et al. 2010). In addition, the positive effect of alkaline pH (pH = 9.0) on carotenoid production was also referred (Pagels et al. 2021). Another change achieved during the PHB production was a significant decrease of the F0 values due to the degradation of the photosystem proteins during the ripening stage which may be connected to the fact that it results from overlapping PSII Chl a and phycobilisome (PBS) fluorescence (Lakatos et al. 2021; Troschl et al. 2018). We consider that the values of F0 may be used as an early indicator of PHB production (Fig. 6B).
The highest PHB production was achieved in the late stationary phase under nutrient depletion which was also described in non-diazotrophic cyanobacteria (Klotz et al. 2016). Several studies also reported PHB production by cyanobacterial strains and summarized the current status of PHB production by cyanobacteria using different types of strategies (Kamravamanesh et al. 2018b; Troschl et al. 2017; Yashavanth et al. 2021). Potential for high PHB production in the Synechocystis sp. MT_a24 culture was reported in a laboratory experiment and a 40-L outdoor photobioreactor (PBR) under N and P deprivations; however, the applicability of the strain culturing in wastewater was not tested (Kamravamanesh et al. 2018a, 2019). Recent studies aimed at PHB production in mass cultivation of cyanobacterial strains (Synechocystis, Arthrospira, Nostoc) are summarized in Table 3. None of the Synechocystis strains has reached a PHB content as high as in this study, even when optimized growth media were used. The highest content of 26.8 ± 0.9% PHB per CDW was reported in Nostoc muscorum (Bhati and Mallick 2015) while the highest cell dry weight of 3.23 g L−1 was achieved in pilot-scale cultivation of the strain Synechocystis sp. PCC 6714 which was also used in this study (Kamravamanesh et al. 2019). Despite the use of an optimized growth medium, only 11.3% PHB per CDW was achieved after 10 days which corresponds to 0.365 g L−1. The explanation for lower PHB production is probably the type of used cultivation unit. Nevertheless, the highest PHB content per CDW (63% in a phototrophic regime without additional carbon source) was found in a genetically improved strain of Synechocystis (Koch et al. 2020b); however, GMO strains have restricted use in open units.
Cyanobacteria are capable to take up various substances such as ammonium, nitrate, phosphate, and also metal ions such as cobalt, copper, chromium, and zinc during their growth in wastewater (Trentin et al. 2019). When producing PHB in cyanobacterial cells, its accumulation occurs under nutrient limitation in the later stage of growth (Troschl et al. 2018). Nitrogen was described as the main limiting factor inducing PHB production in Synechocystis cells; however, phosphorus and sulfate partial limitations also support PHB production.
During the trial, two processes of nitrogen removal from WW were examined: (i) nitrogen consumption by cyanobacteria observed as the biomass increase and (ii) ammonia stripping—conversion of ammonium ions to ammonia gas which was enhanced by the rise of pH level as described by Mohammed-Nour et al. (2019). While the primary nutrients (N and P) were largely removed, the results indicated the presence of organic compounds that remained inaccessible to cyanobacteria. Similar observations have been reported in prior studies (Hu et al. 2012; Yao et al. 2015). PHB production typically occurs when nitrogen is completely depleted (Troschl et al. 2018). Therefore, in this trial, it is likely that non-usable nitrogen residues either minimally or not at all influenced PHB accumulation in the culture. Microscopy (Supplementary Fig. S3) revealed distinctive aspect of cell heterogeneity in terms of PHB content in nitrogen-starved cells, consistent with the findings described by Koch et al. (2020a). High nutrient removal efficiency was also observed in Synechocystis salina during pilot scale cultivation with the final N and P content under the limit of detection when using a dig estate fraction (dilution 1:3) (Meixner et al. 2016). A 66% removal of ammonia (initial content of 600 mg L−1) and 96% removal of phosphorus (initial content of 90 mg L−1) was reported in the culture of Synechocystis sp. PCC 6803 (Trentin et al. 2019). A comparison of the results in this study with the data published elsewhere shows that removal efficiency is influenced not only by the initial concentration of nutrients but also by the N:P ratio. The optimal value is 1:3 for good nutrient-removal capacity and high biomass productivity of microalgae (Yao et al. 2015). As the P:N ratio in urban wastewater is very high, cyanobacteria are not able to reach a phase of phosphorus limitation. This may be one of the factors of lower PHB production (23% per CDW) compared to the previous findings (37% PHB per CDW) when the cultivation was performed with the same strain in the N-P depleted media (Kamravamanesh et al. 2018a).
Data availability
The nucleotide sequence was deposited into the GeneBank database under accession number OQ569478. The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Allen MM, Stanier RY (1968) Simple conditions for growth of unicellular blue-green algae. J Gen Microbiol 51:199–202. https://doi.org/10.1099/00221287-51-2-199
Bhati R, Mallick N (2015) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: process optimization and polymer characterization. Algal Res 7:78–85. https://doi.org/10.1016/j.algal.2014.12.003
Bhati R, Mallick N (2016) Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: a sustainable approach. J Appl Phycol 28:161–168. https://doi.org/10.1007/s10811-015-0573-x
Carneiro M, Ranglová K, Lakatos GE, Câmara Manoel JA, Grivalský T, Kozhan DM, Toribio A, Moreno J, Otero A, Varela J, Malcata FX, Suárez Estrella F, Acién-Fernándéz FG, Molnár Z, Ördög V, Masojídek J (2021) Growth and bioactivity of two chlorophyte (Chlorella and Scenedesmus) strains co-cultured outdoors in two different thin-layer units using municipal wastewater as a nutrient source. Algal Res 56:2–9. https://doi.org/10.1016/j.algal.2021.102299
Carney LT, Lane TW (2014) Parasites in algae mass culture. Front Microbiol 5:278. https://doi.org/10.3389/fmicb.2014.00278
Di Caprio F (2020) Methods to quantify biological contaminants in microalgae cultures. Algal Res 49:101943. https://doi.org/10.1016/j.algal.2020.101943
Drosg B, Fritz I, Gattermayr F, Silvestrini L (2015) Photo-autotrophic production of poly(hydroxyalkanoates) in cyanobacteria. Chem Biochem Eng Q 29:145–156. https://doi.org/10.15255/CABEQ.2014.2254
Forchhammer K, Schwarz R (2019) Nitrogen chlorosis in unicellular cyanobacteria – a developmental program for surviving nitrogen deprivation. Environ Microbiol 21:1173–1184. https://doi.org/10.1111/1462-2920.14447
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9:1–9. https://doi.org/10.1186/s13104-016-2321-y
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made - Supplementary Information. Sci Adv 3:19–24. https://doi.org/10.1126/sciadv.1700782
Grivalský T, Ranglová K, da Câmara JAM, Lakatos GE, Lhotský R, Masojídek J (2019) Development of thin-layer cascades for microalgae cultivation: milestones (review). Folia Microbiol 64:603–614. https://doi.org/10.1007/s12223-019-00739-7
Grivalský T, Ranglová K, Lakatos GE, Manoel JAC, Černá T, Barceló-Villalobos M, Estrella FS, Ördög V, Masojídek J (2022) Bioactivity assessment, micropollutant and nutrient removal ability of Tetradesmus obliquus cultivated outdoors in centrate from urban wastewater. J Appl Phycol 34:2955–2970. https://doi.org/10.1007/s10811-022-02828-6
Grivalský T, Střížek A, Přibyl P, Lukavský J, Čegan R, Hobza R, Hrouzek P (2021) Comparison of various approaches to detect algal culture contamination: a case study of Chlorella sp. contamination in a Phaeodactylum tricornutum culture. Appl Microbiol Biotechnol 105:5189–5200. https://doi.org/10.1007/s00253-021-11396-7
Hu B, Min M, Zhou W, Du Z, Mohr M, Chen P, Zhu J, Cheng Y, Liu Y, Ruan R (2012) Enhanced mixotrophic growth of microalga Chlorella sp. on pretreated swine manure for simultaneous biofuel feedstock production and nutrient removal. Bioresour Technol 126:71–79. https://doi.org/10.1016/j.biortech.2012.09.031
Kaewbai-ngam A, Incharoensakdi A, Monshupanee T (2016) Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour Technol 212:342–347. https://doi.org/10.1016/j.biortech.2016.04.035
Kamravamanesh D, Kiesenhofer D, Fluch S, Lackner M, Herwig C (2019) Scale-up challenges and requirement of technology-transfer for cyanobacterial poly (3-hydroxybutyrate) production in industrial scale. Int J Biobased Plast 1:60–71. https://doi.org/10.1080/24759651.2019.1688604
Kamravamanesh D, Kovacs T, Pflügl S, Druzhinina I, Kroll P, Lackner M, Herwig C (2018a) Increased poly-Β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: mutant generation and characterization. Bioresour Technol 266:34–44. https://doi.org/10.1016/j.biortech.2018.06.057
Kamravamanesh D, Lackner M, Herwig C (2018b) Bioprocess engineering aspects of sustainable polyhydroxyalkanoate production in cyanobacteria. Bioengineering 5:1–18. https://doi.org/10.3390/BIOENGINEERING5040111
Karr DB, Waters JK, Emerich DW (1983) Analysis of poly-, 3-hydroxybutyrate in Rhizobium japonicum bacteroids by ion-exclusion high-pressure liquid chromatography and UV detectiont. Appl Environ Microbiol 46:1339–1344
Kavitha G, Kurinjimalar C, Sivakumar K, Aravind R, Shree CG, Arthi K, Palani P, Kaviyarasan V, Rengasamy R (2016) Mass cultivation of UV-B adapted Arthrospira platensis RRGK under open raceway pond for the production of Poly-β-hydroxy butyrate. Int J Biol Macromol 93:1304–1316. https://doi.org/10.1016/j.ijbiomac.2016.09.105
Klotz A, Georg J, Bučinská L, Watanabe S, Reimann V, Januszewski W, Sobotka R, Jendrossek D, Hess WR, Forchhammer K (2016) Awakening of a dormant cyanobacterium from nitrogen chlorosis reveals a genetically determined program. Curr Biol 26:2862–2872. https://doi.org/10.1016/j.cub.2016.08.054
Koch M, Berendzen KW, Forchhammer K (2020a) On the role and production of polyhydroxybutyrate (PHB) in the cyanobacterium Synechocystis sp. PCC 6803. Life 10:47
Koch M, Bruckmoser J, Scholl J, Hauf W, Rieger B, Forchhammer K (2020b) Maximizing PHB content in Synechocystis sp. PCC 2 6803: development of a new photosynthetic 3 overproduction strain. bioRxiv 2020:10.22.350660
Koch M, Doello S, Gutekunst K, Forchhammer K (2019) PHB is produced from glycogen turn-over during nitrogen starvation in Synechocystis sp. PCC 6803. Int J Mol Sci 20:1942. https://doi.org/10.3390/ijms20081942
Lakatos GE, Ranglová K, Câmara Manoel J, Grivalský T, Masojídek J (2021) Photosynthetic monitoring techniques indicate maximum glycogen accumulation in nitrogen-limited Synechocystis sp. PCC 6803 culture. Algal Res 55: 102271. https://doi.org/10.1016/j.algal.2021.102271
Lapointe A, Spiteller D, Kroth G (2022) High throughput method for extracting polyphosphates from diatoms. Endocytobiosis Cell Res 31:29–38
Lee S-A, Kim M, Kim H-S, Ahn C-Y (2022) Extra benefit of microalgae in raw piggery wastewater treatment: pathogen reduction. Microbiome 10:1–14. https://doi.org/10.1186/s40168-022-01339-3
Ma M, Gong Y, Hu Q (2018) Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res 29:142–153. https://doi.org/10.1016/j.algal.2017.11.024
Masamoto K, Furukawa KI (1997) Accumulation of zeaxanthin in cells of the cyanobacterium, Synechococcus sp. Strain PCC 7942 grown under high irradiance. J Plant Physiol 151:257–261. https://doi.org/10.1016/S0176-1617(97)80250-7
Masojídek J, Ranglová K, Lakatos GE, Benavides AMS, Torzillo G (2021) Variables governing photosynthesis and growth in microalgae mass cultures. Processes 9:820. https://doi.org/10.3390/pr9050820
McAdam B, Brennan FM, Mcdonald P, Mojicevic M (2020) Production of polyhydroxybutyrate (PHB) and factors impacting its chemical andmechanical characteristics. Polymers 12:2908
Meixner K, Fritz I, Daffert C, Markl K, Fuchs W, Drosg B (2016) Processing recommendations for using low-solids digestate as nutrient solution for poly-ß-hydroxybutyrate production with Synechocystis salina. J Biotechnol 240:61–67. https://doi.org/10.1016/j.jbiotec.2016.10.023
Mittermair S, Richter J, Doppler P, Trenzinger K, Nicoletti C, Forsich C, Spadiut O, Herwig C, Lackner M (2021) Impact of exoD gene knockout on the polyhydroxybutyrate overaccumulating mutant Mt_a24. Int J Biobased Plast 3:1–18. https://doi.org/10.1080/24759651.2020.1863020
Mohammed-Nour A, Al-Sewailem M, El-Naggar AH (2019) The influence of alkalization and temperature on ammonia recovery from cow manure and the chemical properties of the effluents. Sustain 11:2441. https://doi.org/10.3390/su11082441
Nishioka M, Nakai K, Miyake M, Asada Y, Taya M (2001) Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol Lett 23:1095–1099. https://doi.org/10.1023/A:1010551614648
Pagels F, Vasconcelos V, Guedes AC (2021) Carotenoids from cyanobacteria: biotechnological potential and optimization strategies. Biomolecules 11:1–21. https://doi.org/10.3390/biom11050735
Rai PK, Lee J, Brown RJC, Kim KH (2021) Micro- and nano-plastic pollution: Behavior, microbial ecology, and remediation technologies. J Clean Prod 291:125240. https://doi.org/10.1016/j.jclepro.2020.125240
Ranglová K, Lakatos GE, Câmara Manoel JA, Grivalský T, Suárez Estrella F, Acién Fernández FG, Molnár Z, Ördög V, Masojídek J (2021) Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res 53:102136. https://doi.org/10.1016/j.algal.2020.102136
Ranglová K, Lakatos GE, Manoel JAC, Grivalský T, Masojídek J (2019) Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol (Praha) 64:615–625. https://doi.org/10.1007/s12223-019-00738-8
Rasoul-Amini S, Ghasemi Y, Morowvat MH, Mohagheghzadeh A (2009) PCR amplification of 18S rRNA, single cell protein production and fatty acid evaluation of some naturally isolated microalgae. Food Chem 116:129–136. https://doi.org/10.1016/j.foodchem.2009.02.025
Rueda E, García-Galán MJ, Díez-Montero R, Vila J, Grifoll M, García J (2020) Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour Technol 295:122233. https://doi.org/10.1016/j.biortech.2019.122233
Schagerl M, Müller B (2006) Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. J Plant Physiol 163:709–716. https://doi.org/10.1016/j.jplph.2005.09.015
Summerfield TC, Sherman LA (2008) Global transcriptional response of the alkali-tolerant cyanobacterium Synechocystis sp. Strain PCC 6803 to a pH 10 environment. Appl Environ Microbiol 74:5276–5284. https://doi.org/10.1128/AEM.00883-08
Takaichi S, Mochimaru M (2007) Carotenoids and carotenogenesis in cyanobacteria: unique ketocarotenoids and carotenoid glycosides. Cell Mol Life Sci 64:2607–2619. https://doi.org/10.1007/s00018-007-7190-z
Touloupakis E, Cicchi B, Benavides AMS, Torzillo G (2016) Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl Microbiol Biotechnol 100:1333–1341. https://doi.org/10.1007/s00253-015-7024-0
Trentin G, Bertucco A, Sforza E (2019) Mixotrophy in Synechocystis sp. for the treatment of wastewater with high nutrient content: effect of CO2 and light. Bioprocess Biosyst Eng 42:1661–1669. https://doi.org/10.1007/s00449-019-02162-1
Troschl C, Meixner K, Drosg B (2017) Cyanobacterial PHA production—review of recent advances and a summary of three years’ working experience running a pilot plant. Bioengineering 4:26. https://doi.org/10.3390/bioengineering4020026
Troschl C, Meixner K, Fritz I, Leitner K, Romero AP, Kovalcik A, Sedlacek P, Drosg B (2018) Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal Res 34:116–125. https://doi.org/10.1016/j.algal.2018.07.011
Valev D, Kurkela J, Tyystjärvi E, Tyystjärvi T (2020) Testing the potential of regulatory sigma factor mutants for wastewater purification or bioreactor run in high light. Curr Microbiol 77:1590–1599. https://doi.org/10.1007/s00284-020-01973-w
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449
Wang Q, Hall CL, Al-Adami MZ, He Q (2010) IsiA is required for the formation of photosystem I supercomplexes and for efficient state transition in Synechocystis PCC 6803. PLoS ONE 5:e10432. https://doi.org/10.1371/journal.pone.0010432
Wellburn AR (1994) The Spectral Determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Yao L, Shi J, Miao X (2015) Mixed wastewater coupled with CO2 for microalgae culturing and nutrient removal. PLoS ONE 10:1–16. https://doi.org/10.1371/journal.pone.0139117
Yashavanth PR, Das M, Maiti SK (2021) Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J Environ Chem Eng 9:105379. https://doi.org/10.1016/j.jece.2021.105379
Zerrouki D, Henni A (2019) Outdoor microalgae cultivation for wastewater treatment. Appl Microalgae Wastewater Treat 2019:81–99. https://doi.org/10.1007/978-3-030-13913-1_5
Acknowledgements
The authors thank Ms. Soňa Pekařová and Mr. Michal Bureš, for technical assistance during experiments and Mr. Miroslav Kajan for consultation on wastewater use.
Funding
This research was supported by the Program INTERREG V-A Austria—Czech Republic 2014–2020 (project Plastocyan ATCZ260).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. TG, AZ, OS, and JM conceived and designed the research. TG, GEL, KŠ, JACM, RB. PD, JK, RK, and KT conducted experiments and analyzed the data. The first draft of the manuscript was written by TG, and all authors commented on the manuscript. JM revised and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors read and approved the manuscript.
Consent for publication
The authors give consent to the publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grivalský, T., Lakatos, G.E., Štěrbová, K. et al. Poly-β-hydroxybutyrate production by Synechocystis MT_a24 in a raceway pond using urban wastewater. Appl Microbiol Biotechnol 108, 44 (2024). https://doi.org/10.1007/s00253-023-12924-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12924-3