Abstract
The microalga Chlamydopodium fusiforme MACC-430 was cultured in two types of outdoor pilot cultivation units—a thin-layer cascade (TLC) and a raceway pond (RWP) placed in a greenhouse. This case study aimed to test their potential suitability for cultivation scale-up to produce biomass for agriculture purposes (e.g., as biofertilizer or biostimulant). The culture response to the alteration of environmental conditions was evaluated in “exemplary” situations of good and bad weather conditions using several photosynthesis measuring techniques, namely oxygen production, and chlorophyll (Chl) fluorescence. Validation of their suitability for online monitoring in large-scale plants has been one of the objectives of the trials. Both techniques were found fast and robust reliable to monitor microalgae activity in large-scale cultivation units. In both bioreactors, Chlamydopodium cultures grew well in the semi-continuous regime using daily dilution (0.20—0.25 day−1). The biomass productivity calculated per volume was significantly (about 5 times) higher in the RWPs compared to the TLCs. The measured photosynthesis variables showed that the build-up of dissolved oxygen concentration in the TLC was higher, up to 125–150% of saturation (%sat) as compared to the RWP (102–104%sat). As only ambient CO2 was available, its shortage was indicated by a pH increase due to photosynthetic activity in the thin-layer bioreactor at higher irradiance intensities. In this setup, the RWP was considered more suitable for scale-up due to higher areal productivity, lower construction and maintenance costs, the smaller land area required to maintain large culture volumes, as well as lower carbon depletion and dissolved oxygen build-up.
Key points
• Chlamydopodium was grown in both raceways and thin-layer cascades in pilot-scale.
• Various photosynthesis techniques were validated for growth monitoring.
• In general, raceway ponds were evaluated as more suitable for cultivation scale-up.
Graphical Abstract










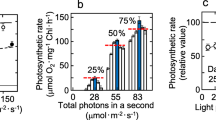
Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Acién FG, Molina E, Reis A, Torzillo G, Zittelli G, Sepúlveda J, Masojídek J (2017) Photobioreactors for the production of microalgae. In: Gonzalez-Fernandez C, Muñoz R (eds) Microalgae-based biofuels and bioproducts. Elsevier-Woodland Publishing, Duxford, U.K, From feedstock cultivation to end-products, pp 1–44
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30:1344–1353
Andersen RA (2005) Algal Culturing Techniques. Appendix – Recipes for freshwater and seawater media. Elsevier Academic Press, Amsterdam, The Netherlands, pp 429-532
Arbib, Z, Maín D, Cano R, Saúco C, Fernandez M, Lata E, Rogalla F (2022) Large-scale demonstration of microalgae-based wastewater biorefineries. In: Demirer GN, Uludag-Demirer S (eds) Integrated wastewater management and valorization using algal cultures. Elsevier, Amsterdam, the Netherlands, pp 215-234
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotech 70:313–321
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Berlin/Heidelberg, Germany, pp 601–652
Borowitzka MA, Vonshak A (2017) Scaling up microalgal cultures to commercial scale. Eur J Phycol 52:407–418
Carneiro M, Ranglová K, Lakatos GE, Câmara Manoel JA, Grivalský T, Kozhan DM, Toribio A, Moreno J, Otero A, Varela J, Xavier Malcata F, Suárez Estrella F, Acién-Fernándéz FG, Molnár Z, Ördög V, Masojídek J (2021) Growth and bioactivity of two chlorophyte (Scenedesmus and Chlorella) strains co-cultured outdoors in two different thin-layer units using municipal wastewater as a nutrient source. Algal Res 56:102299
Chaudry S (2021) Integrating microalgae cultivation with wastewater treatment: a peek into economics. Appl Biochem Biotechnol 193:3395–3406
Enríquez S, Borowitzka MA (2010) The use of the fluorescence signal in studies of seagrasses and macroalgae. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, The Netherlands, pp 187–208
Figueroa FL, Jerez C, Korbee N (2013) Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat Am Aquatic Res 41:801–881
García-González J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthesis electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Grivalský T, Ranglová K, Câmara Manoel JA, Lakatos GE, Lhotský R, Masojídek J (2019) Development of thin-layer cascades for microalgae cultivation: milestones (review). Folia Microbiol 64:603–614
Grivalský T, Ranglová K, Lakatos GE, Câmara Manoel JA, Černá T, Barceló-Villalobos M, Suárez Estrella F, Molnár Z, Ördög V, Masojídek J (2022) Bioactivity assessment, micropollutant and nutrient removal ability of Tetradesmus obliquus cultivated outdoors in centrate from municipal wastewater. J Appl Phycol 32:2955–2970
Havlik I, Lindner P, Scheper T, Reardon KF (2013) On-line monitoring of large cultivations of microalgae and cyanobacteria. Trends Biotechnol 31:406–414
Hofstraat JW, Peeters JC, Snel JFH, Geel C (1994) Simple determination of photosynthetic efficiency and photoinhibition of Dunaliella tertiolecta by saturating pulse measurements. Mar Ecol Prog Ser 103:187–196
Jerez CG, Navarro E, Malpartida I, Rico RM, Masojídek J, Abdala R, Figueroa FL (2014) Hydrodynamics and photosynthesis performance of Chlorella fusca grown in a thin-layer cascade (TLC) system. Aquat Biol 22:111–122
Jerez CG, Malapascua JR, Sergejevová M, Masojídek J, Figueroa FL (2016) Chlorella fusca (Chlorophyta) grown in thin-layer cascades: estimation of biomass productivity by in-vivo chlorophyll a fluorescence monitoring. Algal Res 17:21–30
Kromkamp JC, Forster RM (2003) The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 38:103–112
Kulik MM (1995) The potential for using cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur J Plant Pathol 101:585–599
Kumar J, Ramla A, Mallick D, Mishra V (2021) An overview of some biopesticides and their importance in plant protection for commercial. Plants 10:1185
Lívanský K (1993) Dependence of the apparent CO2 mass transfer coefficient KLa on the nutrient solution pH in outdoor algal culture units. Arch Hydrobiol Suppl Algol Stud 71:111–119
Lívanský K, Doucha J (1996) CO2 and O2 gas exchange in outdoor thin-layer high density microalgal cultures. J Appl Phycol 8:353–358
Malapascua JRF, Jerez CG, Sergejevová M, Figueroa FL, Masojídek J (2014) Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquat Biol 22:123–140
Malapascua JR, Ranglova K, Masojídek J (2019) Photosynthesis and growth kinetics of Chlorella vulgaris R-117 cultured in an internally LED-illuminated photobioreactor. Photosynthetica 57:103–112
Masojídek J, Torzillo G, Koblížek M (2013) Photosynthesis in microalgae. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Publishing Ltd., Oxford, U.K., pp 21–36
Masojídek J, Kopecký J, Giannelli L, Torzillo G (2011) Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J Ind Microbiol Biotechnol 38:307–317
Masojídek J, Vonshak A, Torzillo G (2011) Chlorophyll fluorescence applications in microalgal mass cultures. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications, vol 4. Springer. Dordrecht, The Netherlands, pp 277–292
Masojídek J, Papáček Š, Sergejevová M, Jirka V, Červený J, Kunc J, Korečko J, Verbovikova O, Kopecký J, Štys D, Torzillo G (2003) A closed solar photobioreactor for cultivation of microalgae under supra-high irradiance: basic design and performance. J Appl Phycol 15:239–248
Masojídek J, Ranglová K, Torzillo G, Celis Plá P, Rearte TA, Silva Benavides AM, Neori N, Gómez C, Caporgno MP, Alvarez Gómez F, Abdala R, Miazek K, Fávero Massocato T, Carmo da Silva J, Atzmüller R, Al Mahrouqu H, Suarez Estrella F, Lukeš M, Figueroa FL (2021) Changes in photosynthesis, growth and biomass composition in outdoor Chlorella g120 culture during the metabolic shift from heterotrophic to phototrophic cultivation regime. Algal Res 56:102303
Masojídek J, Ranglová K, Lakatos GE, Silva Benavides AM, Torzillo G (2021) Variables governing photosynthesis and growth in microalgae mass cultures (review). Processes 9:820
Masojídek J, Gómez-Serrano C, Ranglová K, Cicchi B, Encinas Bogeat A, Câmara Manoel JA, Sanchez Zurano A, Silva Benavides AM, Barceló Villalobos M, Robles Carnero VA, Ördög V, Gómez Pinchetti JL, Vörös L, Arbib Z, Rogalla F, Torzillo G, Figueroa FL, Acién-Fernándéz FG (2022) Photosynthesis monitoring in microalgae cultures grown on municipal wastewater as a nutrient source in large-scale outdoor bioreactors. Biology 11:1380
Morales-Amaral M, Gómez-Serrano C, Acién FG, Fernández-Sevilla JM, Molina-Grima E (2015) Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res 12:99–108
Moreno-Garcia L, Adjallé K, Barnabé S, Raghavan GSV (2017) Microalgae biomass production for a biorefinery system: recent advances and the way towards sustainability. Renew Sust Energ Rev 76:493–506
Morillas-España A, Ruiz-Nieto Á, Lafarga T, Acién FG, Arbib Z, González-López CV (2022) Biostimulant capacity of Chlorella and Chlamydopodium species produced using wastewater and centrate. Biology 11:1086
Ördög V, Stirk WA, van Staden J, Novák O, Strnad M (2004) Endogenous cytokinins in three genera of microalgae from Chlorophyta. J Phycol 40:88–95
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ranglová K, Lakatos GE, Câmara Manoel JA, Grivalský T, Suárez Estrella F, Acién Fernández FG, Molnár Z, Ördög V, Masojídek J (2021) Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res 53:102136
Ranglová K, Lakatos GE, Câmara Manoel JA, Grivalský T, Masojídek J (2019) Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol 64:615–625
Rearte A, Celis-Plá P, Neori A, Masojídek J, Torzillo G, Gómez C, Silva Benavides AM, Álvarez-Gómez F, Abdala-Díaz R, Ranglová K, Caporgno M, Massocato TF, Carmo da Silva J, Al-Mahrouqi H, Atzmüller R, Figueroa FL (2021) Diurnal changes of dissolved oxygen gradients in the highly productive strain Chlorella vulgaris R117 cultured in thin-layer cascades: effect on photosynthetic activity. Algal Res 54:102176
Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A (2019) Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9:192
Schreiber U, Endo T, Mi H, Asada K (1995) Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukaryotic algae and Cyanobacteria. Plant Cell Physiol 36:873–882
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Takács G, Gergely I, Molnár Z, Ördög V (2019) Plant biostimulating effects of the green alga Chlamydopodium fusiforme on winter wheat in field experiments. Book of abstract, 9th Symposium on microalgae and seaweed products in plant/soil systems, Mosonmagyaróvár, Hungary, June 25-26, p 34
Torzillo G, Accolla P, Pinzani E, Masojídek J (1996) In situ monitoring of chlorophyll fluorescence to assess the synergistic effect of low temperature and high irradiance stresses in Spirulina cultures grown outdoors in photobioreactors. J Appl Phycol 8:283–291
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Biotechnol Resour 102:1675–1682
Acknowledgments
The authors thank Ms. Marta Barceló, Ms. Cynthia Goméz, and Mr. José Bononi Barufi for their technical assistance.
Funding
This study was funded by the EU Horizon 2020 program, grant no. 727874 and in part by a special grant 2022 to J.M. awarded by the Czech Academy of Sciences to holders of the Doctor of Science degree.
Author information
Authors and Affiliations
Contributions
Conceptualization: JM, CGS, KŠ, FLF, FGAF; methodology: JM, KŠ, CGS, FLF, TG, JCdS; investigation and data analysis: JM, KŠ, CGS, FLF, JCdS, TG; writing—figures and original draft preparation: JM, KŠ, CGS, FLF; writing—review and editing: JM, KŠ, CGS, FLF, FGAF, TG, JCdS; funding acquisition: FGAF, FLF, JM; supervision: JM, FLF, FGAF. All authors read, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article did not contain research involving humans or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masojídek, J., Štěrbová, K., Serrano, C.G. et al. Photosynthetic performance of Chlamydopodium (Chlorophyta) cultures grown in outdoor bioreactors. Appl Microbiol Biotechnol 107, 2249–2262 (2023). https://doi.org/10.1007/s00253-023-12428-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12428-0