Abstract
The choroid plexus (ChP) is an extensively vascularized tissue that protrudes into the brain ventricular system of all vertebrates. This highly specialized structure, consisting of the polarized epithelial sheet and underlying stroma, serves a spectrum of functions within the central nervous system (CNS), most notably the production of cerebrospinal fluid (CSF). The epithelial cells of the ChP have the competence to tightly modulate the biomolecule composition of CSF, which acts as a milieu functionally connecting ChP with other brain structures. This review aims to eloquently summarize the current knowledge about the development of ChP. We describe the mechanisms that control its early specification from roof plate followed by the formation of proliferative regions—cortical hem and rhombic lips—feeding later development of ChP. Next, we summarized the current knowledge on the maturation of ChP and mechanisms that control its morphological and cellular diversity. Furthermore, we attempted to review the currently available battery of molecular markers and mouse strains available for the research of ChP, and identified some technological shortcomings that must be overcome to accelerate the ChP research field. Overall, the central principle of this review is to highlight ChP as an intriguing and surprisingly poorly known structure that is vital for the development and function of the whole CNS. We believe that our summary will increase the interest in further studies of ChP that aim to describe the molecular and cellular principles guiding the development and function of this tissue.
Similar content being viewed by others
Introduction
The choroid plexus (ChP) is a conspicuous tissue within the brain ventricular system of all chordates about the lancelets [1] which is widely recognized as the predominant source of the cerebrospinal fluid (CSF) [2]. This greatly specialized structure protrudes into brain cavities from three distinct brain regions: the pair of Telencephalic ChPs (TelChP), also known as lateral ChP, extends symmetrically into lateral ventricles of the telencephalon, (2) Diencephalic ChP (DiChP), also known as 3 ChP, lays in the 3rd vesicle formed in the diencephalon, and finally, (3) Hindbrain ChP (HbChP), also known as 4th ChP, resides in the 4th cavity within the mesencephalic region. (Fig. 1) Regardless of ChP first observation by the Greek anatomist Herophilos already before our era [3], severe pathologies of this tissue reported at the beginning of the last century [4, 5] and its fairly well-defined structure available from the 1960s [6, 7] the growing interest in ChP among neuroscientists is evident only in the recent decade. Several facts lie at the root of this shift in the perception of ChP tissue and its significance. Besides model and methods availability or sufficiently demonstrated clinical salience, the disclosure of the ChP functional potential to actively secrete morphogens into the CSF and thus participate in the patterning of the adjacent neuronal tissue across the neural tube took the essential lead [8,9,10].
The localization of the ChP secretory system within the developing mouse brain. The set of three distinct ChP types (highlighted in red colour) occupies every ventricle formed within the main brain embryonic regions. This includes lateral ventricles marked by the presence of telencephalic TelChPs, 3rd ventricle characterized by the small DiChP and 4th ventricle fills with the robust HbChP. A growing body of evidence indicated differences between the individual types of ChP, not only in their diverse morphological appearance, which can be appreciated on the coronal (panel in dark green) as well as sagittal sections (panel in dark pink) of the murine brain, but also in the expression profiles of the ChP epithelia. This spatial regionalization translates into differences in the composition of the cerebrospinal fluid (CSF) which ChPs produces into the lumen of the ventricular system. ChP choroid plexus, TelChP telencephalic choroid plexus, DiChP diencephalic choroid plexus, HbChP hindbrain choroid plexus, CSF cerebrospinal fluid (CSF), E embryonic day
This review aspirates to compile the current knowledge about the ChP with an emphasis on its development. Within this manuscript, we attempted to bring a new synthetic view on the most crucial steps of the ChP development and to comprehensively summarize known functions of molecular regulators involved in the ChP ontogenesis. We distinguished and schematically outlined three key processes of ChP development and comprehensively described the key factors and molecules needed for the specification and formation of this tissue. In addition, our review also provides the reader with clearly summarized essential information about the ChP morphology assessment, the experimental models to study the development of ChP, the commonly used molecular markers, and the currently available specific mouse strains used for studies of ChP. We believe that this summary will provide a comprehensive insight into ChP development and morphogenesis, and will stimulate and efficiently direct further research toward our understanding of this crucial secretory tissue.
ChP morphology
ChP strikes the eyes of the neuroscientists certainly by their gross morphological shape in the lumen of ventricles. Within the central nervous system (CNS), this tissue represents rather atypical structures with huge differences in the overall appearance of individual ChPs (Fig. 1). In the murine brain, the HbChP displays the most complex three-dimensional structure with numerous branches bifurcating in the hindbrain cavity whose length and a total number have become evaluation parameters of the HbChP morphology [10]. Moving more anteriorly across the neural tube, the morphology assessment of the frond-like DiChP is still quite challenging and sporadically realized as this miniature structure has less-defined borders [11]. Recently, Langford and colleagues approached this issue by the manual track of Transthyretin + epithelial cells of ChP (ChPEC) on the histological sections with the intention to determine the length of the DiChP [12]. Finally, the tarpaulin-like tissue of TelChPs occupies nearly the entire telencephalic cavities with an exception of their frontal horns [13]. The morphology of this ChP type has been scored as an evaluation of its total area and length within the lumen of the ventricles [10, 12].
Further divergences in the ChP morphology can be found across the individual species, namely between DiChPs and TelChPs of various animals [14]. The striking differences in the morphological appearance have been observed in the human TelChP compared to its murine form. TelChPs within the human brain display a more corrugated character of the tissue resembling the branched morphology of the HbChP (Fig. 2) [15]. However, the molecular mechanism which stands behind this morphological alternation has not been clarified and additional research is needed to determine the regulatory network which participates not only in this phenomenon but also in the general morphological specification of ChP across the ventricles as well as species.
The distinct morphological shapes of TelChPs in mouse and human. The TelChPs outgrows gradually out of the medial telencephalic wall into the lateral ventricles. TelChPs fill up to 63% of the total cavity area in humans [16] while the murine TelChPs occupied only 27% of the mapped area [17]. These differences are due to a more complicated morphology of human TelChPs. TelChP telencephalic choroid plexus, GW gestation week, E embryonic day
Overall, quantitative analysis of ChP morphology is not trivial. It can be performed by measurements on several consecutive slides of the brain sections, which, however, still does not provide an overall view of ChP within the brain ventricular system. To overcome this obstacle, Perin et al. executed the three-dimensional reconstruction of the rat HbChP tissue using the light sheet microscope scans focusing on its total macrostructure as well as on the individual components such as vasculature [18]. Further information can provide an evaluation of the relative position of ChP within the ventricle. For example, the position of HbChP within the 4th ventricle has been used as a differential criterion in the diagnosis of the human posterior fossa malformations like Vermian hypoplasia [19] or Blake's pouch cyst [20].
ChP structure and cellular composition
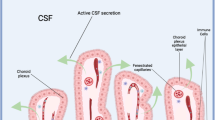
The gross structure of ChP, shortly described below, is conserved across the aforementioned ChP types, as well as species. The ChP tissue is constituted out of two core components—the stromal tissue mass which is enveloped by the single monolayer of polarized cuboidal epithelial cells [21].
Mesodermally derived stroma [22] is composed of a plethora of cell types, which involve fibroblasts, glial, immune, neuronal, or endothelial cells [11]. The last-mentioned cells form an atypical vascular system, which is unique to ChP and has the diaphragmed fenestrations in capillary walls. These modulations ensure the influx of water and small molecules from the blood to epithelial cells which are elemental for the CSF secretion [23, 24]. The formation and effective function of the ChP vascular network is guaranteed by the presence of the smooth muscle cells together with the pericytes that cover endothelial cells [11].
ChP vasculature is connected to the brain circulation system. LatChPs are supplied by the anterior as well as posterior choroidal arteries which are a continuance of the internal carotid and the vertebral artery, respectively. Similarly, the vertebral artery also delivers the necessary blood to the DiChP. Within the hindbrain region, the basilar and vertebral arteries branched to the anterior and posterior inferior cerebellar arteries which outgrow to the whole HbChP [25, 26].
In addition, a broad spectrum of the immune cell types, including monocytes, lymphocytes, basophils, neutrophils, dendritic cells or B cells can be found in the ChP stroma. However, the most abundant immune cell population are macrophages [11] which are derived from the haematopoietic stem cell-derived myeloid cells that are produced within the aorta-gonad-mesonephros region or later in the development by the fetal liver [27]. Interestingly, the apical surface of choroid plexus epithelial cells (ChPEC) is occupied by the specific subpopulation of macrophages termed as epiplexus or Kolmer cells, which scan the content of CSF [28].
The second-mentioned element of the ChP tissue—the ChP epithelium—originates in the neuroectoderm and represents the continuance of the ependymal lining of the ventricular system [29]. The population of ChPEC is distinguishable from others by three major features which are the results of the ChPEC maturation and reflect functions of this secretory tissue. First, the space between ChPEC is sealed by tight junctions which restrain the paracellular transport across the ChPEC and establish the physical barrier between blood and ventricles fulfilled by CSF [30]. Second, the composition and surface of epithelial cells are significantly adjusted to produce CSF. Namely, the epithelial sheet displays higher mitochondrial content [31] together with asymmetrical localisation of various transporters on the apical (e.g., Na+- K+- ATPase or water channels) as well as a basal (e.g., Na+- HCO3− exchanger or anion exchanger 2) side [32,33,34,35]. Third, the apical side of the epithelium is characterized by the presence of membrane modulations such as microvilli or cilia which significantly increase the area out of which the CSF is produced [7]. The most typical molecular markers used to distinguish individual cellular pools of developing ChP are summarized in Table 1.
Despite shared basics, ventricle- or age-dependent variations of individual cell types has been shown by recent single-cell analysis of all ChPs across the mouse lifespan. Interestingly, this study uncovered further regionalization within the ChPEC. These spatial divergences were most typical for the epithelium of the HbChP which could be further subdivided into the rostral (Penk+, Shh+, Wnt5a+ among others) and the caudal (i.e., Fab3+, Acad8+, Rb1+) parts [11]. Similarly, despite the molecular details being missing, even the very early study in humans morphologically distinguished anterior and posterior regions within the epithelium of TelChP [36].
CSF fluid
The ChP is a superior secretory tissue that supplies the brain ventricular system with the CSF, producing up to 80% of its total volume [46]. The human ventricular system is filled with approximately 150 ml of CSF and this volume is daily changed 3–4x [47]. There is a general agreement about the CSF flow. Its directional movement starts from lateral ventricles to the 3rd and 4th cavity through the foramen of Monro and cerebral aqueduct, respectively. Afterward, CSF enters the subarachnoid space and spinal cord via the foramen of Magendie, where it is resorbed back to the bloodstream. On top of that, several studies suggested novel ways of CSF absorption highlighting cervical lymphatics or dural venous plexus [48]. The CSF flow is guaranteed by the synchronic cilia beating of the ependymal lining which is tightly regulated by the circadian rhythm [49] together with brain-derived hormones [50]. Within the zebrafish ventricular system, the motile ependymal cilia appear to regulate the CSF flow merely within the induvial ventricles, while the CSF motion across cavities is generated by the heartbeats along with the body movements [51].
Formerly, CSF was mainly recognized as a clear body liquid serving a mechanistic function within the CNS—ensuring its protection, cleanliness, as well as preservation of intracranial pressure needed for the ventricle expansion and proper brain development [52]. Nowadays, CSF is perceived as a dynamic fluid with a heterogeneous morphogen composition that arises from the divergent secretion activity of each ChP epithelia, which is further described in the chapter ChP-mediated brain patterning. Interestingly, this phenomenon seems to decrease over the lifespan with its peak during embryonic development [53, 54]. Thus, CSF regionalization contributes significantly to the diverse temporal developmental programs of stem cells pools that reside in the interface of individual ventricles [8, 55].
Another noteworthy fact about CSF is its brain nourishing character as this body liquid contains several micronutrients, ions, peptides, or a variety of small RNAs [21, 56]. Besides this, the macronutrients like glucose or lactate pass through the fenestrated capillaries and any imbalance in their concentration levels within the CSF can signify some pathological conditions. For example, decreased CSF level of glucose has been connected to bacterial meningitis [57] or leptomeningeal carcinomatosis [58]. Lower lactate parameters within the CSF have been observed in patients with cerebral hypoxia [59] or neurometabolic disorder [60]. On top of this, ChP tissue tightly coordinates the penetration of the leptin from the blood into the CSF fluid. This circulating hormone stimulates receptors of the hypothalamus which culminates in the control of satiety feeling [61].
Development of the ChP
The ChP ontogenesis is a complex and dynamic process that consists of several crucial consecutive steps leading to the formation of the fully-functioning ChP tissue. These stages will be individually discussed in the sections below and are schematized in Fig. 3 and further expanded to the cellular and molecular level in Fig. 4. Of note, ChP development is predominantly studied in the mouse. However, we would like to appreciate the fact that several noteworthy studies, not further discussed in this review, have been done in other models such as zebrafish (for primary references see [62,63,64,65]). Additional imbalance in the knowledge on ChP development can be found in the levels of published details about the formation of individual ChP types. Namely, the DiChP is repeatedly omitted from the analysis probably due to its unclear morphology. On top of this, the vast majority of the studies focused on the development of ChP were performed on the systemic knock-out models which impoverish our knowledge about the time and cell-type requirements for the specific proteins within the individual developing stages of ChP. Only more recently tissue- and/or time-specific ChP knockout models were reported. To help the reader, we summarize the mouse strains that are most commonly used to specifically study ChP development in Table 2.
The ontogenesis of ChPs. A schematic simplification of ChP development and formation is shown on the coronal brain sections of mouse telencephalon (panels in green, left) and hindbrain (panels in dark pink, right) at E8.5 (A, B), E11.5 (C, D) and E14.5 (E, F). The early specification of the TelChP as well as HbChP epithelial cells (ChPEC) (highlighted in red within panels C–F), takes place in the embryonic roof plate (in grey; except the hatched part in B) (A, B). Further development (panels C,D as close up of neural tube regions indicated by dashed boxes in A, B) of the ChPs is connected with the formation of progenitor domains (orange). They are called cortical hem (CH) in the telencephalon (C) and rhombic lips (RL) in the hindbrain (D). These regions, besides ChPEC, also give rise to the migratory neurons (green). They are represented by Cajal–Retzius cells (from CH) migrating into the developing cortex of the telencephalon (C, E) as well as hindbrain migratory neurons targeting the cerebellum (from upper RL) (D, F). The last step in the ChP ontogenesis is its maturation when ChP gets its typical shape (E, F). Maturation involves further differentiation of ChPEC and the formation of ChP stroma (pink) achieved by the migration of mesoderm-derived stromal cells
Cell types, transcription factors, and signalling pathways involved in the ontogenesis of ChPs. A summary of cell types and key molecular regulators involved in the development of ChP. Schematics directly connects to Fig. 3 that describes the roof plate (A, B; E8.5), progenitor zone (C, D; E11.5), and maturation (E, F; E14.5) stages of ChP development. The development of TelChP (A, C, E; left) and HbChP (B, D, F; right) is outlined separately. For the legend of individual cell types, see the panel on the right. Transcription factors are shown in rounded squares; components of morphogenetic signalling pathways are in circles. For further details and references, see the text in the chapter “Development of the ChP”
Early ChP specifications
The first sights of ChP tissue within the murine brain have been observed at embryonic day (E) 11.5 when the HbChP start to bulge into the 4th ventricle. Shortly after, TelChPs arise from the medial wall of the telencephalon quickly followed by the DiChP formation within the 3rd cavity [7]. Nonetheless, the specification of the ChP occurs earlier in the development as indicated by grafting experiments of future chick ChP which was capable to form ChP tissue out of its original region [66]. This concept was further extended by the in vitro cultivation of the E8.5 and E9.5 mouse prospective ChP regions from relevant brain regions which also resulted in the formation of ChP like structures [29].
ChPs are adjacent to the roof plate which is a fundamental signaling hub that occupies the dorsal midline of the developing CNS [67]. Roof plate cells, which are located ventrally to the prospective neural crest cells within the neural tube [68], settle the dorsal midline region after the emigration of their neighbors [69]. Several studies have focused on this region to resolve the potential role of these apicobasal polarized cells in the ChP specification.
The lineage tracing experiment of Gdf7 + cells occupying the dorsal midline uncovered the contribution of these cells to the epithelium of HbChP as well as DiChP from E9.5. The examination of the TelChP showed the presence of a small Gdf7 + cellular population within the anterior part of the TelChP epithelial sheet. Contrarily, the posterior part of the epithelium lacked any progeny of Gdf7 + cells labeling [70] which suggests the existence of regionalization of the mouse TelChP epithelium as described previously in humans [36]. A further study addressing the involvement of roof plate in the formation of HbChP epithelium divided the hindbrain roof plate into three spatiotemporal regions which emerge from the neuroepithelium at distinct time points. Field 1, positive for Wnt1, colonized the dorsal midline at E8-E9.5 and did not participate in the formation of the ChP epithelium. On the other side, fields 2 and 3 positive for Wnt1 and Gdf7 formed at latter E9.5 from rhombomeres 2–8 and rhombomeres 1, respectively, clearly contributed to the epithelium of HbChP [71]. These observations of Wnt1 significance in the development of HbChP can be further complemented by the phenotype of Wnt1 knock-out mice that lack this tissue [72].
In general, the ChPEC specification requires inhibition of the neural fate within the neuroectoderm from which these cells arise [29]. The regulatory network behind this process has been studied widely in the embryonic telencephalon. For instance, members of the BMP protein family, which are present within the dorsal parts of the telencephalon from E10.5, induce epithelial cell fate within the dorsal midline by the repression of the Lhx1/Foxg1 complex [37, 73,74,75,76]. This observation was validated experimentally in vitro by Watanabe and colleagues who showed that Bmp4 is sufficient to induce ChPEC fate in neural progenitors derived from mouse embryonic stem cells [77]. Furthermore, the misexpression of Bmp receptor Bmpr1 at E10.5 resulted in the expansion of ChPEC, whereas its deletion decreased the number of ChPEC [78]. This specification process is also regulated by the Otx2-Emx2 transcriptional network which is present in this region from E10.5 [79]. The ectopic expression of Emx2 within the dorsal midline has been observed to activate the neuronal cell fate trajectories. The absence of ChPEC in these mice that was accompanied by the decreased levels of Otx2 transcripts directed further research of pro-epithelial factors within the roof plate to Otx2 [80, 81]. Indeed, the early deletion of the Otx2 gene at E9.5 caused the absence of all ChPs, while its later ablation (at E15.5) impaired only the development of the HbChP [38]. Altogether, these studies point to the crucial role of Otx2 in diverse time points of the ChP development.
Text Box: How to study ChP and its development
Molecular markers To distinguish ChP from the surrounding tissue, several representative markers can be used to visualize the ChP epithelium. For instance, the transporter of thyroid hormone thyroxine T4 throughout the CSF—Transthyretin (Ttr)—is exclusively present in ChPEC from the early stages of ChP development, even before the ChP starts to protrude into ventricles, and its expression persists till adulthood [82]. The differentiated epithelial sheet can be also highlighted by the water channel Aqp1 [10] or other proteins essential for the ChP secretory functions like Slc4a10 [45]. The transition zone between the progenitor and fully differentiated epithelial cells can be visualized by ciliogenesis markers like Shisa8, Ccdc67 or Mcidas [11]. For summary overview see Table 1. Mouse models Several tissue-specific mouse models have been developed to study ChP development. A strong emphasis must be placed on the selection of the adequate Cre drivers, as the transcriptomic regionalization of ChP epithelium can significantly influence the outcome of the analysis. The potential disruption of the ChP morphology arising in these models has been evaluated by the manual measurements of several parameters (e.i. total area, length or number of branches [10, 40] on several consecutive tissue slides. In the future, these interminable and challenging quantifications should be replaced by the automatized procedure capable to assess the ChP tissue properties in three dimensions, as lately was shown by Perin and colleagues [18]. For detailed overview of the mouse models see Table 2. ChP organoids The recent generation of the human ChP organoids has uncovered several possible directions of the ChP research, in the terms of the result validation of big-data analyses as well as exploration of ChP functions in vitro or evolutionary studies of ChP development. These TelChP-like protrusions on the cerebelar organoids derived from human pluripotent stem cells displays a high level of similarities to those in vivo regarding the secretome or ChPEC transcriptomic profile. Nonetheless, this state-of-art model lacks the vasculature network within the stroma and as such it is unsuited for the interaction studies of endothelial cells with other cell types within the ChP [41] |
Moving more posteriorly across the neural tube, the recent investigation of the zinc-finger Zfp423, expressed within the hindbrain roof plate from E8.5, revealed its strong pro-epithelial functions. The C-terminal mutants of this transcriptional factor display hypoplastic HbChP and the downregulation of another key player in the HbChPEC specification—Lmx1a [83, 84]. Mutants of this transcriptional factor are characteristic of drastic malformations within the hindbrain region, including an underdeveloped roof plate that culminates in the absence of HbChP [84, 85]. One of the transcriptional targets of Lmx1a is Mafb. The analysis of Mafb-deficient mice from E10.5 showed delayed differentiation of HbChP probably caused by increased apoptosis together with the decrease of the proliferation within this tissue. Interestingly, Mafb mutant tissue had lower levels of Lmx1a [39] which suggested the complex regulatory network required for the specification of HbChPEC within the hindbrain roof plate. Further effort is required to fully comprehend the early specification of HbChPEC.
Progenitor zones of the developing ChP epithelial cells
Later, development (E11.5–E14) of TelChPEC and HbChPEC is linked with two highly proliferative domains that are contiguous to the developing ChP—telencephalic cortical hem and rhombic lip residing within the hindbrain region. These structures represent not only the progenitor zones of ChPEC but also the origins of migratory neurons.
The cortical hem is a dorsal midline derivative whose development is regulated by the balance between functions of Shh signaling [86, 87] and LIM-homeodomain transcription factor Lhx2 [88]. This Wnt/Bmp/Msx-rich region [87] is apart from TelChPEC, one out of three sources of Cajal–Retzius cells that migrate tangentially to the cortex [89] or hippocampal marginal zone [90]. The rhombic lip is a territory placed along the dorsal midline which is further divided into two regions—upper rhombic lip (URL) and lower rhombic lip (LRL) [91]. Apart from the epithelial cells of ChP, the Wls/Math1/Pax6-positive URL [92] gives rise also to tangentially migrating granule progenitor cells of the cerebellum [93], whereas migratory mossy fiber neurons or climbing fiber neurons have been observed to arise from the LRL [94].
A recent study connected these two progenitor domains by a shared expression profile typical of high expression of Rspo genes [11]. Rspo encodes the members of the R-spondin family which act as secreted enhancers of the Wnt/ß-catenin signaling pathways [95]. Moreover, the transcriptomic analysis of all embryonic ChPs identified Rspo2 as a common marker of progenitor cells which, based on the in silico lineage trajectory analysis, can further produce all relevant neurons and epithelia of ChPs [11]. Nonetheless, the regulators that control the balance between neuronal and epithelial differentiation of these progenitors are largely unknown, with few exceptions.
In the cortical hem, the interplay between the Hes-1,-2, and -3 transcriptional factors and Neurogenin2 (Ngn2) contributes to the equilibrium between ChPEC and Cajal–Retzius pools. The Hes mutants displayed upregulation of Ngn2 expression which acts in the favor of Cajal–Retzius cells numbers, while the population of ChPEC decreased [96]. Furthermore, the lineage tracing of Fzd10, encoding a Wnt receptor, showed the exclusive contribution of Fzd10 + cells to the pool of Cajal–Retzius cells [90], indicating that the quantitative balancing of the individual cell types may also occur on the receptor levels and subsequent downstream signaling cascades. Indeed, the most recent analysis of one of the main Wnt signaling branches—canonical Wnt/ß-catenin cascade—within the cortical hem revealed the necessity of its balanced level within this brain region as constitutive activation of ß-catenin enhanced neuronal cell fate trajectories within the cortical hem, to the detriment of the epithelial state [97]. Additionally to this, a recent study pointed out the vital role of double-sex and mab-3 related transcription factor (Dmrt) genes in the formation of hem-derived Cajal–Retzius cells, as the Dmtr1, Dmtr3, and Dmtr1/Dmtr3 double knockout out mice displayed a significant reduction of cortical hem region and lower number of these cells [98]. Unfortunately, the authors did not examine the effect of these transcriptional factors on the ChP epithelial pool.
The regulation cascade which fine-tunes the epithelial vs. nonepithelial pools within the URL is less described as scientists concentrated predominantly on the cerebellum. Still, several studies do exist. For instance, Lmx1a KO mice display several malformations within the cerebellum as well as a significant reduction of HbChP size [85]. The lineage tracing of the Gbx2 + cell progeny showed their contribution both to the granule cell layer of the cerebellum as well as HbChP epithelium. Interestingly, contribution to granule cells persisted to adulthood, whereas contributing to the outgrowing epithelium of HbChP decreased rapidly even during the early embryonic stages [99]. On top of this, a study performed by Huang and colleagues pointed to the role of Shh morphogen in the proliferation rate of LRL progenitors which can significantly affect the size of HbChP [42]. However, other LRL derivatives were not examined in this study.
Overall, the specification of the ChPEC is a complex multistage process that involved a myriad of transcriptional factors or morphogens which ensure the cell fate switch of ChPEC from the initial neuroectodermal character. Furthermore, an additional equitable network of regulators within later progenitor domains of ChP epithelia is needed to complete the differentiation of ChPEC as well as other cell types derived from these brain regions.
The maturation of ChP
Once the ChPEC cells are defined, the whole tissue undergoes the maturation process. Based on the morphological changes of human TelChPEC, this process has been divided into four stages [100]. Epithelial cells of Stage I are characterized by the central localization of nuclei and the absence of apical membrane folding. These cells form pseudostratified epithelium consisting of 2–3 layers. The transition to Stage II involves changes in the shape of epithelial cells to cuboidal and the shift of the nuclei toward the apical side. Additionally, the basal connective tissue starts to form. Stage III is mainly defined by the formation of the cilia on the apical side. Moreover, epithelial cells display a higher number of villi and basally located nuclei. Large modifications also occur within the stroma as the complexity of the vascular network increases gradually. The gradual decrease in the epithelial cell size defines the switch to the last 4th Stage [100].
It is worth mentioning that, under normal circumstances, epithelia of ChPs are quiescent cell populations whose cell division is dramatically reduced early after the maturation [101,102,103]. However, the examination of ChPs in some pathological conditions like stroke [104] or physical brain injuries [105, 106] revealed an increased proliferation rate of ChPEC which led to the speculation that the ChP is a depository of neural progenitor cells in the adult mammalian brain [104]. Interestingly, grafting experiments of HbChP cells into spinal cord lesions significantly enhanced both the tissue repair and overall locomotor movements of the animals suffering from spinal cord injury [107, 108]. Similarly, courses of stroke [109], hydrocephalus [110], or Huntington's disease [111] have been significantly improved after the transplantation of mature ChPEC into the rodent models of these diseases. This highlights the ChP as tissue with promising therapeutic potential in the spectrum of pathological conditions, mainly because of its capacity to produce the astonishing range of proteins and matrix compartments [112].
Overall, the regulatory programs that lead to the shaping and maturation of fully functioning ChP are not extensively described with a few exceptions. For instance, the Sox9–Co9a3 axis has been recently identified as a crucial factor of the proper epithelial–basal lamina assembly whose disruption leads to the destruction of the ChPEC polarity and hyperpermeability of the blood–CSF barrier [113].
Similar polarity defects and decreased epithelial cohesivity have been observed in the HbChP of Wnt5a mutants [12]. Within its epithelium, Meis transcriptional factors are embroiled in the transcription of the Wnt5a from the early stages, starting at E11.5 [40]. The epithelial-specific deletion of the Wnt5a gene at E11.5 culminates into the severe morphological defects of the typical gross appearance of HbChP, including the decrease of its total area, reduced number of branches and their shortening [10, 40]. The severity of these malformations is further aggravated in the systemic Wnt5a knock-out [10, 12, 40] which can draw attention to the Wnt5a produced by stromal cells. Indeed, Dani and colleagues identified the Wnt5a + mesenchymal cluster [11], which suggests that Wnt5a can also mediate the crosstalk between the individual cell types fundamental for the formation of ChP structure.
Further essentiality of the fine-tuned epithelial–stroma interaction in the ChP maturation has been shown by the Shh knock-out mouse model which phenocopies the Wnt5a mutants. Here, the lack of Shh signals from the epithelium between E12.5 and E14.5 leads to the impaired function of pericytes and ultimately the reduced vascular surface area without the changes in the expression levels of typical pro-angiogenic genes like Ang-1, Tie-2, or Vegf [44]. Interestingly, the co-culture of endothelial cells with ChP epithelial cells, which have been observed to express Vegf [115], resulted in the increased numbers of ChP typical fenestrations within the endothelial cell population [116]. All these findings suggest a complex, multi-step maturation process of the ChP vasculature which involved a variety of signaling cascades needed during the different embryonic time points as the small number of fenestrated vessels have not been observed as early as E16.5 in the rat model [117].
Our understanding of the interplay between all compartments of maturating ChP can be further enhanced by recent bioinformatic analysis of the potential cell–cell interaction network across the ChP tissue. This extensive prediction, based on the ligand and receptor expression patterns within the individual cell types, highlighted mesenchymal cells (i.e., fibroblast and pericytes) as an organizing center that presumably communicates with the remaining cell types via several distinct signaling pathways including Wnt, Bmp, or Notch protein families [11].
ChP-mediated brain patterning
Besides the blood–CSF barrier formation or the CSF secretory function, the ChP has nowadays been appreciated also as the orchestrator of the long-range signaling in the developing brain. This function of ChP is ensured by the production of numerous signaling molecules into the CSF.
The initial work demonstrating the importance of ChP induced patterning within the embryonic telencephalon comes from Lehtinen et al. These authors identified the presence of Igf2 within the meninges and epithelium of the TelChP, and proved that its release into the CSF culminates in the enhanced proliferation of stem cells within the ventricular–subventricular zone (V-SVZ) [8]. Further patterning of this brain region was reported in the recent study where ChP-derived Semaphorins (Sema)/Neuropilins (Nrp) complexes were identified in the CSF. Here, Gerstmann and colleagues showed a decreased rate of murine progenitor differentiation caused by the Sema3b/Nrp2 induced enhancement of their adhesion properties. In turn, Sema3F/Nrp1 complex seems to decrease cell detachment, thus inducing neuron differentiation [118]. More investigation on this issue has been performed on adult samples. For instance, Otx2 knock-out mice display lower numbers of newborn neurons which integrate into the olfactory bulb. This is caused by the impairment of their migration properties which is connected to the alternation of extracellular matrix within the V-SVZ of animals lacking the CSF-derived Otx2 protein [9]. Furthermore, the transport of one of the most emblematic markers of ChPEC—Transthyretin—across the lateral ventricle seems to be fundamental to maintaining the balance in the differentiation of neurogenic as well as oligodendrogenic cell types within the lateroventral—SVZ progenitor niche [119]. Intriguingly, the recent study by Arnaud et al. also showed the interplay between TelChP and the hippocampus. Shortly, the elevated pool of App within the adult ChP leads to the impaired proliferation within hippocampus stem cells niche and behavioral defects in reversal learning [120]. Another brain region that is sensitive to the external stimuli sent out of ChP is the progenitor niche of the cerebellum. In detail, the Wnt5a protein [10] and Shh [55] have been observed to be emitted from the epithelial monolayer of HbChP and transported across the 4th ventricle to regulate cerebellar proliferation.
The secretome analysis of the media from TelChP and HbChP explants has revealed the presence of nearly 200 proteins that are likely to be produced by ChPs in vivo. The authors highlighted several proteins with the potential to participate in the further brain patterning (e.g., Ctsb or Ctsd in the case of TelChP or HbChP-derived Ec-sod or Penk) [54]; however, these findings need further validations in the identical model.
Aging of the ChP and its related diseases
Severe alterations have been observed within the structure of ChP during aging. One of the first described is the so-called Biondi bodies [121]. These filamentous, lipid droplets-associated structures develop preferably in the cytoplasm of aged human ChPEC and it is speculated that these bodies have a destructive impact on the epithelial sheet [122]. Furthermore, the transcriptomic analysis of the adult and aged mouse ChP showed the expression shift related to the IL-1B signaling within the aging macrophages, endothelial, as well as mesenchymal cells. The authors ascribe this observation to enhance macrophage migration and subsequent infiltration of the CSF–blood barrier occurring in older individuals [11].
Similarly, the changes in the status or gene expression pattern of individual ChP cell types have been connected to the presence of various infectious agents in the body, for which the ChP represents the gateway to the CNS. For instance, the Zika virus disturbs the brain cortex via the ChP pericytes’ infection, impairment of the ChP epithelium, and subsequent infiltration into the CSF [123]. Single-cell analysis of human ChP tissue from COVID-19-positive patients revealed inflammatory associated transcriptional changes within the ChP epithelium [124] which is in line with the previous study performed on the latest cutting-edge model in the ChP field—human ChP organoids [41]. Here, the authors showed the leakage of the CSF–blood barrier as well as the inflammatory transition of ChPEC induced by the COVID-19 infection [125]. Intriguingly, the recent study by Carloni and colleagues uncovers the defense mechanism within the ChP tissue restricting the agens entrance and further spreading across the CNS. The authors, focusing on inflammatory bowel diseases, first detected the upregulation of the Wnt/ß-catenin signaling pathway in the ChP endothelial cells from infected animals causing the increased permeability of the ChP vascular network. To prevent further infections, the recruitment of inflammatory cells occurs resulting in the shutdown of the CSF–blood barrier [126].
Beside the entering point, ChP may also act as a reservoir of infection agents as Liu et al. recently showed studying the Ebola virus. Within this study, the authors showed that even after the monoclonal antibody-based treatment, the virus particles were detected within the ChP macrophage cell pool and their subsequent activity resulted in the lethal recrudescence of Ebola diseases [127].
In addition to mental disorders, which have been linked to ChP tissue dysfunction, e.g., schizophrenia [128], bipolar disorder [129], depression [130] anxiety [131] or Alzheimer's disease [132], notorious disorders of this structure are ChP carcinoma and ChP sarcoma. These ChP-based tumors are rare diseases (accounting for 2–4% of intracranial tumors in children and 0.5% in adults [133]) that are most typical for the TelChP and HbChP. Interestingly, 80% of TelChP tumors have been observed in children, while the incidence of HbChP tumors is more uniformly distributed across age groups [134]. Characteristic clinical features of ChP tumors, which have been allied to several genetic conditions, such as Aicardi syndrome [135], Li–Fraumeni syndrome [136], or Von Hippel–Lindau disease [137], are hydrocephalus and increased intracranial pressure which manifest in vomiting or headaches [138]. On the molecular level, ChP tumors have been connected to the alternation in the TP53 [139], PDGFR [140], Notch [141], or Shh signaling cascade [142].
Apart from the aforementioned syndromes, mouse models of ciliopathies such as Bardet–Biedl syndrome [143], Joubert syndrome [144], or Meckel–Gruber syndrome [145], which have already been associated with the ChP cyst [146], show cilia loss at the epithelial monolayer of the ChP. This is accompanied by the disruption of the ChPEC integrity and hydrocephalus. Altogether, these studies underline the relevance of the ChP in the research and/or diagnosis of these ciliopathies.
Conclusion and future directions
The enormous effort has been recently put into the ChP research with the intention to fully comprehend every aspect of this secretory organ—its functions, morphogenesis, as well as mechanisms that result in the onset of ChP-related diseases. The range of models used in the ChP field is growing rapidly and includes zebrafish line with fluorescent ChPECs [63], the time- and/or tissue-specific conditional mouse knockouts [10, 55], or human ChP organoids [41]. This is accompanied by the boost of transcriptomic [11, 54, 128] and proteomic datasets [41, 54] of ChP tissue or CSF. Despite all of this, many key issues related to the ChP development, and its functions remain unresolved. First, are there any additional molecular mechanisms of ChP epithelium specification which would be shared by all ChP types? Then, is the predicted common origin of ChPs in the Rspo2 + progenitors evolutionary conserved? What is the full extent of the epithelium–stroma interactions and what is its impact on the morphology of individual ChPs? What is the extent of regionalization of CSF within the individual ventricles? How is such diversity maintained? What is the full scope of brain regions which respond to the biomolecules produced by the ChP? Overall, further studies within the basic research framework are needed to put the entire picture of developmental processes and the functioning of this unique structure together. Only once we broaden our basic knowledge of ChP, we can understand better the onset of the ChP pathologies, and ultimately develop the effective treatments of ChP-related diseases.
Availability of data and materials
Not relevant.
Change history
18 June 2023
Figure placement of 4 has been updated and accidentally placed box text has been updated.
References
Brocklehurst G (1979) The significance of the evolution of the cerebrospinal fluid system. Ann R Coll Surg Engl 61:349–356
Davson H, Segal MB (1996) Physiology of the CSF and blood-brain barriers. CRC Press, Boca Raton
Dohrmann GJ, Bucy PC (1970) Human choroid plexus: a light and electron microscopic study. J Neltrosurg 33:506/516. https://doi.org/10.3171/jns.1970.33.5.0506
Dandy WE (1918) Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg 68:569–579. https://doi.org/10.1097/00000658-191812000-00001
Somerford AE (1933) A case of papilloma of the choroid plexus. Arch Dis Child 8:53–56. https://doi.org/10.1136/adc.8.43.53
Tennyson VM, Pappas GD (1964) Fine structure of the developing telencephalic and myelencephalic choroid plexus in the rabbit. J Comp Neurol 123:379–411. https://doi.org/10.1002/cne.901230307
Sturrock RR (1979) A morphological study of the development of the mouse choroid plexus. J Anat 129:777–793
Lehtinen MK, Zappaterra MW, Chen X et al (2011) The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905. https://doi.org/10.1016/j.neuron.2011.01.023
Planques A, Oliveira Moreira V, Dubreuil C et al (2019) OTX2 signals from the choroid plexus to regulate adult neurogenesis. eNeuro 6:1–11. https://doi.org/10.1523/ENEURO.0262-18.2019
Kaiser K, Gyllborg D, Procházka J et al (2019) WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis. Nat Commun. https://doi.org/10.1038/s41467-019-09298-4
Dani N, Herbst RH, McCabe C et al (2021) A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell 184:3056-3074.e21. https://doi.org/10.1016/j.cell.2021.04.003
Langford MB, O’Leary CJ, Veeraval L et al (2020) WNT5a regulates epithelial morphogenesis in the developing choroid plexus. Cereb Cortex 30:3617–3631. https://doi.org/10.1093/cercor/bhz330
Javed K, Reddy V, Lui F (2021) Neuroanatomy, Choroid Plexus. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK538156/. Accessed 27 Nov 2021
Netsky MG, Shuangshoti S (1975) The choroid plexus in health and disease. University Press of Virginia, Charlottesville
Roy A, Gonzalez-Gomez M, Pierani A et al (2014) Lhx2 regulates the development of the forebrain hem system. Cereb Cortex 24:1361–1372. https://doi.org/10.1093/cercor/bhs421
Voetmann E (1949) On the structure and surface area of the human choroid plexuses. Ugeskr Laeger 111:1051
Knudsen PA (1964) The surface area of choroid plexus in normal mouse embryos. Cells Tissues Organs 58:355–367. https://doi.org/10.1159/000142595
Perin P, Rossetti R, Ricci C et al (2021) 3D reconstruction of the clarified rat hindbrain choroid plexus. Front Cell Dev Biol 9:1–14. https://doi.org/10.3389/fcell.2021.692617
Paladini D, Donarini G, Parodi S et al (2019) Hindbrain morphometry and choroid plexus position in differential diagnosis of posterior fossa cystic malformations. Ultrasound Obstet Gynecol 54:207–214. https://doi.org/10.1002/uog.20120
Volpe P, De Robertis V, Volpe G et al (2021) Position of the choroid plexus of the fourth ventricle in first- and second-trimester fetuses: a novel approach to early diagnosis of cystic posterior fossa anomalies. Ultrasound Obstet Gynecol 58:568–575. https://doi.org/10.1002/uog.23651
Spector R, Keep RF, Robert Snodgrass S et al (2015) A balanced view of choroid plexus structure and function: Focus on adult humans. Exp Neurol 267:78–86. https://doi.org/10.1016/j.expneurol.2015.02.032
Catala M (1998) Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: the ventricular system, meninges and choroid plexuses. Arch Anat Cytol Pathol 46:153–169
Schmidley JW, Wissig SL (1986) Basement membrane of central nervous system capillaries lacks ruthenium red-staining sites. Microvasc Res 32:300–314. https://doi.org/10.1016/0026-2862(86)90067-1
Ek CJ, Habgood MD, Dziegielewska KM, Saunders NR (2003) Structural characteristics and barrier properties of the choroid plexuses in developing brain of the opossum (Monodelphis domestica). J Comp Neurol 460:451–464. https://doi.org/10.1002/cne.10661
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93(4):1847–1892. https://doi.org/10.1152/physrev.00004.2013
Zagórska-Świezy K, Litwin JA, Gorczyca J et al (2008) Arterial supply and venous drainage of the choroid plexus of the human lateral ventricle in the prenatal period as revealed by vascular corrosion casts and SEM. Folia Morphol 67(3):209–213
Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15(5):300–312. https://doi.org/10.1038/nrn3722
Pietzsch-Rohrschneider I (1980) The development of epiplexus cells (Kolmer cells) in the choroid plexus of the fourth ventricle of the mouse. A scanning and transmission electron microscopic study. Z Mikrosk Anat Forsch 94:316–326
Thomas T, Dziadek M (1993) Capacity to form choroid plexus-like cells in vitro is restricted to specific regions of the mouse neural ectoderm. Development 117:253–262. https://doi.org/10.1242/dev.117.1.253
Møllgård K, Lauritzen B, Saunders NR (1979) Double replica technique applied to choroid plexus from early foetal sheep: completeness and complexity of tight junctions. J Neurocytol 8:139–149. https://doi.org/10.1007/BF01175557
Cornford EM, Varesi JB, Hyman S et al (1997) Mitochondrial content of choroid plexus epithelium. Exp Brain Res 116:399–405. https://doi.org/10.1007/PL00005768
Praetorius J, Nejsum LN, Nielsen S (2004) A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol 286:C601–C610. https://doi.org/10.1152/ajpcell.00240.2003
Masuzawa T, Sato F (1983) The enzyme histochemistry of the choroid plexus. Brain 106:55–99. https://doi.org/10.1093/brain/106.1.55
Bouzinova EV, Praetorius J, Virkki LV et al (2005) Na+-dependent HCO3- uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol - Cell Physiol 289:1448–1456. https://doi.org/10.1152/ajpcell.00313.2005
Johansson PA, Dziegielewska KM, Ek CJ, Habgood MD, Mollgard K, Potter A et al (2005) Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res 322:353–364. https://doi.org/10.1007/s00441-005-1120-x
Bailey P (1915) Morphology of the roof plate of the forebrain and the lateral choroid plexuses in the human embryo. J Comp Neurol 26:79–120. https://doi.org/10.1002/cne.900260104
Hébert JM, Mishina Y, McConnell SK (2002) BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 35:1029–1041. https://doi.org/10.1016/S0896-6273(02)00900-5
Johansson PA, Irmler M, Acampora D et al (2013) The transcription factor Otx2 regulates choroid plexus development and function. Dev 140:1055–1066. https://doi.org/10.1242/dev.090860
Koshida R, Oishi H, Hamada M et al (2017) MafB is required for development of the hindbrain choroid plexus. Biochem Biophys Res Commun 483:288–293. https://doi.org/10.1016/j.bbrc.2016.12.150
Kaiser K, Jang A, Kompanikova P et al (2021) MEIS-WNT5A axis regulates development of fourth ventricle choroid plexus. Development 148(10):dev192054. https://doi.org/10.1242/dev.192054
Pellegrini L, Bonfio C, Chadwick J et al (2020) Human CNS barrier-forming organoids with cerebrospinal fluid production. Science (80-). https://doi.org/10.1126/science.aaz5626
Huang X, Ketova T, Fleming JT et al (2009) Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development 136:2535–2543. https://doi.org/10.1242/dev.033795
Kratzer I, Vasiljevic A, Rey C et al (2012) Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier. Histochem Cell Biol 138:861–879. https://doi.org/10.1007/s00418-012-1001-9
Nielsen CM, Dymecki SM (2010) Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev Biol 340:430–437. https://doi.org/10.1016/j.ydbio.2010.01.032
Christensen IB, Wu Q, Bohlbro AS et al (2020) Genetic disruption of slc4a10 alters the capacity for cellular metabolism and vectorial ion transport in the choroid plexus epithelium. Fluids Barriers CNS 17:1–18. https://doi.org/10.1186/s12987-019-0162-5
Redzic ZB, Preston JE, Duncan JA et al (2005) The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol 71:1–52. https://doi.org/10.1016/S0070-2153(05)71001-2
Johanson CE, Duncan JA, Klinge PM et al (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:1–32. https://doi.org/10.1186/1743-8454-5-10
Brinker T, Edward Stopa JM, Klinge P (2014) A new look at cerebrospinal fluid movement. Fluids Barriers CNS 11:1–16. https://doi.org/10.1186/2045-8118-11-16
Faubel R, Westendorf C, Bodenschatz E, Eichele G (2016) Cilia-based flow network in the brain ventricles. Science (80-) 353:176–178. https://doi.org/10.1126/science.aae0450
Conductier G, Brau F, Viola A et al (2013) Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat Neurosci 16:845–847. https://doi.org/10.1038/nn.3401
Olstad EW, Ringers C, Hansen JN et al (2019) Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Curr Biol 29:229-241.e6. https://doi.org/10.1016/j.cub.2018.11.059
Desmond ME, Jacobson AG (1977) Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev Biol 57:188–198. https://doi.org/10.1016/0012-1606(77)90364-5
Zappaterra MD, Lisgo SN, Lindsay S et al (2007) A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res 6:3537–3548. https://doi.org/10.1021/pr070247w
Lun MP, Johnson MB, Broadbelt KG et al (2015) Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J Neurosci 35:4903–4916. https://doi.org/10.1523/JNEUROSCI.3081-14.2015
Huang X, Liu J, Ketova T et al (2010) Transventricular delivery of sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A 107:8422–8427. https://doi.org/10.1073/pnas.0911838107
Gallego JA, Gordon ML, Claycomb K et al (2012) In vivo MicroRNA detection and quantitation in cerebrospinal fluid. J Mol Neurosci 47:243–248. https://doi.org/10.1007/s12031-012-9731-7
Cameron PD, Boyce JMH, Ansari BM (1993) Cerebrospinal fluid lactate in meningitis and meningococcaemia. J Infect 26:245–252. https://doi.org/10.1016/0163-4453(93)95253-F
Bruna J, González L, Miró J et al (2009) Leptomeningeal carcinomatosis prognostic implications of clinical and cerebrospinal fluid features. Cancer 115:381–389. https://doi.org/10.1002/cncr.24041
Fernandez F, Verdu A, Quero J et al (1986) Cerebrospinal fluid lactate levels in term infants with perinatal hypoxia. Pediatr Neurol 2:39–42. https://doi.org/10.1016/0887-8994(86)90038-x
Hoffmann GF, Surtees RA, Wevers RA (1998) Cerebrospinal fluid investigations for neurometabolic disorders. Neuropediatrics 29:59–71. https://doi.org/10.1055/s-2007-973538
Zlokovic BV, Jovanovic S, Miao W et al (2000) Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology 141:1434–1441. https://doi.org/10.1210/endo.141.4.7435
Bill BR, Balciunas D, McCarra JA et al (2008) Development and notch signaling requirements of the zebrafish choroid plexus. PLoS ONE 3:1–9. https://doi.org/10.1371/journal.pone.0003114
Henson HE, Parupalli C, Ju B, Taylor MR (2014) Functional and genetic analysis of choroid plexus development in zebrafish. Front Neurosci 8:1–19. https://doi.org/10.3389/fnins.2014.00364
Van Leeuwen LM, Evans RJ, Jim KK et al (2018) A transgenic zebrafish model for the in vivo study of the blood and choroid plexus brain barriers using claudin 5. Biol Open. https://doi.org/10.1242/bio.030494
García-Lecea M, Kondrychyn I, Fong SH et al (2008) In vivo analysis of choroid plexus morphogenesis in zebrafish. PLoS ONE. https://doi.org/10.1371/journal.pone.0003090
Wilting J, Christ B (1989) An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res 255:487–494. https://doi.org/10.1007/BF00218783
Chizhikov VV, Millen KJ (2005) Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol 277:287–295. https://doi.org/10.1016/j.ydbio.2004.10.011
Krispin S, Nitzan E, Kassem Y et al (2010) Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development 137(4):585–595. https://doi.org/10.1242/dev.041509
Rekler D, Kalcheim C (2021) From neural crest to definitive roof plate: the dynamic behavior of the dorsal neural tube. Int J Mol Sci. https://doi.org/10.3390/ijms22083911
Currle DS, Cheng X, Hsu CM et al (2005) Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development 132(15):3549–3559
Hunter NL, Dymecki SM (2007) Molecularly and temporally separable lineages comprise the hindbrain roof plate and contribute differentially to the choroid plexus. Dev 134:3449–3460. https://doi.org/10.1242/dev.003095
McMahon AP, Bradley A (1990) The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62:1073–1085. https://doi.org/10.1016/0092-8674(90)90385-R
Monuki ES, Porter FD, Walsh CA (2001) Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-lhx2 pathway. Neuron 32:591–604. https://doi.org/10.1016/S0896-6273(01)00504-9
Porter FD, Drago J, Xu Y et al (1997) Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 124:2935–2944. https://doi.org/10.1242/dev.124.15.2935
Furuta Y, Piston DW, Hogan BLM (1997) Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124:2203–2212. https://doi.org/10.1242/dev.124.11.2203
Hanashima C, Fernandes M, Hebert J et al (2007) The role of Foxg1 and dorsal midline signaling in the generation of Cajal–Retzius subtypes. J Neurosci 27(41):11103–11111. https://doi.org/10.1523/JNEUROSCI.1066-07.2007
Watanabe M, Kang YJ, Davies LM et al (2012) BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J Neurosci 32:15934–15945. https://doi.org/10.1523/JNEUROSCI.3227-12.2012
Panchision DM, Pickel JM, Studer L et al (2001) Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev 15:2094–2110. https://doi.org/10.1101/gad.894701
Boncinelli E, Gulisano M, Spada F et al (1995) Emx and Otx gene expression in the developing mouse brain. Ciba Found Symp. https://doi.org/10.1002/9780470514795.ch6
von Frowein J, Wizenmann A, Götz M (2006) The transcription factors Emx1 and Emx2 suppress choroid plexus development and promote neuroepithelial cell fate. Dev Biol 296:239–252. https://doi.org/10.1016/j.ydbio.2006.04.461
Yoshida M, Suda Y, Matsuo I et al (1997) Emx1 and Emx2 functions in development of dorsal telencephalon. Development 124(1):101–111. https://doi.org/10.1242/dev.124.1.101
Stauder AJ, Dickson PW, Aldred AR et al (1986) Synthesis of transthyretin (pre-albumin) mRNA in choroid plexus epithelial cells, localized by in situ hybridization in rat brain. J Histochem Cytochem 34:949–952. https://doi.org/10.1177/34.7.3458812
Casoni F, Croci L, Vincenti F et al (2020) ZFP423 regulates early patterning and multiciliogenesis in the hindbrain choroid plexus. Dev. https://doi.org/10.1242/dev.190173
Millonig JH, Millen KJ, Hatten ME (2000) The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature 403:764–769. https://doi.org/10.1038/35001573
Chizhikov VV, Lindgren AG, Mishima Y et al (2010) Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc Natl Acad Sci U S A 107:10725–10730. https://doi.org/10.1073/pnas.0910786107
Himmelstein DS, Bi C, Clark B et al (2010) Balanced Shh signaling is required for proper formation and maintenance of dorsal telencephalic midline structures. BMC Evol Biol 10(1):118. https://doi.org/10.1186/1471-213X-10-118
Grove EA, Tole S, Limon J et al (1998) The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125(12):2315–2325
Bulchand S, Grove EA, Porter FD et al (2001) LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech Dev 100(2):165–175. https://doi.org/10.1016/S0925-4773(00)00515-3
Borrell V, Marín O (2006) Meninges control tangential migration of hem-derived Cajal–Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci 9:1284–1293. https://doi.org/10.1038/nn1764
Gu X, Liu B, Wu X et al (2011) Inducible genetic lineage tracing of cortical hem derived Cajal–Retzius cells reveals novel properties. PLoS ONE 6:1–9. https://doi.org/10.1371/journal.pone.0028653
Awatramanil R, Soriano P, Rodriguez C et al (2003) Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat Genet 35:70–75. https://doi.org/10.1038/ng1228
Yeung J, Ha TJ, Swanson DJ et al (2014) Wls provides a new compartmental view of the rhombic lip in mouse cerebellar development. J Neurosci 34(37):12527–12537. https://doi.org/10.1523/JNEUROSCI.1330-14.2014
Alder J, Cho NK, Hatten ME (1996) Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron 17:389–399. https://doi.org/10.1016/S0896-6273(00)80172-5
Landsberg RL, Awatramani RB, Hunter NL et al (2005) Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron 48:933–947. https://doi.org/10.1016/j.neuron.2005.11.031
Han XH, Jin YR, Seto M, Yoon JK (2011) A WNT/β-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem 286:10649–10659. https://doi.org/10.1074/jbc.M110.169391
Imayoshi I, Shimogori T, Ohtsuka T, Kageyama R (2008) Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development 135:2531–2541. https://doi.org/10.1242/dev.021535
Parichha A, Suresh V, Chatterjee M et al (2022) Constitutive activation of canonical Wnt signaling disrupts choroid plexus epithelial fate. Nat Commun 13:633. https://doi.org/10.1038/s41467-021-27602-z
Kikkawa T, Sakayori N, Yuuki H et al (2020) Dmrt genes participate in the development of Cajal–Retzius cells derived from the cortical hem in the telencephalon. Dev Dyn 249(6):698–710
Hagan N, Guarente J, Ellisor D, Zervas M (2017) The temporal contribution of the Gbx2 lineage to cerebellar neurons. Front Neuroanat. https://doi.org/10.3389/fnana.2017.00050
Dziegielewska KM, Ek J, Habgood MD, Saunders NR (2001) Development of the choroid plexus. Microsc Res Tech 52:5–20. https://doi.org/10.1002/1097-0029(20010101)52:1%3c5::aid-jemt3%3e3.3.co;2-a
Altma J, Das GD (1965) Autoradiographic and histoloaical evidence of postnatal hippocampal neurogenesis in rats. J Comp Neu 124:319–3:31/335
Korzhevskii DE (2000) Proliferative zones in the epithelium of the choroid plexuses of the human embryo brain. Neurosci Behav Physiol 30:509–512. https://doi.org/10.1007/BF02462607
Chauhan AN (1979) Lewis PD (1979) a quantitative study of cell proliferation in ependyma and choroid plexus in the postnatal rat brain a. Neuropathol Appl Neurobiol 5:303–309
Li Y, Chen J, Chopp M (2002) Cell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in rats. J Neurol Sci 193:137–146. https://doi.org/10.1016/S0022-510X(01)00657-8
Barkho BZ, Monuki ES (2015) Proliferation of cultured mouse choroid plexus epithelial cells. PLoS ONE 10:1–14. https://doi.org/10.1371/journal.pone.0121738
Chouaf-Lakhdar L, Fèvre-Montange M, Brisson C et al (2003) Proliferative activity and nestin expression in periventricular cells of the adult rat brain. NeuroReport 14:633–636. https://doi.org/10.1097/00001756-200303240-00022
Ide C, Kitada M, Chakrabortty S et al (2001) Grafting of choroid plexus ependymal cells promotes the growth of regenerating axons in the dorsal funiculus of rat spinal cord: a preliminary report. Exp Neurol 167:242–251. https://doi.org/10.1006/exnr.2000.7566
Kanekiyo K, Nakano N, Noda T et al (2016) Transplantation of choroid plexus epithelial cells into contusion-injured spinal cord of rats. Restor Neurol Neurosci 34:347–366. https://doi.org/10.3233/RNN-150546
Borlongan CV, Skinner SJM, Geaney M et al (2004) Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke 35:2206–2210. https://doi.org/10.1161/01.STR.0000138954.25825.0b
Johanson CE, Vıo K, Guerra M (2020) Organ culture and grafting of choroid plexus into the ventricular CSF of normal and hydrocephalic HTx rats. J Neuropathol Exp Neurol 79(6):626–640. https://doi.org/10.1093/jnen/nlaa028
Borlongan CV, Thanos CG, Skinner SJM et al (2008) Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington’s disease. Cell Transplant 16:987–992. https://doi.org/10.3727/000000007783472426
Thanos CG, Bintz BE, Goddard M et al (2011) Functional modulation of choroid plexus epithelial clusters in vitro for tissue repair applications. Cell Transplant 20:1659–1672. https://doi.org/10.3727/096368911X564985
Vong KI, Ma TC, Li B et al (2021) SOX9-COL9A3-dependent regulation of choroid plexus epithelial polarity governs blood-cerebrospinal fluid barrier integrity. Proc Natl Acad Sci U S A 118:1–11. https://doi.org/10.1073/pnas.2009568118
Lewis AE, Vasudevan HN, O’Neill AK et al (2013) The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Dev Biol 379:229–234. https://doi.org/10.1016/j.ydbio.2013.04.026
Breier G, Albrecht U, Sterrer S et al (1992) Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 114(2):521–532. https://doi.org/10.1242/dev.114.2.521
Esser S, Wolburg K, Wolburg H et al (1998) Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 140(4):947–959. https://doi.org/10.1083/jcb.140.4.947
Keep RF, Jones HC (1990) A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res Dev Brain Res 56(1):47–53. https://doi.org/10.1016/0165-3806(90)90163-S
Gerstmann K, Kindbeiter K, Telley T, et al (2020) An unconventional cerebrospinal fluid-derived Semaphorin-signalling regulates apical progenitor dynamics in the developing neocortex. bioRxiv. https://doi.org/10.1101/2020.05.20.106526
Vancamp P, Gothié JD, Luongo C et al (2019) Gender-specific effects of transthyretin on neural stem cell fate in the subventricular zone of the adult mouse. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-56156-w
Arnaud K, Moreira VO, Vincent J et al (2021) Choroid plexus APP regulates adult brain proliferation and animal behavior. Life Sci Alliance 4:1–11. https://doi.org/10.26508/lsa.202000703
Biondi G (1932) Ein neuer histologiseher Befund am Epithel des Plexus ehorioideus. Neur u Psych 144:161–165
Kiktenko AI (1986) Biondi bodies in the choroid plexus epithelium of the human brain—a scanning electron-microscopic study. Cell Tissue Res 244:239–240. https://doi.org/10.1007/BF00218405
Kim J, Alejandro B, Hetman M et al (2020) Zika virus infects pericytes in the choroid plexus and enters the central nervous system through the blood-cerebrospinal fluid barrier. PLoS Pathog 16:1–27. https://doi.org/10.1371/journal.ppat.1008204
Yang AC, Kern F, Losada PM et al (2021) Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595:565–571. https://doi.org/10.1038/s41586-021-03710-0
Pellegrini L, Albecka A, Mallery DL et al (2020) SARS-CoV-2 infects brain choroid plexus and disrupts the blood-CSF-barrier. bioRxiv. https://doi.org/10.1101/2020.08.20.259937
Carloni S, Bertocchi A, Mancinelli S et al (2021) Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 374:439–448. https://doi.org/10.1126/science.abc6108
Liu J, Trefty JC, Babka AM et al (2022) Ebola virus persistence and disease recrudescence in the brains of antibody-treated nonhuman primate survivors. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abi5229
Kim S, Hwang Y, Lee D, Webster MJ (2016) Transcriptome sequencing of the choroid plexus in schizophrenia. Transl Psychiatry 6:e964. https://doi.org/10.1038/tp.2016.229
Lizano P, Lutz O, Ling G et al (2019) Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry 176:564–572. https://doi.org/10.1176/appi.ajp.2019.18070825
Turner CA, Thompson RC, Bunney WE et al (2014) Altered choroid plexus gene expression in major depressive disorder. Front Hum Neurosci 8:1–8. https://doi.org/10.3389/fnhum.2014.00238
Kertser A, Baruch K, Cooper I, Schwartz M (2017) Severe psychological stress impairs choroid plexus gateway activity for leukocyte trafficking. Brain Behav Immun 66:e10. https://doi.org/10.1016/j.bbi.2017.07.049
Lee JH, Ostalecki C, Oberstein T et al (2022) Alzheimer’s disease protease-containing plasma extracellular vesicles transfer to the hippocampus via the choroid plexus. EBioMedicine 77:103903. https://doi.org/10.1016/j.ebiom.2022.103903
Smith AB, Smirniotopoulos JG, Horkanyne-Szakaly I (2013) From the radiologic pathology archives: Intraventricular neoplasms: Radiologic-pathologic correlation. Radiographics 33:21–43. https://doi.org/10.1148/rg.331125192
Paulus W, Brandner S, Louis DN et al (2007) WHO classification of tumours of the central nervous system. IARC, Lyon, pp 82–85
Taggard DA, Menezes AH (2000) Three choroid plexus papillomas in a patient with Aicardi syndrome: a case report. Pediatr Neurosurg 33:219–223. https://doi.org/10.1159/000055956
Cruz O, Caloretti V, Salvador H et al (2021) Synchronous choroid plexus papilloma and Wilms tumor in a girl, disclosing a Li-Fraumeni syndrome. Hered Cancer Clin Pract 19:1–6. https://doi.org/10.1186/s13053-020-00158-7
Blamires TL, Maher ER (1992) CHOROID PLEXUS PAPILLOMA a new presentation a/von Hippel-Lindau (VHL) disease. Eye. https://doi.org/10.1038/eye.1992.18
Bettegowda C, Adogwa O, Mehta V et al (2012) Treatment of choroid plexus tumors: a 20-year single institutional experience. J Neurosurg Pediatr 10:398–405. https://doi.org/10.3171/2012.8.PEDS12132
Tabori U, Shlien A, Baskin B et al (2010) TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol 28:1995–2001. https://doi.org/10.1200/JCO.2009.26.8169
Nupponen NN, Paulsson J, Jeibmann A et al (2008) Platelet-derived growth factor receptor expression and amplification in choroid plexus carcinomas. Mod Pathol 21:265–270. https://doi.org/10.1038/modpathol.3800989
Beschorner R, Waidelich J, Trautmann K et al (2013) Notch receptors in human choroid plexus tumors. Histol Histopathol 28(8):1055–1063. https://doi.org/10.14670/HH-28.1055
Li L, Grausam KB, Wang J et al (2016) Sonic Hedgehog promotes proliferation of Notch-dependent monociliated choroid plexus tumour cells. Nat Cell Biol 18:418–430. https://doi.org/10.1038/ncb3327
Swiderski RE, Agassandian K, Ross JL et al (2012) Structural defects in cilia of the choroid plexus, subfornical organ and ventricular ependyma are associated with ventriculomegaly. Fluids Barriers CNS 9:1–13. https://doi.org/10.1186/2045-8118-9-22
Hynes AM, Giles RH, Srivastava S et al (2014) Murine Joubert syndrome reveals Hedgehog signaling defects as a potential therapeutic target for nephronophthisis. Proc Natl Acad Sci U S A 111:9893–9898. https://doi.org/10.1073/pnas.1322373111
Shim JW, Territo PR, Simpson S et al (2019) Hydrocephalus in a rat model of Meckel Gruber syndrome with a TMEM67 mutation. Sci Rep 9:1–17. https://doi.org/10.1038/s41598-018-37620-5
Panduranga C, Kangle R, Badami R, Patil P (2012) Meckel-Gruber syndrome: report of two cases. J Neurosci Rural Pract 3:56–59. https://doi.org/10.4103/0976-3147.91943
Acknowledgements
The authors would like to thank all Bryjalab members for their support. Furthermore, the authors would like to appreciate the Allen Institute whose publicly accessible histological atlases were used as templates for the schematic drawings of choroid plexuses.
Funding
P.K. was supported by Operational Programme Research, Development and Education (No. CZ.02.2.69 / 0.0 / 0.0 / 19_073 / 0016943 & CZ.02.1.01/0.0/0.0/16_025/0007381). P.K. is the holder of a Brno Ph.D. talent scholarship, which is funded by the municipality of the city of Brno, Czech Republic. V.B. is supported by the Czech Science Foundation (GX19-28347X).
Author information
Authors and Affiliations
Contributions
Text conceptualization, writing, and editing: P.K. and V.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
Not relevant.
Consent for publication
All authors have read and agreed with the published version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kompaníková, P., Bryja, V. Regulation of choroid plexus development and its functions. Cell. Mol. Life Sci. 79, 304 (2022). https://doi.org/10.1007/s00018-022-04314-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04314-1