Abstract
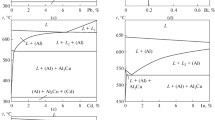
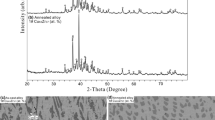
The Al–Cu binary system has been investigated intensively in the past as key binary system for many important industrial alloys. With respect to new experimental results about phase equilibria in Al–Cu system, it was found necessary to prepare new CALPHAD-type thermodynamic description of the Al–Cu system. In course of this work, the known crystallography of several intermetallic phases and the information about site occupations in particular crystallographic positions were implemented in the new thermodynamic phase descriptions. The intermetallic phases were furthermore modelled with all their different temperature modifications. Very good agreement with the experimental results was reached both, for the Al–Cu phase diagram and for the calculated thermodynamic properties, namely the enthalpy of mixing, enthalpies of formation and thermodynamic activities.









Similar content being viewed by others
References
Zobač O, Kroupa A, Zemanová A, Richter KW (2019) Experimental description of the Al–Cu binary phase diagram. Met Mater Trans A 50:3805–3815
Kaufman L, Nesor H (1978) Coupled phase diagrams and thermochemical data for transition metal binary systems-V. CALPHAD 2:325–348
Murray JL (1985) The aluminium—copper system. Int. Met. Rev. 30:211–234
Chen S-W, Chuang Y-Y, Austin Chang Y, Chu M (1991) Calculation of phase diagrams and solidification paths of Al-rich Al-Li-Cu alloys. Met Trans A 22:2837–2848
Saunders N (1998) System Al–Cu, COST 507: thermochemical database for light metal alloys, volume 2: definition of thermodynamical and thermophysical properties to provide a database for the development of new light alloys. In: Ansara I, Dinsdale AT, Rand MH (Eds), European Commission, Brussels
Witusiewicz VT, Hecht U, Fries SG, Rex S (2004) The Ag–Al–Cu system: Part I: reassessment of the constituent binaries on the basis of new experimental data. J Alloy Compd 385:133–143
Liang SM, Schmid-Fetzer R (2015) Thermodynamic assessment of the Al–Cu–Zn system, part II: Al–Cu binary system. CALPHAD 51:252–260
Ponweiser N, Lengauer CL, Richter KW (2011) Re-investigation of phase equilibria in the system Al–Cu and structural analysis of the high-temperature phase η1-Al1-δCu. Intermetallics 19:1737–1746
Goedecke T, Sommer F (1996) Solidification behaviour of the Al2Cu phase. Z Metall 87:581–586
Gulay LD, Harbrecht B (2004) The crystal structure of ζ1-Al3Cu4. J Alloy Compd 367:103–108
Flandorfer H, Rechchach M, Elmahfoudi A, Bencze L, Popovič A, Ipser H (2011) Enthalpies of mixing of liquid systems for lead free soldering: Al–Cu–Sn system. J Chem Thermodyn 43:1612–1622
Heyer E (1989) University of Vienna, private communication to [14]
Kawakami M (1930) A further investigation of the heat of mixture in molten metals. Sci Rep Tohoku Imp Univ Ser 1(19):521–549
Witusiewicz V, Stolz UK, Arpshofen I, Sommer F (1998) Thermodynamics of liquid Al–Cu–Zr alloys. Z Metall 89:704–713
Stolz UK, Arpshofen I, Sommer F, Predel B (1993) Determination of the enthalpy of mixing of liquid alloys using a high-temperature mixing calorimeter. J Phase Equilib 14:473–478
Sandakov VM, Esin YO, Geld PV, Shantarin VD (1971) Heats of formation of liquid copper–aluminium alloys. Russ J Phys Chem 45:1150–1152
Kanibolotsky D, Bieloborodova O, Kotova N, Lisnyak V (2002) Thermodynamic properties of liquid Al–Si and Al–Cu alloys. J Therm Anal Calorim 70:975–983
Grube G, Hantelmann P (1942) Polarisationsmessungen bei der elektrolytischen Raffination des Aluminiums nach dem Dreischichtenverfahren, I. Mitteilung. Z Elektrochem 48:399–409
Wilder TC (1965) The thermodynamic properties of the liquid aluminum–copper system. Trans Met Soc AIME 233:1202–1208
Mitani H, Nagai H (1969) Determination of the activities of aluminum and cupper in liquid Al–Cu Binary alloys by the bubbling method. J Jpn Inst Met 33:344–348
Batalin GI, Beloborodova EA, Vasilev VN (1972) Thermodynamic properties of molten alloys of aluminum with copper. Ukr Khim Zh (Russ Ed) 38:920–923
Perakis J, Chatillon C, Pattoret A (1973) High-temperature mass spectrometry. Thermodynamic properties of aluminum–copper liquid alloys. C R Acad Sci Ser C 276:1513–1516
Layner DI, Ostrovskaya LM, Serebryannikova OS (1976) Activities of components in liquid Cu–Al alloys. Izv Akad Nauk SSSR Met 15:15–18
Oyamada H, Nagasaka T, Hino M (1998) Activity measurement of the constituents in liquid Cu–Al alloy with mass-spectrometry. Mater Trans, JIM 39:1225–1229
W.Oelsen, W. Middel, Zur Thermochemie, I. Der Legierungen, Unmittelbare Bestimmung der Bildungswaermender Legierungsreihen Kobalt–Silizium, Eisen–Aluminium, Kobalt–Aluminium, Nickel–Aluminium, Kupfer–Aluminium und Antimon–Zink fuer den Gusszustand, Mitt. K. W. I für Eisenforsch., 19(1937), 1–26
Kubaschewski O, Heymer G (1960) Heats of formation of transition-metal aluminides. Trans Faraday Soc 56:473–478
Sinvhal RC, Khangaonkar PR (1967) Heats of formation of some aluminum alloys by differential calorimetry. Trans Indian Inst Met 20:107–110
Hair J, Downie DB (1973) Thermodynamic properties of the Cu–Al system:correlation with bonding mechanisms. Faraday Symp Chem Soc 8:56–63
Lukas HL, Fries SG, Sundman B (2017) Computational thermodynamics: the calphad method. Cambridge University Press, New York
Saunders N, Miodownik AP (1998) Calphad (A Comprehensive Guide). Pergamon Press, Oxford
Andersson J-O, Helander T, Höglund L, Shi P, Sundman S (2002) Thermo-calc and DICTRA, computational tools for materials science. CALPHAD 26:273–312
Cao W, Chen SL, Zhang F, Wu K, Yang Y, Chang YA, Schmid-Fetzer R, Oates WA (2009) PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multicomponent phase diagram calculation and materials property simulation. CALPHAD 33:328–342
Redlich O, Kister A (1948) Thermodynamics of nonelectrolyte solutions—X-Y-T relations in a binary system. Ind Eng Chem 40:341–345
Gulay LD, Harbrecht B (2003) The crystal structure of ζ2-Al3Cu4-δ. Z Anorg Allg Chem 629:463–466
Dinsdale AT, Kroupa A, Watson A, Vřešťál J, Zemanová A, Brož P (2012) The thermodynamic database COST MP0602 for materials for high-temperature lead-free soldering. J Min MetallSect B 48:339–346. https://doi.org/10.2298/JMMB120711043K
Dinsdale AT, Kroupa A, Watson A, Vřešťál J, Zemanová A, Brož P (2012) Handbook of high-temperature lead-free solders: Vol. 1, Atlas of Phase Diagrams, COST MP0602 ACTION, COST office 2012, The Czech Republic, ISBN 978-80-905363-1-9
Dinsdale AT (2020) SGTE Unary Database, Version 4.4, www.sgte.org. Accessed 2020
El-Boragy M, Szepan R, Schubert K (1972) Kristallstruktur von Cu3Al2+ (h) und CuAl (r). J Less-Common Met 29:133–140
Kuwano N, Doi T, Eguchi T (1977) Period of antiphase and tetragonality in the α2 phase of Cu-Al alloys. Trans. JIM 18:807–815
Acknowledgements
This study was supported by the Austrian Science Found (FWF) under the Lisa-Meitner Project M2293-N34.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: P. Nash.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kroupa, A., Zobač, O. & Richter, K.W. The thermodynamic reassessment of the binary Al–Cu system. J Mater Sci 56, 3430–3443 (2021). https://doi.org/10.1007/s10853-020-05423-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05423-7