Abstract
Global chronic nitrogen (N) deposition to forests can alleviate ecosystem N limitation, with potentially wide ranging consequences for biodiversity, carbon sequestration, soil and surface water quality, and greenhouse gas emissions. However, the ability to predict these consequences requires improved quantification of hard-to-measure N fluxes, particularly N gas loss and soil N retention. Here we combine a unique set of long-term catchment N budgets in the central Europe with ecosystem 15N data to reveal fundamental controls over dissolved and gaseous N fluxes in temperate forests. Stream leaching losses of dissolved N corresponded with nutrient stoichiometry of the forest floor, with stream N losses increasing as ecosystems progress towards phosphorus limitation, while soil N storage increased with oxalate extractable iron and aluminium content. Our estimates of soil gaseous losses based on 15N stocks averaged 2.5 ± 2.2 kg N ha−1 yr−1 and comprised 20% ± 14% of total N deposition. Gaseous N losses increased with forest floor N:P ratio and with dissolved N losses. Our relationship between gaseous and dissolved N losses was also able to explain previous 15N-based N loss rates measured in tropical and subtropical catchments, suggesting a generalisable response driven by nitrate (NO3−) abundance and in which the relative importance of dissolved N over gaseous N losses tended to increase with increasing NO3− export. Applying this relationship globally, we extrapolated current gaseous N loss flux from forests to be 8.9 Tg N yr−1, which represent 39% of current N deposition to forests worldwide.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nitrogen availability limits net primary productivity of many terrestrial ecosystems (Vitousek and Howarth 1991, LeBauer and Treseder 2008). Because most plant species are adapted to low N availability, maintaining high global plant biodiversity largely relies on the continued scarcity of this essential nutrient (Suding et al 2005, Bobbink et al 2010). Preindustrial ecosystem N supply was sustained by inputs from natural N deposition and biological nitrogen fixation (BNF), estimated at 40–290 Tg yr−1 (Cleveland et al 1999, Galloway et al 2004, Vitousek et al 2013). Since the onset of the industrial and agricultural revolutions, human activities have more than doubled the rate of reactive N formation (Galloway et al 2008). Global N emissions are currently estimated to be 115 Tg yr−1, of which 74 Tg yr−1 deposits onto continental areas (Tan et al 2018), with 23 Tg yr−1 landing on forests (Schwede et al 2018). Deposited N can be either retained within terrestrial ecosystems, lost by hydrologic pathways (mainly as nitrate, NO3 − or dissolved organic N, DON), or returned to atmosphere in gaseous forms (largely as N2 and the greenhouse gas N2O). Gaseous N losses are typically estimated either based on steady state mass balance calculations, or by spatial extrapolation of point measurements. Both methods are subject to measurement difficulties (particularly for the large and opposing fluxes of BNF and denitrification to N2) and additional uncertainty is created by the high spatiotemporal heterogeneity of these fluxes (Groffman et al 2006). Isotope ratios of ecosystem N pools provide an alternative, more powerful tool to inform N balances and net losses, as they integrate the signals associated with isotope fractionation during various transformations (Kjønaas et al 1993, Högberg 1997, Gundersen et al 1998b, Templer et al 2007, Craine et al 2009, Hobbie and Ouimette 2009). The process of denitrification preferentially consumes the lighter isotope of N (14N rather than 15N), and so ecosystem 15N natural abundance has been employed to estimate the fraction of total N losses that are gaseous (Houlton et al 2006, 2015, Houlton and Bai 2009, Vitousek et al 2013, Fang et al 2015).
Despite decades of elevated N deposition, forests in the temperate zone usually retain the majority of deposited N, especially in low-fertility ecosystems with a high elemental ratio of soil C to N (Gundersen et al 1998a, Lovett et al 2002, Dise et al 2009, Oulehle et al 2017, Tahovská et al 2020). Nonetheless, accumulation of ecosystem N relative to other nutrients (C, phosphorus (P), or base cations) should eventually alleviate N limitation (Rowe et al 2008, Braun et al 2010, Crowley et al 2012) with consequences for future trajectory of forests as a major terrestrial C sink (Thomas et al 2015, Schulte‐Uebbing and De Vries 2018, Wieder et al 2019). Here, we utilised a unique set of long-term and consistent N budget measurements from forested catchments spanning moisture, fertility and N deposition gradients in managed forests in temperate central Europe to examine which environmental controls best predicted ecosystem N retention and losses by both gaseous and hydrologic pathways. We tested which soil properties best predicted soil N storage, and whether ecosystem C:N:P stoichiometry fundamentally controls total ecosystem N losses. In addition, we combined our results with published 15N-based N flux estimates to compute the gaseous N flux from global forest ecosystems. Understanding the drivers that control regional to global N balance presents a critical challenge for projecting forest carbon sinks, emissions of N2O, and the avoidance of land and water eutrophication.
2. Methods
2.1. Study sites
The 12 catchments used in this study are located within the mountain landscapes of the Czech Republic (table 1). All but two catchments have acidic soils developed over acidic bedrock; out of 93 soil pits assessed in this study, 66% were described according to the World Reference Base soil classification as Cambisols, 13% as entic Podzols, 12% as haplic Podzols, 7% as Stagnosols and 2% as gleyic Stagnosols. Norway spruce dominates the catchment vegetation, but deciduous trees are common at two catchments, and one catchment is predominantly alpine. Catchment areas ranged from 21 to 254 ha (mean = 102 ha) and mean elevations ranged from 471 to 1301 m. Calculated runoff to precipitation ratio (runoff ratio), considered to be a measure of catchment wetness, varied from 0.08 to 0.84 (mean = 0.38). For the period 1994–2016 total inorganic N deposition (Oulehle et al 2017) averaged 12 kg N ha−1 yr−1 and ranged from 7 to 17 kg N ha−1 yr−1. The δ15N of inorganic N (both NO3 − and NH4) in precipitation was collated from previous studies in the territory of the Czech Republic (Buzek et al 1998, Novak et al 2016).
Table 1. Catchment environmental characteristics, average (standard error) nutrients pools in soil and foliage, average (standard error) δ15N in foliage and soil and measured average (1994–2016) annual dissolved nitrogen (N) fluxes and calculated gaseous N losses across catchments. Averages and standard errors calculated from indicated number of soil samples (n).
| Anenský potok | Červík | Lesní potok | Liz | Loukov | Lysina | Modrý potok | Na zeleném | Pluhův Bor | Polomka | Salačova Lhota | Uhlířská | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catchment | |||||||||||||
| Area | ha | 26 | 181 | 63 | 94 | 54 | 25 | 254 | 60 | 21 | 66 | 200 | 180 |
| Elevation | m a.s.l. | 520 | 802 | 471 | 942 | 577 | 881 | 1301 | 786 | 764 | 614 | 640 | 818 |
| Air temp. | °C | 8.3 | 6.8 | 8.7 | 7.0 | 6.1 | 6.3 | 3.3 | 6.8 | 6.8 | 7.7 | 7.6 | 5.6 |
| Runoff ratio | mm mm−1 | 0.08 | 0.56 | 0.17 | 0.44 | 0.15 | 0.47 | 0.84 | 0.25 | 0.36 | 0.34 | 0.12 | 0.76 |
| Mineral soil Alo/Feo | t ha−1 | 25 | 23 | 22 | 28 | 17 | 20 | 34 | 33 | 25 | 28 | 20 | 36 |
| Forest floor total exchangeable acidity | meq kg−1 | 25 | 69 | 31.0 | 53 | 33 | 91 | 81 | 44 | 37.0 | 45 | 43 | 131 |
| N deposition | kg ha−1 yr−1 | 13 | 14 | 7.3 | 10 | 10 | 12 | 14 | 11 | 8.5 | 13 | 10 | 17 |
| Nutrients | n = 5 | n = 10 | n = 6 | n = 9 | n = 7 | n = 5 | n = 9 | n = 7 | n = 5 | n = 7 | n = 10 | n = 8 | |
| FH horizon N | t ha−1 | 0.6 (0.2) | 1.1 (0.1) | 0.8 (0.3) | 0.7 (0.1) | 0.6 (0.2) | 1.2 (0.2) | 1.5 (0.3) | 0.6 (0.1) | 1.6 (0.4) | 0.9 (0.1) | 1.1 (0.1) | 2.6 (0.2) |
| Mineral soil N | t ha−1 | 4.3 (0.7) | 3.7 (0.4) | 3.6 (0.4) | 3.9 (0.3) | 2.4 (0.3) | 3.3 (0.1) | 5.3 (0.6) | 5.3 (0.8) | 3.6 (0.3) | 4.4 (0.4) | 2.5 (0.2) | 5.0 (0.4) |
| FH horizon C | t ha−1 | 16 (4.2) | 25 (2.9) | 22 (6.9) | 17 (2.4) | 16 (5.0) | 28 (6.0) | 30 (5.6) | 14 (3.1) | 43 (11.3) | 22 (2.9) | 29 (3.6) | 56 (5.0) |
| FH horizon P | kg ha−1 | 41 (10.4) | 69 (6.6) | 50 (12.6) | 37 (3.7) | 37 (10.5) | 102 (17.9) | 117 (20.4) | 39 (7.2) | 78 (19.0) | 55 (6.9) | 52 (6.8) | 201 (24.7) |
| FH horizon Pex | kg ha−1 | 2.1 (0.3) | 3.4 (0.7) | 2.7 (0.5) | 1.8 (0.2) | 2.3 (0.6) | 4.1 (1.0) | 2.5 (0.6) | 1.5 (0.2) | 3.2 (0.4) | 2.3 (0.5) | 3.4 (0.5) | 4.7 (0.6) |
| Needles C | % | 49.9 (0.4) | 50.0 (0.1) | 49.1 (0.1) | 50.0 (0.1) | 49.9 (0.1) | 49.6 (0.2) | 49.7 (0.2) | 49.9 (0.5) | 49.4 (0.1) | 49.3 (0.1) | 49.4 (0.2) | 50.7 (0.1) |
| Needles N | % | 1.38 (0.1) | 1.38 (0.03) | 1.33 (0.04) | 1.27 (0.02) | 1.33 (0.05) | 1.40 (0.06) | 1.41 (0.03) | 1.33 (0.06) | 1.24 (0.04) | 1.53 (0.05) | 1.35 (0.03) | 1.41 (0.06) |
| Needles P | % | 0.14 (0.01) | 0.14 (0.01) | 0.14 (0.01) | 0.14 (0.02) | 0.18 (0.01) | 0.15 (0.01) | 0.12 (0.004) | 0.14 (0.004) | 0.11 (0.01) | 0.13 (0.01) | 0.13 (0.005) | 0.16 (0.01) |
| δ15N | |||||||||||||
| Needles | ‰ | −5.77 (0.77) | −4.22 (0.24) | −5.63 (0.25) | −4.69 (0.21) | −5.98 (0.51) | −4.55 (0.36) | −4.42 (0.38) | −4.37 (0.26) | −4.65 (0.16) | −5.33 (0.22) | −6.42 (0.37) | −4.57 (0.17) |
| FH horizon | ‰ | −4.38 (0.52) | −1.92 (0.27) | −5.01 (0.25) | −3.02 (0.20) | −5.13 (0.52) | −1.98 (0.28) | −0.58 (0.53) | −2.60 (0.30) | −2.98 (0.08) | −4.06 (0.26) | −4.79 (0.21) | −1.86 (0.48) |
| Mineral soil | ‰ | 2.18 (0.68) | 2.63 (0.29) | 1.53 (0.42) | 2.65 (0.49) | 1.76 (0.50) | 1.84 (0.43) | 4.49 (0.59) | 2.30 (0.36) | 2.50 (0.31) | 2.84 (0.52) | 2.52 (0.28) | 2.31 (0.54) |
| Ecosystem (N stock weighted) | ‰ | −0.12 | 0.47 | −0.98 | 0.26 | −1.62 | −0.61 | 2.95 | 0.79 | −0.10 | −0.15 | −1.66 | 0.74 |
| Stream NO3 (volume weighted) | ‰ | 13.5 | 5.3 | 5.2 | 1.1 | N.D. | N.D. | 0.5 | −1.0 | 0.1 | −0.4 | 11.6 | 4.5 |
| Nitrogen fluxes (1994–2016) | |||||||||||||
| N-NO3 leaching | kg ha−1 yr−1 | 0.62 | 2.66 | 0.54 | 1.40 | 0.03 | 0.31 | 5.09 | 1.11 | 2.20 | 3.78 | 0.27 | 2.98 |
| DON leachinga | kg ha−1 yr−1 | 0.20 | 0.95 | 0.35 | 0.58 | 0.10 | 1.89 | 2.18 | 1.18 | 2.20 | 1.34 | 0.22 | 2.35 |
| Denitrification | kg ha−1 yr−1 | 1.3 | 3.9 | 0.8 | 1.5 | 0.1 | 1.8 | 7.5 | 1.6 | 2.9 | 3.1 | 0.5 | 5.2 |
2.2. Soil sampling and analysis
Soils were sampled in 2015 using quantitative soil pits up to 40 cm depth (Huntington et al 1988). Soil samples were taken during the growing season and the number of soil pits per catchment varied from 5 to 10 according to catchment size. The positions of soil sampling pits were chosen to characterize topography and forest vegetation within catchment. At each location, soil profiles were sampled in a 0.5 m2 frame as follows: L (litter) layer; F + H (fermented + humus) layers combined; and mineral soil in specified depths of 0–10 cm, 10–20 cm, 20–40 cm (where 0 cm is the top of the mineral soil layer). All material from each layer was weighed and sieved in the field (1 cm), separated into stones, soil <1 cm and coarse roots. The soil samples were weighed and then returned to the laboratory where it was sieved after air-drying (mesh size of 5 mm for organic horizons and 2 mm for mineral horizons). Soil moisture was determined gravimetrically by drying at 105 °C. Air-dried soil samples were analysed for total organic C and total N by dry combustion with a CNS elemental analyser (Thermo Scientific FLASH 2000). Total P was analysed in FH horizon after digestion spectrophotometrically, available P was determined in Mehlich extract by the molybdate method. Oxalate-extractable Fe and Al were determined in 0.2 M ammonium oxalate/oxalic acid solution at pH 3. The concentrations of oxalate-extractable Fe and Al (Feo and Alo) in the extracts were determined by ICP-OES (Thermo Elemental Intrepid II). Total exchangeable acidity (TEA) was determined by titration of 0.1 M BaCl2 extracts.
2.3. Biomass sampling and analysis
Forest inventory data were measured within forest plots located in circles (500 m2) with soil pit in the centre, thus directly related to the soil chemistry. For each forest plot, three dominant Norway spruce trees were selected for chemical analysis of foliage (1st and 2nd needle year) and wood (bulk wood core). Chemical analysis consisted of total C, N and P concentrations. Total above- and belowground biomass pools were calculated according to an allometric equation derived for Norway spruce (Wirth et al 2004) using measured site DBH.
2.4. Stream N fluxes
Stream N fluxes represented long-term averages of available measurements, covering 1994–2016 for NO3 − and 2006–2016 DON losses. Losses of NH4 were negligible and not included (Oulehle et al 2017). Nitrate (NO3) was measured by high-performance liquid chromatography (Knauer 1000), DON was calculated as a difference between total dissolved N (chemiluminiscence detection after sample combustion; Tekmar-Dohrmann Apollo 9000) and the sum of NO3 − and NH4 + (indophenol blue colorimetry method).
2.5. Stable N isotope analysis
All samples (leaves, soils, and wood) were analysed for their stable N isotope composition with an isotope ratio mass spectrometer ISOPRIME100 (Isoprime, UK) interfaced with a Vario PYRO cube Elemental Analyzer (Elementar Analysensysteme, Germany). The system was calibrated by the certified reference materials with known isotopic ratio from the International Atomic Energy Agency (IAEA, Vienna, Austria). δ15N values were referenced to caffeine (IAEA-600) and potassium nitrate (USGS32) standards. Nitrogen isotope composition was measured as δ15N (‰) following the formula δ15N = [(Rsample/Rstandard) − 1] × 1000, where Rsample is the ratio of 15N:14N in the investigated sample and Rstandard is the ratio of 15N:14N in atmospheric reference N2 (purity of 99.999%).
Selected stream water samples from 2018 only were analysed for their 15/14NO3 − ratio using a diffusion method (Goerges and Dittert 1998). Analysis of total N and 15N/14N ratio were performed on IR-MS (Delta X Plus, Finnigan, Germany) connected to the NC analyser (Elementar analyser FLASH 2000, Thermo Fisher Sci., Germany). A calculated blank correction was used (Stark and Hart 1996).
2.6. Denitrification calculation
We applied an isotopic modelling approach (Houlton et al 2007, Houlton and Bai 2009, Soper et al 2018, Yu et al 2019) to estimate the proportion (fgas) and flux of N lost via denitrification across our catchments:
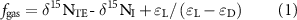
in which δ15NTE and δ15NI represent 15N isotopic ratios of the total ecosystem (soil + vegetation) and atmospheric N inputs, respectively. For each site, differences between measured stream δ15NO3
− and ecosystem δ15N were calculated to estimate the isotope effect ( ) associated with dissolved N leaching (
) associated with dissolved N leaching ( L, assuming zero effect of δ15N-DON). Therefore
L, assuming zero effect of δ15N-DON). Therefore  L represents the combined weighted isotope effect associated with N-NO3
− + DON leaching (mean
L represents the combined weighted isotope effect associated with N-NO3
− + DON leaching (mean  L = 2.7‰, SE = 1.1‰). We used a range of assumed denitrification fractionation effects (
L = 2.7‰, SE = 1.1‰). We used a range of assumed denitrification fractionation effects ( D) between −14 and −18‰ (Houlton and Bai 2009, Fang et al
2015, Yu et al
2019). Published data (Buzek et al
1998, 2012, Novak et al
2016) indicate that precipitation δ15N inputs (δ15NI), including both NH4 and NO3
− average between −4‰ and −8‰, with a central value of −6‰ and so we used this range to assess model sensitivity to these inputs.
D) between −14 and −18‰ (Houlton and Bai 2009, Fang et al
2015, Yu et al
2019). Published data (Buzek et al
1998, 2012, Novak et al
2016) indicate that precipitation δ15N inputs (δ15NI), including both NH4 and NO3
− average between −4‰ and −8‰, with a central value of −6‰ and so we used this range to assess model sensitivity to these inputs.
3. Results
3.1. Ecosystem N stock
Total ecosystem N content in 12 intensively studied Czech catchments varied from 4.4 to 7.9 t N ha−1 across catchments, with soil N comprising the largest N pool (averaging 82% of total ecosystem N, range 72%–96%) (table 1). Total mineral soil N content increased with the amount of oxalate extractable aluminium and iron (Alo and Feo; p < 0.001, r = 0.92; figure 1). That is, mineral soil physicochemical properties largely determined the whole-ecosystem N stock. This relationship was independent of fine soil mass and reflected greater contributions of Spodosols (entic Podzols and haplic Podzols) at catchments with larger mineral N pools.
Figure 1. Ecosystem nitrogen pools (t N ha−1) across catchments related to the pool (t ha−1) of oxalate extractable aluminium (Alo) and iron (Feo). Regression line (R2 = 0.86, p < 0.001) displayed.
Download figure:
Standard image High-resolution image3.2. Dissolved N losses
Stream total dissolved N (TDN) losses were strongly negatively correlated with forest floor C:N ratio (p = 0.005, r = −0.75, figure 2(a)) and positively with forest floor N:Pavailable ratio (p < 0.001, r = 0.94, figure 2(b)). Nitrate (NO3) stream fluxes dominated over DON fluxes at the majority of catchments (table 1), with an average N-NO3/TDN ratio of 0.6. TDN losses were unrelated to foliar C:N, but increased significantly with foliar N:P ratio (p = 0.004, r = 0.76). Measured TDN runoff fluxes averaged 2.9 ± 2.3 kg N ha−1 yr−1 and accounted for 23% ± 17% of measured N deposition across our catchments (table 1).
Figure 2. Measured dissolved N losses (a), (b) and calculated gaseous N losses (c), (d) related to forest floor C:N and N:Pavailable ratios. Regression lines and confidence bands (α = 0.05) displayed.
Download figure:
Standard image High-resolution imageAdditional examination of factors that predict forest floor stoichiometry revealed that forest floor C:N decreased (p < 0.001, r = −0.83) and N:Pavailable increased (p < 0.001, r = 0.88) with increasing catchment wetness (here, as runoff ratio), while the ratio of N:Ptot in forest floor was unrelated to catchment wetness (supplementary table 1 (available online at stacks.iop.org/ERL/16/064025/mmedia)). Canopy N properties correlated significantly with only one environmental variable, as foliar N concentration increased with N deposition (p = 0.025, r = 0.64). Foliar N:P ratio closely related to forest floor N:Pavailable ratio (p = 0.002, r = 0.80) suggesting that canopy enrichment of N over P strengthened with the increasing imbalance of forest floor N relative to available P (supplemetary figure 1).
3.3. Gaseous N losses
Whole-ecosystem δ15N signature averaged 0‰ (range from −1.7 to +2.9 ‰), and increased significantly with stream TDN losses (p = 0.001, r = 0.81; supplementary figure 2(A)) and consequently with catchment wetness (p = 0.002, r = 0.78; supplementary figure 2(B)). On average, TDN leaching was isotopically heavier than N remaining in the ecosystem (fractionation effect of leaching,  L, of +2.7‰), thus ecosystem 15N enrichment likely arose as consequence not from leaching but from a highly fractionating process such as denitrification. Isotopic estimates of denitrification fluxes across our catchments were sensitive to the values assigned to denitrification fractionation effect (
L, of +2.7‰), thus ecosystem 15N enrichment likely arose as consequence not from leaching but from a highly fractionating process such as denitrification. Isotopic estimates of denitrification fluxes across our catchments were sensitive to the values assigned to denitrification fractionation effect ( D) and precipitation δ15N (supplementary figure 3). When the
D) and precipitation δ15N (supplementary figure 3). When the  D value was set to −16‰ and the precipitation input δ15N was set at −6‰ (see section 2), average gaseous N loss was estimated to be 47% of total N loss (range = 38%–62%). Alternatively, using the
D value was set to −16‰ and the precipitation input δ15N was set at −6‰ (see section 2), average gaseous N loss was estimated to be 47% of total N loss (range = 38%–62%). Alternatively, using the  D of −48‰ calculated for soils incubated under laboratory conditions (Wang et al
2018) estimated gaseous N loss amounted to only 17% of total loss. Precisely measured hydrologic losses of N for the study sites (averaging 2.9 ± 2.3 kg N ha−1 yr−1), allow translation of fractional N losses to an average calculated denitrification flux of 2.5 ± 2.2 kg N ha−1 yr−1 (table 1), amounting to 20% ± 14% of measured N deposition. Calculated gaseous N fluxes thus increased with increasing TDN fluxes (p < 0.001, R2 = 0.88), and the magnitudes of both N losses were associated with forest floor C:N and N:Pavailable ratios (figure 2), both of which provide metrics of ecosystem N saturation.
D of −48‰ calculated for soils incubated under laboratory conditions (Wang et al
2018) estimated gaseous N loss amounted to only 17% of total loss. Precisely measured hydrologic losses of N for the study sites (averaging 2.9 ± 2.3 kg N ha−1 yr−1), allow translation of fractional N losses to an average calculated denitrification flux of 2.5 ± 2.2 kg N ha−1 yr−1 (table 1), amounting to 20% ± 14% of measured N deposition. Calculated gaseous N fluxes thus increased with increasing TDN fluxes (p < 0.001, R2 = 0.88), and the magnitudes of both N losses were associated with forest floor C:N and N:Pavailable ratios (figure 2), both of which provide metrics of ecosystem N saturation.
3.4. Ecosystem N mass balance
Measured average N deposition across our catchments over the period 1994–2016 was 12 kg N ha−1 yr−1, and ranged from 7.3 to 17 kg N ha−1 yr−1 (table 1). Based on our mass balances, catchment N retention (i.e. N deposition—stream TDN—gaseous N loss) was relatively high (mean = 6.3 kg N ha−1 yr−1 or 57% of N deposition) but with striking variation from 0% to 97%. In contrast to dissolved and gaseous losses, N retention was significantly correlated only with forest floor C:Pavailable (p = 0.019, r = −0.66) and N:Pavailable (p = 0.014, r = −0.68) but not with forest floor C:N ratio, suggesting a dominant role of soil available P in determining ecosystem N retention (supplementary figure 4).
3.5. Global estimates of N losses in forests
We sought to test whether the tight relationship between measured stream TDN losses and estimated gaseous N losses was consistent across forested biomes worldwide. We identified 11 additional forested catchments where stream inorganic N export was measured alongside N gaseous estimates based on the soil 15N isotopic balance method, spanning a broad ecological and climatic range (four tropical (Fang et al
2015, Soper et al
2018), five subtropical (Yu et al
2019), and two temperate (Fang et al
2015)). Thus our dataset more than doubles the total number of catchments globally for which a full isotopic mass balance is possible. Based on the combined dataset, we observed a remarkably consistent relationship between stream NO3
− export and denitrification flux, spanning environmentally diverse forest ecosystems (with annual air temperature range from 3 °C to 26 °C and precipitation range from 615 mm to 3220 mm) and variations in N flux of 2–3 orders of magnitude (figure 3). Based on this apparently general relationship, gaseous N flux (in kg N ha−1 yr−1) is predictable from stream NO3
− flux using a power function ( , where a = 1.74 and b= 0.62; figure 3). The relationship also shows that gaseous N loss dominates over dissolved inorganic N loss when N losses are low, and the relative importance of gaseous loss decreases with increasing NO3
− export and with overall N loss.
, where a = 1.74 and b= 0.62; figure 3). The relationship also shows that gaseous N loss dominates over dissolved inorganic N loss when N losses are low, and the relative importance of gaseous loss decreases with increasing NO3
− export and with overall N loss.
Figure 3. Calculated gaseous N fluxes based on soil 15N/14N enrichment model (Houlton and Bai 2009) related to measured NO3 − stream export. Data comprised results from temperate zone (this study—black circles), subtropical zone in China (Yu et al 2019) (white diamonds), temperate and tropical zone in Japan and China (Fang et al 2015) (grey squares) and one lowland tropical catchment in Costa Rica (Soper et al 2018, grey triangle). Linear regression line (R2 = 0.84), confidence band (α = 0.05) and 1:1 line displayed.
Download figure:
Standard image High-resolution imageExtrapolating this cross-biome relationship globally, we calculated the global forest denitrification flux based on published NO3 stream exports from 100 catchments covering tropical (n = 28), subtropical (n = 9), temperate (n = 31) and boreal (n = 32) forest biomes (supplementary table 2). Weighted by biome area, our calculated current stream TDN flux from global forests is 13.5 Tg N yr−1 (table 2). Calculated area-weighted N gaseous losses increased in order boreal (0.7 kg N ha−1 yr−1) < temperate (2.7 kg N ha−1 yr−1) < tropical/subtropical (3.3 kg N ha−1 yr−1) forests, resulting in total annual flux of 8.9 Tg N yr−1 (table 2). This analysis predicts that total dissolved and gaseous N losses (22.4 Tg N yr−1) nearly balance N deposition to global forests (23.1 Tg N yr−1), that do also receive uncertain amounts of N input from N fixation. Gaseous losses comprised 40% of the total N loss.
Table 2. Calculated nitrogen fluxes in global forests. The contemporary N deposition fluxes were derived from Schwede et al (2018), current stream N fluxes were calculated according literature search (supplementary table 2) and gaseous N fluxes according to this study. N fixation also contributes N to forests. Its fluxes are highly uncertain and are not included here.
| Area | Deposition | Stream TDN | Stream NO3 | Denitrification | |
|---|---|---|---|---|---|
| Forest biome | Million km2 | kg N ha−1 yr−1 (Tg yr−1) | |||
| Boreal | 4.6 | 1.2 (0.6) | 1.6 (0.7) | 0.2 (0.1) | 0.7 (0.3) |
| Temperate | 7.2 | 7.3 (5.3) | 2.9 (2.1) | 2.0 (1.4) | 2.7 (1.9) |
| Tropical/Subtropical | 20.4 | 8.5 (17.3) | 5.3 (10.7) | 2.8 (5.7) | 3.3 (6.6) |
| Total forests | 32.2 | 7.2 (23.1) | 4.2 (13.5) | 2.2 (7.2) | 2.8 (8.9) |
4. Discussion
Assessments of the consequences of forest N retention and loss at both regional to global scales have long been hampered by the difficulty of measuring N gas losses and N retention in soil. This analysis, based on detailed ecosystem N and δ15N measurements across a dozen well-studied catchments in central Europe, provides novel insights into the mineralogical and stoichiometric controls on soil N retention and loss fluxes. Combining these results with those from forests from other biomes suggests a globally consistent relationship between N gas and NO3 − leaching losses. This relationship likely reflects mechanistic drivers depending on nitrate surplus in ecosystems, and enables broader extrapolation to estimate N gas losses from forests worldwide.
4.1. Forest soils are important long-term N sink
Soils comprised the largest N store within all of our study catchments, as is typical of most forests (Vesterdal et al 2008, Cremer et al 2016). Storage capacity for N in the mineral soil appears to be controlled by soil physicochemical properties, particularly aluminium- and iron-oxyhydroxides, which also have been shown to predict soil carbon stocks across the U.S. and to immobilise dissolved organic matter within mineral soil horizons (Kalbitz et al 2000, Bingham and Cotrufo 2016). Our results (figure 1) suggest that organic matter was preferentially protected in spodic horizons by interaction with poorly crystalline minerals represented by the oxalate soluble Al and Fe fraction (Kleber et al 2005, Spielvogel et al 2008). The occurrence of Spodosols generally increases with elevation across our catchments (Chuman et al 2021), and so do Alo + Feo stocks. Thus, catchment soils with high rates of mineral weathering, e.g. those with more water availability and dissolved organic matter leaching, are predisposed to accumulate relatively large quantities of organic N due to the development of soils with high sorption capacities in spodic horizons.
4.2. Soil C:N:P stoichiometry determines N losses
Catchment wetness was also the strongest correlate among environmental variables influencing forest floor C:N:P stoichiometry, which in turn governed N losses. Across our environmental gradient, wetter forests are associated with progressively thicker forest floor (Oulehle et al 2008) (moder/mor like organic horizons) and more acidic conditions (table 1). Increasing exchangeable acidity (Al and H+) promotes stabilization of organic P in the forest floor, thus negatively affecting P availability (Tahovská et al 2018). Reduced P availability associated with increased catchment wetness combined with increasing N deposition to result in surpluses of forest floor N over C and available P. These responses are consistent with the N saturation hypothesis (Aber et al 1989, 1998) that predicts eventual increases in nitrification and NO3 − loss to surface waters as a result of chronic N deposition, with new information on soil P limitation and N gas losses. Our data suggest that dissolved N losses in runoff are tightly linked to forest floor nutrient stoichiometry, with observed increases in N leaching as forest floor C:N ratio decreases (Gundersen et al 1998a, Lovett et al 2002, Dise et al 2009) and N:Pavailable ratio increases (figure 2). The strikingly tight relationship between dissolved NO3 − losses and forest floor N:Pavailable further suggests that chronic N enrichment leads to progressive P limitation. This observation is consistent with forest growth responses from Switzerland (Braun et al 2010) and canopy chemistry from the Adirondack region in the US (Crowley et al 2012) suggesting shifts towards P limitation as consequence of increasing N deposition.
Compared across catchments, total ecosystem 15N enrichment increased with catchment wetness, which pointed to the importance of gaseous losses. This ecosystem 15N enrichment could not be produced by N deposition, which is isotopically lighter than ecosystem N, or by leaching, which is isotopically heavier. By contrast, gaseous losses usually have large fractionation factors (Mariotti et al
1981, Denk et al
2017), which enrich the N that remains in the ecosystem. Denitrification can occur even in well-drained soils, in low-oxygen microsites or in temporarily or locally developed episodes (McClain et al
2003, Wexler et al
2014). Denitrification estimates calculated by isotopic model approaches (Houlton and Bai 2009, Fang et al
2015) are challenged by uncertainties associated with the assumed fractionation effects of the N loss processes. Across our catchments, the isotopic effect of dissolved N leaching varied between −1.6 and 10.7‰, with an average of +2.7‰. The large isotope effect associated with denitrification used in our study (range of −14 to −18‰) and elsewhere (Houlton and Bai 2009, Wexler et al
2014, Weintraub et al
2016, Soper et al
2018, Yu et al
2019) resulted in calculated average gaseous losses of 2.5 ± 2.2 kg N ha−1 yr−1 across our catchments. This assigned  D between −14 and −18‰ is less fractionating than the denitrification isotope effect calculated for forest soils under laboratory conditions with an abundant supply of NO3
− (−48‰ on average; Wang et al
2018). Underexpression of the
D between −14 and −18‰ is less fractionating than the denitrification isotope effect calculated for forest soils under laboratory conditions with an abundant supply of NO3
− (−48‰ on average; Wang et al
2018). Underexpression of the  D under field conditions compared to laboratory conditions is expected to arise from more effective nitrate consumption (e.g. Högberg 1997, Houlton et al
2006). Nevertheless, a correct understanding of isotopic fractionation and its variability during denitrification poses a critical challenge for further application of the 15N natural abundance method.
D under field conditions compared to laboratory conditions is expected to arise from more effective nitrate consumption (e.g. Högberg 1997, Houlton et al
2006). Nevertheless, a correct understanding of isotopic fractionation and its variability during denitrification poses a critical challenge for further application of the 15N natural abundance method.
Combining measured stream TDN fluxes with isotope-model derived denitrification estimates (figure 2) suggested non steady state N ecosystem balance, as the sum of these losses were less than N deposition inputs, yielding an N retention term by difference. The long-term accumulation of N in the soil derived from N deposition with a distinct δ15N signature (usually negative δ15N) (Bragazza et al 2005, Solga et al 2006) could impact the isotopic model calculations, leading to underestimates of the proportion of N losses occurring as N gases. Nonetheless, calculated N retention was strongly related to the forest floor concentration of available P and its ratios to total C and N, indicating an important role of P limitation in the capacity of forest ecosystem to retain N from deposition.
4.3. Globally consistent gaseous and dissolved losses alongside increasing N availability gradient
Across forest biomes, denitrification fluxes derived from ecosystem δ15N values increased with ecosystem NO3 − export (figure 3). The same overall relationship between N gas and NO3 − leaching loss held across tropical, subtropical and temperate forests, indicating a generalized response to increased NO3 − availability within ecosystems. Moreover, increasing N losses associated with progressive P scarcity (widening of N/P ratios in both forest floor and foliage) across our temperate forest gradient are generally consistent with observed increases of foliar N:P from high to low latitudes (McGroddy et al 2004) and increasing N losses towards tropical regions (Hedin et al 2009). The largest N loss fluxes to dissolved N and denitrification were calculated for the tropical/subtropical forest biome, followed by temperate and boreal biomes. The relative importance of dissolved N over gaseous N losses tended to increase with increasing NO3 − export, indicating that denitrification dominates when N losses are low but that denitrification capacity may be exceeded in N saturated ecosystems (figure 3).
Systematic evidence of increased N losses with increasing N availability in our regional analysis of temperate forest catchments match broader ecological observations of decreased N use efficiency in high-N forests (Vitousek 1982). Contemporary global average TDN export from catchments across different forest biomes (supplementary table 2), ranges from 5.3 kg N ha−1 yr−1 in tropical/subtropical forests to 1.6 kg N ha−1 yr−1 in boreal forests, with a global average of 4.2 kg N ha−1 yr−1, i.e. 13.5 Tg N yr−1 (table 2).
N gas losses have long been challenging to constrain, as they include the greenhouse gas N2O produced by both nitrification and denitrifiaiton, as well as production of elemental N2. Given the chain of possible N transformations as mineral N availability increases, including the coupled processes of nitrification and denitrification (both associated with formation of the greenhouse gas N2O) it is possible to constrain estimates of gaseous N losses based on hydrological NO3 − losses, which are routinely measured as part of catchment monitoring programs. Extrapolating our empirical relationship between N gas and NO3 − fluxes to forests worldwide yields an estimated values of 8.9 Tg N yr−1 (2.8 kg N ha−1yr−1). This estimate of N gas loss is much lower than past N-budget-based estimates of denitrification N losses from non-agricultural soils worldwide (58 Tg N yr−1) based on N mass balances that include highly uncertain and widely varying N fixation fluxes (Van Drecht et al 2003, Seitzinger et al 2006). Bouwman et al (2013) further provided denitrification estimate of 28 Tg yr−1 for non-agricultural soils applying revised (and much lower) estimates for BNF (Vitousek et al 2013) as N input to their mass balance calculations. This revised rate of denitrification recalculated to forest land is then similar to our independent denitrification estimate for forested land, thereby supporting the evidence of lower BNF in natural ecosystems (Vitousek et al 2013, Sullivan et al 2014) compared to earlier assessments (Cleveland et al 1999, Galloway et al 2004).
5. Conclusions
We combined nitrogen flux and isotope mass balances from a network of intensively studied catchments to reveal the underpinning role of soil P availability and N deposition in defining temperate forests N retention capacity. Increasing excess of soil N over available P, reflected in foliar N:P ratio, was accompanied by enhanced dissolved and gaseous N losses. Increasing catchment wetness corresponded to lower available P concentration in the forest floor, likely because of effective soil P stabilization due to changing soil acidity. These limitations on P availability, combined with N inputs from deposition, resulted in increasing gaseous and dissolved N losses along gradients of wetness and N deposition. This pattern is likely attributable to variation in soil nitrate production driven by excess N availability over soil C and P availability. Combining our results with 15N-based N flux estimates available from other temperate and tropical forests, we present a surprisingly consistent relationship between N gas loss and nitrate export for forests across the world, and estimate that N gas loss and dissolved TDN export counterbalance 39% and 59% respectively of worldwide N deposition to forests.
Acknowledgement
We are indebted to several research institutes (Czech Geological Survey, Academy of Sciences of the Czech Republic, Forestry and Game Management Research Institute and Czech Hydrometeorological Institute) for long lasting support of catchment monitoring. Concept of this work was developer under the GeoMicLink project sponsored by Czech Science Foundation (GACR) (No. 20-19471S), 15N isotope analysis were supported by the GACR project No. 18-17295S. The authors are thankful to I. Roshka and N. Pernicová for their laboratory work on 15N analyses. This study contributes to the SustES - Adaptation strategies for sustainable ecosystem services and food security under adverse environmental conditions (CZ.02.1.01/0.0/0.0/16_019/0000797) and Czech research infrastructure for systems biology C4SYS (project No. LM2015055).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.




