Abstract
C-TERMINALLY ENCODED PEPTIDE (CEP) and cytokinin hormones act over short and long distances to control plant responses to environmental cues. CEP and cytokinin pathway mutants share phenotypes, however, it is not known if these pathways intersect. We show that CEP and cytokinin signalling converge on CEP DOWNSTREAM (CEPD) glutaredoxins to inhibit primary root growth. CEP inhibition of root growth was impaired in mutants defective in trans-zeatin (tZ)-type cytokinin biosynthesis, transport, perception, and output. Concordantly, mutants affected in CEP RECEPTOR 1 showed reduced root growth inhibition in response to tZ, and altered levels of tZ-type cytokinins. Grafting and organ-specific hormone treatments showed that tZ-mediated root growth inhibition involved CEPD activity in roots. By contrast, root growth inhibition by CEP depended on shoot CEPD function. The results demonstrate that CEP and cytokinin pathways intersect, and utilise signalling circuits in separate organs involving common glutaredoxin genes to coordinate root growth.
Similar content being viewed by others
Introduction
To ensure their survival under diverse conditions, plants evolved complex intercellular communication mechanisms that enable them to sense and respond to environmental cues1. To help achieve this, plants have co-opted metabolite- and peptide-hormone pathways to control a myriad of adaptive responses2,3,4. There is a growing number of interactions established between peptide hormones and metabolite hormone pathways. These pathways involve local and systemic signalling to coordinate growth and stress responses throughout the plant5,6. One example is the interaction between cytokinin and CLAVATA3/Endosperm surrounding region-related (CLE) hormone pathways which together regulate shoot meristem size7 and protoxylem vessel formation8, however little is known about interactions between cytokinin and other peptide hormone pathways.
C-TERMINALLY ENCODED PEPTIDEs (CEPs) play a key role in plant responses to the environment9. The Arabidopsis thaliana genome has 12 canonical CEP genes that encode propeptide precursors from which one or more 15-amino acid CEP peptide hormones are excised10,11. Mature, root-derived CEPs then enter the xylem stream and travel shootward12,13,14. CEP gene expression responds to several environmental and nutritional cues including low nitrogen and high carbon10,15,16. CEPs interact with CEP RECEPTOR 1 (CEPR1) in Arabidopsis12 and its orthologue COMPACT ROOT ARCHITECTURE 2 (CRA2) in Medicago truncatula17,18,19. CEP-CEPR1 signalling inhibits root growth12,16,18,20, modulates root system architecture and auxin transport21, and promotes root nodulation18,22,23, shoot growth12, and resource allocation for seed yield24. In addition, the interaction of CEP1 with CEPR1 promotes nitrate uptake in roots via a systemic mechanism12 by upregulating the genes encoding the phloem-mobile class III glutaredoxins CEP DOWNSTREAM 1 (CEPD1) and CEPD2. CEPD1 and CEPD2 subsequently travel rootward to positively regulate high affinity nitrate transporter gene expression and protein function in the roots25,26.
Like CEPs, cytokinins also control plant growth in response to the environment and changes in nutritional status27,28,29,30. ISOPENTENYLTRANSFERASE 3 (IPT3), IPT5, and IPT7 are major contributors to the biosynthesis of isopentenyladenine (iP)- and trans-zeatin (tZ)-type cytokinins31. The P450 enzymes CYP735A1 and CYP735A2 catalyse the conversion of iP-type to tZ-type cytokinins primarily in roots32,33. The transporter ATP-BINDING CASSETTE G14 (ABCG14) then enables the shootward translocation of tZ34,35. Cytokinins are perceived by ARABIDOPSIS HISTIDINE KINASE (AHK) receptors36,37. Double-knockout mutants in AHK2 and AHK3 have increased root system growth and diminished shoot growth37,38, which resembles the phenotype of cepr1 knockout mutants12,16. After AHKs perceive cytokinin, a number of type-B response regulators (ARRs) with redundant functions mediate the transcriptional responses to cytokinin39,40. Class III glutaredoxins also appear to play a role in mediating cytokinin-dependent responses, with several acting downstream of type-B ARRs to inhibit root growth in response to high nitrogen41. In addition, several class III glutaredoxins also are implicated in plant responses to low nitrogen status42. Recently, Ota et al.43 demonstrated that ABCG14 is necessary for the full upregulation of particular class III glutaredoxin genes under low nitrogen, including CEPD1 and CEPD2. Given that CEPs and cytokinin have similar roles in inhibiting root growth, promoting shoot growth, and in managing whole plant responses to nitrogen status44,45,46, it is possible that these pathways intersect.
In this paper, we define a point of intersection of the CEP and cytokinin pathways. Firstly, we used biochemical, genetic and grafting approaches to assess the requirement of cytokinin pathway components for CEP activity and vice versa. We found that mutants affected in tZ synthesis, transport, perception, and signalling showed altered sensitivity to CEP-mediated inhibition of primary root growth and, correspondingly, CEP receptor mutants showed altered sensitivity to tZ. Grafting showed that CEP activity depends on AHK2 and AHK3 in both the root and shoot. In addition, we determined using mass spectrometry that cepr1 mutants had altered cytokinin content, particularly in the level of tZ-type cytokinins. Finally, we showed that tZ and CEP inhibition of primary root growth requires CEPD glutaredoxins. CEPD1 transcript and GFP-CEPD1 levels responded to tZ treatment, and CEPD1 expression was basally altered in ahk2,3. Surprisingly, grafting and organ-specific hormone treatments showed that root growth inhibition by tZ required root CEPD activity, whereas CEPD activity in the shoot was required for CEP responses. Together, these data indicate that CEP and tZ converge on CEPD activity in different organs to inhibit primary root growth. We propose a model where root and shoot signalling circuits involving common glutaredoxin gene targets integrate CEP and tZ pathways to mediate plant responses to environmental and nutritional cues.
Results and discussion
CEP inhibition of primary root growth depends on the cytokinin pathway
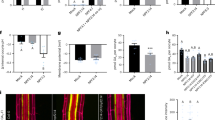
We first assessed if mutants affected in cytokinin synthesis or transport showed altered sensitivity to CEP3 peptide mediated inhibition of primary root growth10,20 (Fig. 1; Supplementary Fig. 1). We identified a partial insensitivity to CEP3 in mutants affected in iP and tZ-type cytokinin biosynthesis (i.e. ipt3,5,7), and tZ-type synthesis (cyp735a1,2) and transport (abcg14) (Fig. 1). Next, we examined how cytokinin perception via AHKs contribute to CEP3 sensitivity (Fig. 2; Supplementary Fig. 2). A double knockout mutant affecting AHK2 and AHK3 showed partial insensitivity to CEP3, whereas there was no change in CEP3 sensitivity in the other single or double cytokinin receptor mutants (Fig. 2a,b). To further assess the level of CEP3 insensitivity in ahk2,3, we measured relative root growth inhibition to increasing concentrations of CEP3. The ahk2,3 mutant was circa 100-fold less sensitive to CEP3 than wild type (Fig. 2c).
a Representative images and (b) relative primary root length for cytokinin biosynthesis or transport mutants grown on medium with or without CEP3 peptide (10−6 M) for 10 days in comparison to Col-0 and cepr1-3 (n = 7–8 plants). Root length expressed as a percentage of seedlings grown on medium without CEP3 (control) for each respective genotype. Letters show significant differences (ANOVA followed by Tukey HSD test, p < 0.05). Scale bar = 1 cm. Box plot centre line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. See Supplementary Fig. 1 for associated absolute root growth measurements. Exact p values and sample sizes for each treatment group are provided in the Source Data file.
a Representative images and (b) relative primary root length for cytokinin perception mutants grown on medium with or without CEP3 peptide (10−6 M) for 10 days in comparison to Col-0 and cepr1-3 (n = 8 plants). c Sensitivity of ahk2,3 to increasing CEP3 concentrations (0, 10−8, 10−7 or 10−6 M) at 10 days of growth (n = 17-21 plants). Root length expressed as a percentage of seedlings grown on medium without CEP3 for each respective genotype. d Effect of CEP3 overexpression in wild-type and ahk2,3. Primary root length after 7 days growth for CEP3ox plants was normalised to their respective background control (Col-0 or ahk2,3) (n = 12 plants). e Primary root length for reciprocal hypocotyl grafts between CEP3 overexpressing plants in the Col-0 and ahk2,3 backgrounds (n = 15–22 plants). Primary root length measured 12 days post grafting. f Representative images and (g) relative primary root length of gain-of-function AHK mutants grown on medium with or without CEP3 peptide (10−6 M) for 12 days in comparison to Col-0. Letters in (b-e, g) indicate significant differences (ANOVA followed by Tukey HSD test, p < 0.05). Scale bars = 1 cm. Box plot centre line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. See Supplementary Fig 2 for associated absolute root growth measurements and representative images. Exact p values and sample sizes for each treatment group are provided in the Source Data file.
We then used a CEP3 overexpressing line (CEP3ox) crossed into the ahk2,3 mutant background as an independent strategy to assess the interaction between CEP signalling and AHK2,3. The ahk2,3 plants overexpressing CEP3 showed a smaller reduction in root length than CEP3-overexpressing Col-0 (Fig. 2d). To determine if root or shoot ahk2,3 dampened the effect of CEP3 overexpression, we reciprocally grafted the CEP3ox line (i.e. wild type for AHK2,3) with CEP3 overexpressing ahk2,3 plants (ahk2,3/CEP3ox; Fig. 2e). CEP3ox plants with ahk2,3 in roots only had increased primary root growth relative to CEP3ox plants with wild type AHK2,3 in both roots and shoots, which supported a role for root AHK2,3 function downstream of CEP3 (Fig. 2e). Maximal root growth, however, was observed in CEP3ox plants with ahk2,3 in both roots and shoots, implying that shoot AHK2,3 also contributes to root growth inhibition (Fig. 2e). Together, these data support a role for AHK2 and AHK3 activity in both the root and shoot in mediating CEP3-sensitivity for root growth inhibition.
Given that both CEP20 and cytokinin signalling47 decrease primary root meristem cell number, we also assessed whether CEP inhibition of meristem cell number depended on AHK2,3 (Supplementary Figs. 3, 4). Meristem cell number in ahk2,3 was insensitive to CEP3 addition (Supplementary Fig. 3), and partially insensitive to CEP3 overexpression (Supplementary Fig. 4).
To test further that cytokinin signalling through AHK2,3 plays a specific role in the sensitivity of plants to CEPs, we assessed CEP sensitivity in the constitutively-active gain-of-function mutants in either AHK2 (rock2) or AHK3 (rock3)48. Consistently, the rock2 and rock3 mutants showed increased CEP sensitivity (Fig. 2f,g). The results observed with the loss- and gain-of-function cytokinin receptor mutants support a role for AHK2 and AHK3 in CEP-mediated inhibition of root growth. Collectively, these results indicate that the full CEP-mediated inhibition of primary root growth involves tZ synthesis and transport, and cytokinin perception by AHK2 and AHK3.
We then assessed the requirement for type-B response regulators in mediating CEP inhibition of root growth. We tested the CEP sensitivity of two high-order type-B response regulator (arr) mutants39,40. The arr1,2,12 mutant was partially insensitive to CEP3, whereas the arr1,2,10 mutant exhibited no alteration to CEP sensitivity compared to the wild type (Supplementary Fig. 5). These results support a role for particular type B ARRs in CEP-mediated inhibition of root growth.
CEP and cytokinin signalling pathways intersect
To investigate whether tZ inhibition of root growth requires CEP signalling, we assessed the sensitivity of cepr1 mutants to tZ inhibition of primary root growth (Fig. 3; Supplementary Fig. 6). The cepr1-3 mutant showed a circa 10-fold reduction in sensitivity to tZ (Fig. 3a, b). At 10 nM tZ, there was a clear differential sensitivity of cepr1-3 compared to the wild type, and a reduced sensitivity at 100 nM tZ.
a Representative images and b relative primary root length for Col-0 and cepr1-3 in response to increasing concentrations of tZ (0, 10, 100 nM) at 12 dg. Root length expressed as a percentage of seedlings grown on medium without tZ for each respective genotype (n = 7 plants). c CEP and tZ signalling is defective in cepr1-3. Primary root length was measured for Col-0 and cepr1-3 plants grown on medium containing CEP3 (1 µM), tZ (10 nM), combined CEP3 and tZ, or control medium (no CEP or tZ) for 12 days (n = 7 plants). Root length expressed as a percentage of the control plants. Letters represent significant differences (ANOVA followed by Tukey HSD test, p < 0.05). Scale bar = 1 cm. Box plot centre line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. See Supplementary Fig. 6 for associated absolute root growth measurements and representative images. Exact p values are provided in the Source Data file.
To determine whether tZ and CEP interact, we tested the sensitivity of wild type and cepr1-3 to tZ (10 nM), and CEP3 (1 µM) alone or in combination. Whilst tZ and CEP3 affected root growth additively in the wild type, cepr1-3 was insensitive to CEP3 and/or tZ treatment (Fig. 3c). This indicates that whilst the effects of CEP3 and cytokinin are additive in the wild type, signalling through CEPR1 affects both tZ and CEP-dependent signalling. Collectively, these results indicate an intersection of CEP and cytokinin mediated inhibition of primary root growth.
Cytokinin homoeostasis is perturbed in cepr1 plants
Mutants affected in cytokinin transport, perception and signalling have perturbed cytokinin content34,35,37,49. To examine if CEPR1 affects cytokinin homoeostasis, we measured cytokinin levels in roots and shoots of cepr1 (Table 1; Supplementary Table 1; Supplementary Data 1). Notably, there were significant perturbations to total root and shoot tZ-type cytokinin levels in cepr1-3 (Col-0) and cepr1-1 (No-0) compared to wild type (Table 1). Specifically, tZ-type cytokinins were elevated in the roots of both cepr1 mutants. The effects of cepr1 knockout on tZ-type cytokinins in the shoot were ecotype-dependent, with a higher amount in cepr1-3 and a lower amount in cepr1-1 compared to wild type. In addition, there were some ecotype-dependent perturbations in other cytokinin types in cepr1 mutants. The altered cytokinin homoeostasis in cepr1 supports an impairment of cytokinin signalling in these mutants, particularly in tZ-type cytokinin responses. The higher amount of tZ-type cytokinins in cepr1 roots is consistent with feedback upregulation of tZ biosynthesis resulting from impaired tZ signalling in cepr1 plants, akin to the increased root tZ levels observed in ahk2,3 mutants37,50.
CEPD1 expression is induced by tZ and affected by AHK2,3 activity
CEP- and tZ-dependent pathways intersect to inhibit root growth, however, it is unknown how the two pathways converge. Recently, Ota et al.43 showed that low nitrogen-dependent upregulation of the glutaredoxin-encoding genes CEPD1 and CEPD2 involved the cytokinin transporter ABCG14. Therefore, we evaluated whether CEPD expression depended on cytokinin signalling (Fig. 4). Firstly, we determined if CEPD1 and CEPD2 expression in roots or shoots was regulated by tZ treatment (Fig. 4a–c). tZ treatment upregulated the cytokinin marker gene ARR5 in both roots and shoots at 8 h post treatment, confirming that a cytokinin response was elicited (Fig. 4a). Simultaneously, CEPD1 was significantly upregulated in roots by tZ (Fig. 4b), whereas CEPD2 expression was not significantly altered (Fig. 4c). Moreover, GFP-CEPD1 fluorescence was increased in the primary root vasculature region in response to tZ or CEP3 treatment (Fig. 4d, e). In addition, CEPD1 transcript levels were basally lower in the ahk2,3 background compared to wild type but CEPD1 transcription remained responsive to CEP3 treatment (Fig. 4f). Additionally, we tested if CEP3 peptide treatment upregulates CEPDs in the shoot, as has been shown for the CEP1 peptide25. CEP3 upregulated CEPD1 and CEPD2 transcripts in shoots (Fig. 4g). Moreover, CEP3 treatment, but not tZ treatment, upregulated GFP-CEPD1 fluorescence in the cotyledon vasculature (Fig. 4h, i). These data suggest that CEPD1 transcript levels in roots are induced by tZ and are affected by AHK2,3 activity, and that CEP3 can increase CEPD expression in both roots and shoots.
a ARR5, (b) CEPD1, and (c) CEPD2 expression in roots and shoots in response to tZ treatment (8 h). n = 3 biologically independent samples containing ~50 roots, or ~30 shoots. d Representative images and (e) fluorescence intensity of GFP-CEPD1 in response to tZ or CEP3 treatment (24 h) in the primary root vasculature region (white dashed box). n = 10-16 plants. f CEPD1 expression in roots responds to CEP treatment (8 h) and is basally reduced in ahk2,3. g CEPD1 and CEPD2 expression in the shoots respond to CEP3 treatment (8 h). n = 3 biologically independent samples containing ~50 roots, or ~20 shoots. h Representative images and i GFP-CEPD1 fluorescence in the cotyledon above background (pixels with intensity value above 26,000) in response to tZ or CEP3 treatment (24 h). n = 12-14 cotyledons. Five (d, e, h, i) or 6 day old (a–c, f, g) seedlings were transferred for specified durations to medium with DMSO (control), tZ (10 nM), or CEP3 (1 µM). Significant differences determined by a two-sided two-sample t test without adjustments for multiple comparisons; *p < 0.05, **p < 0.01, ***p < 0.001 (a–c, e, g, i) or ANOVA followed by Tukey HSD test, p < 0.05 (f). Exact p values for (f) are provided in the Source Data file. Box plot centre line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. Scale bars: (d) = 100 µm; (i) = 200 µm. Exact sample sizes for each treatment group for (d) and (h) are provided in the Source Data file.
tZ sensitivity is affected by root CEPD activity
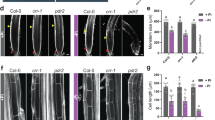
Given that CEPD expression is affected by cytokinin signalling, we assessed whether CEPDs are functionally involved in root growth inhibition in response to tZ (Fig. 5; Supplementary Fig. 7). The cepd1,2 double mutant (No-0 ecotype) was partially insensitive to tZ application with respect to inhibition of primary root growth (Fig. 5a, b) and meristem cell number (Supplementary Fig. 8). As CEPDs are phloem-mobile, shoot-to-root signals25, we determined via reciprocal hypocotyl grafting if root or shoot CEPD activity was required for root growth inhibition by tZ. Surprisingly, having a cepd1,2 root genotype was sufficient to impart a reduced sensitivity to tZ (Fig. 5c). To further test if local or long-distance signalling was involved in tZ-dependent root growth inhibition, we utilised a segmented agar plate set-up to specifically treat roots or shoots with tZ (Fig. 5d). tZ treatment of roots was sufficient for root growth inhibition, and the extent of root growth inhibition was reduced in cepd1,2 (Fig. 5d). Therefore, in addition to its known role as a shoot-derived signal in the CEP pathway, cytokinin-mediated inhibition of root growth depends on a local signalling circuit involving root-derived CEPD.
a Representative images and (b) relative primary root growth for No-0, cepr1-1, and the cepd1,2 double mutant seedlings 3 days after transfer to medium with or without tZ (10 nM) (n = 11–24 plants). Horizontal bars in (a) indicate the length of the primary root on the day of transfer (i.e. 6 days growth). Root growth post transfer expressed as a percentage of seedlings grown on solvent control (DMSO) for each respective genotype. c tZ sensitivity of reciprocal hypocotyl grafts between No-0 and cepd1,2. Primary root growth 3 days post transfer to tZ (5 nM) expressed as a percentage of seedlings transferred to DMSO (control) for each respective graft combination (n = 4–8 plants). d Relative primary root growth for No-0 and cepd1,2 seedlings 3 days post transfer to segmented plates for tZ treatment of roots and/or shoots. See lower panel for diagrammatic representation of the segmented agar plate set-up. Plants were grown for 6 days before transfer to segmented plates with tZ (10 nM) or solvent control (DMSO) infused in each plate segment. Plants were positioned such that roots only were in contact with the bottom segment, and shoots only were in contact with the top segment. Root growth post transfer expressed as a percentage of the DMSO control for each respective genotype (n = 13–18 plants). Letters in (b–d) show significant differences (ANOVA followed by Tukey HSD test, p < 0.05). Scale bar = 1 cm. Box plot centre line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. See Supplementary Fig 7 for associated absolute root growth measurements and representative images. Exact p values and sample sizes for each treatment group are provided in the Source Data file.
Shoot CEPD activity is required for CEP inhibition of primary root growth
Whilst shoot CEPD function is important for CEP signalling25, a role for root CEPDs is plausible given root CEPD1 transcripts are upregulated by CEP (Fig. 4f) and are downregulated in Arabidopsis cepr116, and Medicago cra2 lines23. Therefore, we investigated if CEPDs were involved in CEP-dependent root growth inhibition and whether this required root or shoot CEPD function (Fig. 6, Supplementary Fig. 9). The cepd1,2 double mutant was partially insensitive to CEP3 application (Fig. 6a, b). Reciprocal hypocotyl grafting demonstrated that shoot cepd1,2 was sufficient to diminish sensitivity to CEP3 (Fig. 6c). We next confirmed using a segmented agar plate set-up that shoot-applied CEP3 was sufficient to impart maximal root growth inhibition (Supplementary Fig. 9d, e), and that cepd1,2 was less sensitive to shoot applied CEP3 (Fig. 6d). These results demonstrate that the CEP and cytokinin pathways converge on CEPD activity in different organs to modulate root growth.
a Representative images and (b) relative primary root growth for No-0, cepr1-1, and the cepd1,2 double mutant seedlings 3 days after transfer to medium with or without CEP3 (10−6 M) (n = 12–18 plants). Horizontal bars in (a) indicate the length of the primary root on the day of transfer (i.e. 6 days growth). Root growth post transfer expressed as a percentage of seedlings grown on medium without CEP3 (control) for each respective genotype. c CEP3 sensitivity of reciprocal hypocotyl grafts between No-0 and cepd1,2. Root growth 3 days post transfer to treatment plates expressed as a percentage of seedlings grown on medium without CEP3 (control) for each respective graft combination (n = 5–8 plants). d A diagrammatic representation (upper) and relative primary root growth (lower) for No-0 and cepd1,2 seedlings 3 days post transfer to segmented plates for selective CEP3 treatment of shoots. Plants were grown for 6 days before transfer to segmented plates with CEP3 (10−6 M) or solvent control (water) infused in the top agar segment. Plants were positioned such that shoots but not roots were in contact with the top segment. Root growth post transfer expressed as a percentage of the water control for each respective genotype (n = 22–23 plants). Letters in (b–d) show significant differences (ANOVA followed by Tukey HSD test, p < 0.05). Scale bar = 1 cm. Box plot centre line, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range. See Supplementary Fig 9 for associated absolute root growth measurements, representative images, and extended data. Exact p values and sample sizes for each treatment group are provided in the Source Data file.
Environmental cues trigger cytokinin and CEP pathways that converge on CEPDs to control root growth
CEPs and cytokinins control related processes in plants including root growth10,16,20,37,51, root nodule formation18,52, nutrient mobilisation and utilisation16,24,53, auxin transport21,54 and yield24,55. Until now, definitive links or overlaps between cytokinin and CEP signalling remained obscure or had not been functionally verified.
From the results here and from prior work10,12,15,16,23,25,43,45, we propose the following model where CEP and tZ pathways converge on CEPD glutaredoxins (Fig. 7). Environmental or internal (e.g. nutrient status) stimuli trigger the production of CEP and tZ-type cytokinin signals in roots. These can act locally in roots, or enter the xylem stream for translocation to the shoot. CEP perception by CEPR1 inhibits root growth via shoot-derived CEPDs, whereas tZ perception by AHK2,3 inhibits root growth locally via root-derived CEPDs. Crosstalk occurs between the CEP and tZ pathways as (i) CEP-CEPR1 signalling results in feedback inhibition of tZ levels in roots, (ii) CEP-CEPR1 regulates root CEPD expression16,23, thus affecting tZ-sensitivity, and (iii) tZ transport to the shoot impacts shoot CEPD expression under low nitrogen43, affecting CEP-sensitivity. CEPDs therefore act as a convergence point to integrate CEP and tZ-type cytokinin signalling to fine tune root growth in response to a spectrum of stimuli. Given that cepd1,2 mutants are not fully insensitive to CEP or tZ treatment, this suggests other molecular components also contribute to CEP and cytokinin downstream signalling. It is plausible that additional type III glutaredoxin members41,43, or other yet to be determined signalling components, contribute to CEP/cytokinin signalling and crosstalk in addition to CEPDs. Establishing the generalisability of CEPD’s contribution to CEP and cytokinin signalling will also require future studies utilising cepd mutants in different Arabidopsis ecotypes as well as in different plant species. Whether hormone crosstalk in control of root growth also involves cytokinin-dependent regulation of CEP gene expression, as recently shown in legume nodulation56, remains an open question. From a nitrogen centric perspective, the convergence of low nitrogen (i.e. CEP)- and high nitrogen (i.e. cytokinin)- induced hormone pathways on class III glutaredoxin activity implies that plants utilise common gene targets to slow root growth under disparate conditions such as under nitrate starvation or excess. More broadly, as both CEP and cytokinin are induced by elevated sugar16,57, it is possible that these pathways converge on CEPD glutaredoxin function to balance root growth and nitrogen acquisition with the availability of photosynthetically-derived carbon.
In the shoot, the perception of root-derived CEP by CEPR1 results in increased CEPD1,2 glutaredoxin production (red text). Shoot derived CEPD1,2 travels to the root via the phloem. In the root, tZ signals locally through AHK2,3 to transcriptionally induce local CEPD1 production (black text). It is possible that tZ signals through CEPD2 (grey text) locally via other mechanisms (e.g. post-translationally). The pool of shoot- and root-derived CEPD (orange box with broken lines) ultimately integrates root growth responses with whole-of-plant nutritional and environmental stress status. tZ trans-zeatin, AHK ARABIDOPSIS HISTIDINE KINASE, CEP C-TERMINALLY ENCODED PEPTIDE, CEPR CEP RECEPTOR, CEPD CEP DOWNSTREAM.
Methods
Plant materials and growth conditions
In the Arabidopsis thaliana Col-0 ecotype, the cepr1-3 (467C01; GABI-Kat)16,58, CEP3ox10, ahk2-5, ahk3-7, cre1-2, ahk2-5 ahk3-7, ahk2-5 cre1-2 and ahk3-7 cre1-237, abcg14-2[ 34,35, ipt3-2 ipt5-2 ipt7-131, cyp735a1-2 cyp735a2-233, rock2-1 and rock3-148 lines were used. The arr triple mutants (arr1,2,10 and arr1,2,12; Col-0) were generated by crossing arr1-3 and arr10-5 or arr12-139, with arr2 (GK-269G01)59. The ahk2,3/CEP3ox line was generated by crossing CEP3ox10 and ahk2-5 ahk3-737, and F3 or F4 progeny homozygous for all transgenes were used. In the Nossen (No-0) ecotype, the cepr1-1 (RATM11-2459; RIKEN)12,60 cepd1-1 cepd2-125, and GFP-CEPD1 (in cepd1-1 cepd2-1)25 lines were used. Sterilised seeds were grown on solidified media (1% Type M agar) containing ½ strength Murashige–Skoog (MS) basal salts (Sigma) at pH 5.7. Medium was supplemented with 1% w/v sucrose for experiments in Figs. 1–3, and Supplementary Figs. 1, 2 and 4. Medium for all other experiments contained no added sucrose, unless otherwise indicated. Plates were grown in chambers at 22 °C with a 16 h photoperiod with 100–120 µmol m−2 s−1 light. Roots were scanned on a flatbed scanner and primary root lengths were measured using ImageJ with the SmartRoot plugin61.
CEP peptide and trans-zeatin treatments
Synthetic AtCEP3 (TFRhyPTEPGHShyPGIGH) was dissolved in H2O10. CEP3 peptide was synthesised by GL Biochem, Shanghai, and the structure was validated independently by mass spectrometry. trans-zeatin (Sigma) was dissolved in DMSO. CEP3 and trans-zeatin were added to media after autoclaving and used at the concentrations indicated.
Grafting
Grafting was performed as described previously21. Briefly, seedlings were grown for 6 days on ½ MS with 0.5% sucrose prior to hypocotyl grafting. Both cotyledons were removed then scions were cut and transplanted to the new rootstock hypocotyl. Grafts recovered for five days before subsequent plate transfers. For Fig. 2e, grafted plants were transferred to ½ MS medium with 1% sucrose and grown for a further 7 days before primary root length was measured. For Fig. 5c and Fig. 6c, plants were first transferred to ½ MS medium (no sucrose) for 3 days, and then to ½ MS medium (no sucrose) treatment plates with or without tZ (5 nM) or CEP3 (1 µM), respectively. Plants were grown for a further 3 days on treatment plates before primary root growth post transfer was measured.
Microscopy
Root analyses were conducted with a Leica DM5500 microscope. For enumeration of cortical cells in the meristematic zone, primary root tips were stained with propidium iodide (100 µM, 2 min) and imaged using excitation at 540-580 nm and emission at 592–668 nm16,20. For quantification of GFP-CEPD1 fluorescence, the primary root vasculature region was imaged using excitation at 490-510 nm and emission at 520–550 nm. Mean pixel intensity was determined using ImageJ (http://rsb.info.nih.gov/ij/). Cotyledon analyses were conducted with a Zeiss LSM800 with Airyscan confocal microscope (Centre for Advanced Microscopy, ANU) using excitation at 488 nm, emission at 509 nm and detection at 410-550 nm. Pixels in the region corresponding to the cotyledon with intensity values above 26000 were counted using ImageJ, as this threshold minimised the presence of background fluorescence outside the cotyledon vasculature.
Quantification of endogenous cytokinins
The concentrations of endogenous cytokinins were determined in roots and shoots of 6-d-old Arabidopsis plants grown on ½ MS plates. For each sample, pooled roots or shoots (7 to 25 mg fresh weight) were snap frozen in liquid nitrogen and subsequently freeze-dried. Five independent biological replicates were analysed for each genotype and tissue. Sample extraction and purification were performed according to the method described previously62, with modifications63. Briefly, samples (approx. 20 mg fresh weight) were homogenized and extracted in 1 mL of modified Bieleski buffer (60% MeOH, 10% HCOOH and 30% H2O) together with a cocktail of stable isotope-labelled internal standards (0.2 pmol of cytokinin bases, ribosides, N-glucosides, and 0.5 pmol of cytokinin O-glucosides, nucleotides per sample added). The extracts were applied onto an Oasis MCX column (30 mg/1 mL, Waters) conditioned with 1 mL each of 100% MeOH and H2O, equilibrated sequentially with 1 mL of 50% (v/v) nitric acid, 1 mL of H2O, and 1 mL of 1 M HCOOH, and washed with 1 mL of 1 M HCOOH and 1 mL 100% MeOH. Analytes were then eluted by two-step elution using 1 mL of 0.35 M NH4OH aqueous solution and 2 ml of 0.35 M NH4OH in 60% (v/v) MeOH solution. The eluates were then evaporated to dryness in vacuo and stored at −20 °C. Quantification of endogenous cytokinins was done by ultra-high performance liquid chromatography–fast scanning tandem mass spectrometry, using stable isotope-labelled cytokinin standards62. Separation was performed on an Acquity UPLC® i-Class System (Waters, Milford, MA, USA) equipped with an Acquity UPLC BEH Shield RP18 column (150 × 2.1 mm, 1.7 μm; Waters), and the effluent was introduced into the electrospray ion source of a triple quadrupole mass spectrometer Xevo™ TQ-S MS (Waters), operating in multiple reaction monitoring (MRM) mode. Cytokinin concentrations were determined using MassLynx software (version 4.2; Waters) using stable isotope dilution method (Supplementary Data 1). Five independent biological replicates were performed, including two technical replicates of each.
qRT-PCR
Total RNA was isolated from harvested tissue snap frozen in liquid nitrogen using a modified Trizol extraction method using columns from the RNeasy plant mini kit (QIAGEN)10. cDNA synthesis was performed using oligo(dT)12–18 primers and Superscript III reverse transcriptase (Invitrogen). qRT–PCR was conducted using Fast SYBR Green fluorescent dye (Applied Biosystems) and samples were run on a ViiA 7 Real-Time PCR System (Applied Biosystems) according to manufacturer’s specifications. Data were analysed using the ΔΔCT method64, with EF1α (At1g07920) expression used for normalisation65. Primers used are listed in Supplementary Table 2.
Segmented agar plate assays
For segmented agar plates, a 5 mm trench in the agar was excavated 18 mm from the top of the plate. Solutions of CEP, tZ, or solvent controls were applied to final specified concentrations, spread evenly across the root or shoot agar segment, and allowed to diffuse overnight. After 6 days of growth on standard agar plates, plants were transferred to segmented plates and positioned such that roots only were in contact with the bottom segment, and shoots only were in contact with the top segment.
Accession numbers
Arabidopsis Genome Initiative locus codes are as follows: CEPR1, AT5G49660; CEPD1, AT1G06830; CEPD2, AT2G47880; AHK2, AT5G35750; AHK3, AT1G27320.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Norman, J. M. V., Breakfield, N. W. & Benfey, P. N. Intercellular communication during plant development. Plant Cell 23, 855–864 (2011).
Tavormina, P., De Coninck, B., Nikonorova, N., De Smet, I. & Cammue, B. P. A. The plant peptidome: an expanding repertoire of structural features and biological functions. Plant Cell 27, 2095–2118 (2015).
Verma, V., Ravindran, P. & Kumar, P. P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16, 86 (2016).
Roy, S., Lundquist, P., Udvardi, M. & Scheible, W.-R. Small and Mighty: Peptide hormones in plant biology. Plant Cell 30, tpc.118.tt0718 (2018).
Murphy, E., Smith, S. & Smet, I. D. Small signaling peptides in Arabidopsis development: How cells communicate over a short distance. Plant Cell 24, 3198–3217 (2012).
Shabala, S., White, R. G., Djordjevic, M. A., Ruan, Y.-L. & Mathesius, U. Root-to-shoot signalling: integration of diverse molecules, pathways and functions. Funct. Plant Biol. 43, 87–104 (2016).
Gordon, S. P., Chickarmane, V. S., Ohno, C. & Meyerowitz, E. M. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. 106, 16529 (2009).
Kondo, Y., Hirakawa, Y., Kieber, J. J. & Fukuda, H. CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling. Plant Cell Physiol. 52, 37–48 (2011).
Taleski, M., Imin, N. & Djordjevic, M. A. CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J. Exp. Bot. 69, 1829–1836 (2018).
Delay, C., Imin, N. & Djordjevic, M. A. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. J. Exp. Bot. 64, 5383–5394 (2013).
Ogilvie, H. A., Imin, N. & Djordjevic, M. A. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes. BMC Genomics 15, 870 (2014).
Tabata, R. et al. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346 (2014).
Okamoto, S., Suzuki, T., Kawaguchi, M., Higashiyama, T. & Matsubayashi, Y. A comprehensive strategy for identifying long-distance mobile peptides in xylem sap. Plant J. 84, 611–620 (2015).
Patel, N. et al. Diverse peptide hormones affecting root growth identified in the Medicago truncatula secreted peptidome. Mol. Cell. Proteom. 17, 160 (2018).
Imin, N., Mohd-Radzman, N. A., Ogilvie, H. A. & Djordjevic, M. A. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot. 64, 5395–5409 (2013).
Chapman, K., Taleski, M., Ogilvie, H. A., Imin, N. & Djordjevic, M. A. CEP–CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. J. Exp. Bot. 70, 3955–3967 (2019).
Huault, E. et al. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLOS Genet 10, e1004891 (2014).
Mohd-Radzman, N. A. et al. Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiol. 171, 2536 (2016).
Lee, H.-C. et al. A new method to visualize CEP hormone–CEP receptor interactions in vascular tissue in vivo. J. Exp. Bot. 72, 6164–6174 (2021).
Delay, C. et al. CEP3 levels affect starvation-related growth responses of the primary root. J. Exp. Bot. 70, 4763–4774 (2019).
Chapman, K. et al. CEP receptor signalling controls root system architecture in Arabidopsis and Medicago. N. Phytol. 226, 1809–1821 (2020).
Laffont, C. et al. Independent regulation of symbiotic nodulation by the SUNN negative and CRA2 positive systemic pathways. Plant Physiol. 180, 559 (2019).
Gautrat, P., Laffont, C. & Frugier, F. Compact root architecture 2 promotes root competence for nodulation through the miR2111 systemic effector. Curr. Biol. 30, 1339–1345.e3 (2020).
Taleski, M., Chapman, K., Imin, N., Djordjevic, M. A. & Groszmann, M. The peptide hormone receptor CEPR1 functions in the reproductive tissue to control seed size and yield. Plant Physiol. 183, 620–636 (2020).
Ohkubo, Y., Tanaka, M., Tabata, R., Ogawa-Ohnishi, M. & Matsubayashi, Y. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat. Plants 3, 1–6 (2017).
Ohkubo, Y., Kuwata, K. & Matsubayashi, Y. A type 2C protein phosphatase activates high-affinity nitrate uptake by dephosphorylating NRT2.1. Nat. Plants 7, 310–316 (2021).
Werner, T. & Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 12, 527–538 (2009).
Kieber, J. J. & Schaller, G. E. Cytokinins. Arab. Book 12, e0168 (2014).
Cortleven, A. et al. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 42, 998–1018 (2019).
Naulin, P. A. et al. Nitrate induction of primary root growth requires cytokinin signaling in Arabidopsis thaliana. Plant Cell Physiol. 61, 342–352 (2019).
Miyawaki, K. et al. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl Acad. Sci. 103, 16598–16603 (2006).
Takei, K., Yamaya, T. & Sakakibara, H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J. Biol. Chem. 279, 41866–41872 (2004).
Kiba, T., Takei, K., Kojima, M. & Sakakibara, H. Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev. Cell 27, 452–461 (2013).
Zhang, K. et al. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat. Commun. 5, 1–12b (2014).
Ko, D. et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. 111, 7150–7155 (2014).
Stolz, A. et al. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 67, 157–168 (2011).
Riefler, M., Novak, O., Strnad, M. & Schmülling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18, 40–54 (2006).
Chang, L., Ramireddy, E. & Schmülling, T. Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signalling genes. J. Exp. Bot. 64, 5021–5032 (2013).
Mason, M. G. et al. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17, 3007–3018 (2005).
Argyros, R. D. et al. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20, 2102–2116 (2008).
Patterson, K. et al. Nitrate-regulated glutaredoxins control Arabidopsis primary root growth. Plant Physiol. 170, 989–999 (2016).
Jung, J.-Y., Ahn, J. H. & Schachtman, D. P. CC-type glutaredoxins mediate plant response and signaling under nitrate starvation in Arabidopsis. BMC Plant Biol. 18, 281 (2018).
Ota, R., Ohkubo, Y., Yamashita, Y., Ogawa-Ohnishi, M. & Matsubayashi, Y. Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nat. Commun. 11, 1–9 (2020).
Landrein, B. et al. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. 115, 1382–1387 (2018).
Poitout, A. et al. Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. Plant Cell 30, 1243–1257 (2018).
Oldroyd, G. E. D. & Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 368, eaba0196 (2020).
Dello Ioio, R. et al. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 17, 678–682 (2007).
Bartrina, I. et al. Gain-of-function mutants of the cytokinin receptors AHK2 and AHK3 regulate plant organ size, flowering time and plant longevity. Plant Physiol. 173, 1783–1797 (2017).
Hirose, N., Makita, N., Kojima, M., Kamada-Nobusada, T. & Sakakibara, H. Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 48, 523–539 (2007).
Monden, K. et al. Root-specific reduction of cytokinin perception enhances shoot growth in Arabidopsis thaliana. Plant Cell Physiol. 63, 484–493 (2022).
Werner, T., Motyka, V., Strnad, M. & Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. 98, 10487–10492 (2001).
Plet, J. et al. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 65, 622–633 (2011).
Werner, T. et al. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 59, 2659–2672 (2008).
Růžička, K. et al. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc. Natl. Acad. Sci. 106, 4284–4289 (2009).
Bartrina, I., Otto, E., Strnad, M., Werner, T. & Schmülling, T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23, 69–80 (2011).
Laffont, C. et al. The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat. Commun. 11, 3167 (2020).
Kiba, T., Takebayashi, Y., Kojima, M. & Sakakibara, H. Sugar-induced de novo cytokinin biosynthesis contributes to Arabidopsis growth under elevated CO 2. Sci. Rep. 9, 1–15 (2019).
Kleinboelting, N., Huep, G., Kloetgen, A., Viehoever, P. & Weisshaar, B. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40, D1211–D1215 (2012).
Nitschke, S. et al. Circadian stress regimes affect the circadian clock and cause jasmonic acid-dependent cell death in cytokinin-deficient Arabidopsis plants. Plant Cell 28, 1616–1639 (2016).
Bryan, A. C., Obaidi, A., Wierzba, M. & Tax, F. E. XYLEM INTERMIXED WITH PHLOEM1, a leucine-rich repeat receptor-like kinase required for stem growth and vascular development in Arabidopsis thaliana. Planta 235, 111–122 (2012).
Lobet, G., Pagès, L. & Draye, X. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiol. 157, 29–39 (2011).
Svačinová, J. et al. A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: pipette tip solid-phase extraction. Plant Methods 8, 17 (2012).
Antoniadi, I. et al. Cell-type-specific cytokinin distribution within the Arabidopsis primary root Apex. Plant Cell 27, 1955–1967 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Czechowski, T., Stitt, M., Altmann, T., Udvardi, M. K. & Scheible, W.-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17 (2005).
Acknowledgements
This work was supported by an Australian Research Council grant to MAD (DP200101885) and by the Ministry of Education, Youth and Sports of the Czech Republic (European Regional Development Fund-Project “Plants as a tool for sustainable global development” No. CZ.02.1.01/0.0/0.0/16_019/0000827). We thank Bernd Weisshaar (MPI for Plant Breeding Research, Cologne) for the T-DNA mutant line 467C01, and RIKEN (Japan) for providing the RATM11-2459, cepd1-1 cepd2-1 and GFP-CEPD1 lines. The authors acknowledge the instruments and expertise of Microscopy Australia at the Centre for Advanced Microscopy, Australian National University, a facility that is funded by the University and the Federal Government through NCRIS. We thank Sören Werner and Gabi Grüschow for generating the arr mutants. The authors give sincere thanks to Hana Martínková and Ivan Petřík for their help with cytokinin analyses.
Author information
Authors and Affiliations
Contributions
M.F., M.A.D., M.T. and K.C. conceived and designed experiments; T.S. provided plant materials; K.C., M.T. and M.F. performed experiments; O.N. conducted the cytokinin quantification; M.T., M.F., M.A.D and K.C. analysed the data. M.A.D., M.T., K.C. and M.F. wrote the manuscript; T.S. and O.N. participated in drafting.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Margaret Frank and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taleski, M., Chapman, K., Novák, O. et al. CEP peptide and cytokinin pathways converge on CEPD glutaredoxins to inhibit root growth. Nat Commun 14, 1683 (2023). https://doi.org/10.1038/s41467-023-37282-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-37282-6
This article is cited by
-
Small secreted peptides (SSPs) in tomato and their potential roles in drought stress response
Molecular Horticulture (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.