Abstract
The study aims to characterise the changes in elemental composition in the river sediments of streams influenced by mine waters enriched with radionuclides. The study took place in the vicinity of Ostrava, a city located in a coal mining region in the Czech Republic, namely the Upper Silesian Coal Basin. River sediments and waters of the Karvinský potok and Stružka streams were investigated. Field measurements were made for ambient dose equivalent rate (ADER). Laboratory gamma spectrometry and X-ray fluorescence were used to determine the content of radionuclides and elemental composition in river sediments. Water samples were analysed for the content of major ions and radionuclides. The field ADER measurement proved elevated content of radionuclides with values exceeding 1,000 nSv/h in both streams. The discharged mine waters were Na–Cl type, containing an 226Ra (0.68–0.70 Bq/l) as a dominant radionuclide. Laboratory measurements of radionuclides in bottom sediments proved that the prevailing source of radiation are 226Ra and 232Th in both streams. The calculated enrichment factors showed extreme values for Sr, Cr, Pb, Zn, Cu, and Mo. The precipitation reactions forming Ca-minerals (calcite and aragonite), Fe-bearing minerals (hematite, goethite and amorphous Fe(OH)3) and hausmannite were found to be the primary geochemical process underway in the studied riverine systems. The correlation between elements and radionuclides demonstrated the significant role of geochemical barriers that lead to the precipitation of radionuclides from solution. The results show that the precipitation takes place preferentially in places where other waters enter the stream, or where recent organic matter is present.
Similar content being viewed by others
Introduction
Neutral or alkaline mine water is much less common in mining areas than acid mine ones. Thus, a limited number of studies on the distribution of elements in river environments influenced by neutral/alkaline mine water has been published (Dahrazma and Kharghani 2012; Kelly et al. 2006). Coal, depending on the coalification stage, contains several potentially risk elements. Among the elements of greatest concern are chalcophilic elements—As, Cd, Hg, Pb or Se (Swaine 1990; Suárez-Ruíz et al. 2006). Increased levels of radium (Ra) or uranium (U) have also been documented in the hard-coal coal basins (Bondaruk et al. 2015; Gombert et al. 2019; Janson et al. 2009; Schmid and Wiegand 2003).
Radium is an alkaline earth element with four natural isotopes (223Ra, 224Ra, 226Ra and 228Ra), of which only two—228Ra (β) and 226Ra (α)—are significant in terms of environmental protection. Other Ra isotopes have a very short half-life and/or extremely low concentration. These two isotopes are part of the decay series of primordial radionuclides: 228Ra with a half-life of 5.75 years is a part of the thorium series (with parental isotope 232Th) and 226Ra with a half-life of 1600 y is a part of the uranium series (with parental isotope 238U) (Bourdon et al. 2003; USEPA 2004; IAEA 2014). The activity of Ra isotopes in rocks and minerals is usually the same as that of its parent (Th or U), i.e., it is in secular equilibrium. The shift of this equilibrium is observed in the waters of the hypergenic zone (Chevychelov et al. 2021).
The 226Ra and 228Ra concentrations in the environment are extremely low. Carmichael (1989) stated that the concentration of 226Ra in the earth's crust was 0.9 ng/kg (33 Bq/kg); its concentration in soils was determined by Bowen (1979) to be 0.8 ng/kg. Activities in river water generally range between 0.5 and 20 mBq/L for 226Ra, though enhanced concentrations (of up to 300 mBq/L) have been reported (Vandenhove et al. 2010).
Uranium (U) and Th in the Earth's crust and soils are present in mg/kg, which are much greater concentrations than those of Ra. Rudnick and Gao (2003) stated that the abundance of U for the upper crust was 2.7 mg/kg (33 Bq/kg). In soils, the U concentration varies greatly—from 0.1 to 53.2 mg/kg. Thorium (Th) concentrations in rocks and soils are generally higher than those of U. According to Rudnick and Gao (2003), the concentration of Th is 10.5 mg/kg (43 Bq/kg) in the upper crust and is 3 to 4 times higher than that of U (Ahrland et al. 1973).
It was expected that the content of radioactive elements in the overburden is negligible compared to sulphates, chlorides, and other potentially hazardous elements such as Cd or Pb (Cravotta 2008; Chalupnik et al. 2017; Gombert et al. 2019). However, the increased level of naturally occurring radionuclides e.g. in shales, bituminous shales, igneous silicate rocks or phosphate rocks is documented across the world (Chalupnik et al. 2017; Paridaens and Vanmarcke 2001; Pujol and Sanchez-Cabeza 2000). Thus, the extraction of such raw materials can lead to the accumulation of radionuclides in tailings, fly ash or river sediments, even though the limits for the entry of radionuclides into the environment are met (Chalupnik et al. 2001; Lauer et al. 2017; Papastefanou 2010).
The first data on radioactivity in the Upper Silesian Coal Basin mines come from Poland and date back to the 1970s (Tomza and Lebecka 1981). Since 1989, the coal industry in Poland has been required to monitor the radioactivity of discharged mine waters and sediments (Chalupnik et al. 2001; Lebecka et al. 1994). Data on the occurrence of radionuclides in the surface water in the Czech part of the Upper Silesian Coal Basin (USCB) are available only to a limited extent in the monitoring schemes of state institutions (Hanslík et al. 2005). Besides the aforementioned radionuclides, the discharged mine waters contain elevated concentrations of Fe, Mn, Sr and Ba (Vöröš et al. 2021). Both Sr and Ba belong to alkaline earth metals and behave similarly to Ra. The similarity of element behaviour is used to monitor the fate of radium in the riverbed, but detailed studies evaluating elemental composition and radioactivity monitoring are scarce (Iyengar 1990). The main aim of the present study is to describe the changes in the elemental composition of the riverine system influenced by mine water in the Upper Silesian Basin.
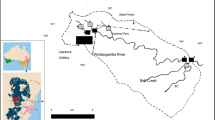
The studied area
The studied streams are situated in the Czech part of the Upper Silesian Coal Basin (USCB), which, with a total area of 7,500 km2 (from which only 1,550 km2 is in the Czech Republic), is one of the biggest hard coal basins in Europe and has a long mining history. The basin is divided into the Ostrava and Karviná sub-basins (Dopita and Kumpera 1993). The present USCB is only a denudation remnant of what was initially a much larger basin structure filled with Devonian and Lower to Upper Carboniferous sediments (Jirásek et al. 2018; Geršlová et. al. 2016).
Karvinský potok
Karvinský potok is located in the Karviná sub-basin and its channel was artificially modified. The stream’s length is 8.4 km and it begins as a stream draining an old reclaimed coal heap. The mine water discharge is located 2.6 km away from the beginning of the stream and enters a small artificial pond (approximate dimensions: 15 × 60 m) with a bottom and sides made of concrete (points S1-2 and S1-3). Moving down the stream, the broad floodplain area (points S1-4) is followed by a channelled section; at sampling location S1-8 the river bed is lined with a concrete bottom and banks and flows to Lake Kozinec, which is a subsidence lake. Downstream of the lake, Karvinský potok is a regulated stream and takes the form of a straightened channel with steep banks. The natural part of Karvinsky potok has an occasional flow that works mainly during the rainy season, otherwise the mine waters are the main water source (Fig. 1).
Stružka
Stružka is located in the Ostrava sub-basin. The total length of the stream is 14.2 km; it is affected by mine subsidence with a total grade of 60 m and is naturally low in water. The beginning of Stružka has characteristics of a dale with many rather small rapids and erratic bottoms. After 270 m from the mine water discharge, another mine water-influenced stream flows into the Stružka. Approximately 1.4 km from the mine waters discharge, there is a wastewater treatment plant, which represents a significant water input. The remaining part of the stream is a regulated stream bed with stabilized banks. The discharged mine water represents approximately 1/4 of the total volume of water. The estimate is based on average volumes of discharged water and measured flow data during the year.
Methodology and equipment
Two streams into which mine water is discharged were selected – Karvinský potok and Stružka. To evaluate the mine water’s influence and its extent, conductivity, pH and radioactivity levels were monitored directly in the field (Tables 1 and 2, Fig. 2). Eighteen samples of river sediments, 12 samples of surface water and 2 samples of discharged mine water were collected from both streams. The content of 7 radionuclides and 19 elements was determined in the river sediments and the content of main ions and radionuclides was determined in the water samples.
Field measurements
The in-situ measurement of ADER was done using a gamma spectrometer GT-40 (Georadis Ltd.) equipped with a NaI (Tl) detector with a volume of 345 cm3, diameter 76 mm (cylinder). GT-40 is a device capable of accurate measurement of ADER values from 20.0 nSv/h to 0.5 mSv/h within an energy range of 25 keV to 3 MeV. The in-situ measurement was done by walking directly in the streams and the device was carried in hand partially submerged under the water so the detector would be still at the same distance from the sediment. Conductivity, pH and temperature were measured by Multimeter WTW Multi 350i (with accuracy ± 0.01 for pH, ± 0.5% for conductivity and ± 0.1 °C for temperature) equipped with probe WTWSenTix 41 for pH and temperature and probe WTW TetraCon 325 for conductivity measurements. The conductivity, temperature and pH of stream water were measured in a single day during active mine water discharging straight into the streams.
Sampling and sample preparation
The samples of sediments from the streams were taken at places with elevated ADER values. The samples were dark brown to black clayey silty sand with a rare occurrence of bigger grains. Sample S1-2 contains corroded fragments of piping (up to 2 cm in size). The fine-grained river sediments were taken with a scoop and placed in plastic bags. Dry samples were sieved with a mesh size of 2 cm to remove plant debris and larger detrital pieces. The obtained fraction was used for the laboratory gamma spectrometry and particle size distribution. The Marinelli beakers with a volume of 600 ml were filled and hermetically sealed for 30 days to reach the secular equilibrium for 226Ra and 222Rn. For the XRF analyses, the homogenized samples were crushed to a fraction less than 0.2 mm.
Samples of water were collected into plastic sampling bottles directly from the streams and were transferred to the laboratory within 24 h and immediately analysed.
Laboratory measurements
Water samples
226Ra was analysed by the JKA 300 analyser equipped with probe NS 9502 E using an accredited radiochemical methodology. The 1 L of water is chemically processed and the residue after evaporation is subsequently mixed with ZnS(Ag) and measured. For 226Ra each sample was measured twice with the efficiency of 0.66 for 2000 s and the background of 23 imps. According to the activity of the sample, the average uncertainty is up to 20%.
The content of chlorides was determined by the precipitation with silver nitrate via titration with a measurement uncertainty of 10%. The equivalence point was determined potentiometrically (electrode pH/mV meter Seven2Go S2 Std-Kit Mettler Toledo). The concentration of bicarbonate ions was analysed by potentiometric method at pH 4.5 and pH 8.3 with 10% measurement uncertainty. The content of sulphates ions was determined by precipitation of barium ions under moderate acid condition with 10% measurement uncertainty. Barium sulphate was filtrated and weighed. The Na+, K+, Ca2+, Mg2+ and Ba2+ contents were determined by atomic absorption spectrometer Agilent 280FS AA. For each element the appropriate wavelength was used. All methods meet requirements for accredited analyses in accordance with ISO/IEC 17025 (control of calibration, blanks and standards measurements, interlaboratory comparisons).
The resulting charge balance errors were at the level of ± 10%.
Sediment samples
Particle size distribution was carried out on sieved homogenized samples using the CILAS 1064 instrument.
Gamma spectrometry was done by HPGe (high purity germanium) coaxial spectrometers with high resolution and efficiency of 4% (HK-8G with carbon window), 25% (HK-1G and HK-2G with beryllium window), 30%, 35% and 40% and with 8,192 channels to 3,000 keV. The measurement time was at least 24 h. Activity of 7 radionuclides was determined: 226Ra, 228Ra, 238U, 228Th, 232Th and 210Pb with deviations of up to 12% for 226Ra, 10% for 228Ra, 36% for 235U, 31% for 238U, 10% for 228Th, 10% for 232Th and 75% for 210Pb. We used the following sources as a standard for energy and efficiency calibration for laboratory gamma spectrometric determination of the concentration of radionuclides: Activity standard: Type CBSS 2; produced by the Czech Metrological Institute, a mixture of radionuclides – Am-241; Cd-109; Ce-139; Co-57; Co-60; Cs-137; Ba-133; Sr-85; Y-88; Cr-51 a Pb-210. Activity standard: type MBSS 2X; produced by the Czech Metrological Institut; a mixture of radionuclides – Ra-226 a Th-23.
The elemental composition was measured by XRF Delta Premium with Rh source, in the settings of Geochem Vanad. Metranal 19, Metranal 34, Nist 2702, Nist 2781 and IRM 5718As were used as reference materials. For each sample, the measurement time was 300 s. The content of 19 elements (Si, Al, Fe, Ti, K, Ca, Mn, Sr, S, Cr, Zr, Rb, Ni, Zn, Cu, Pb, As, Mo, Th) was used for further evaluation. The LODs declared by the InnovXSystem is as follows for Si < 0.5%, Al < 0.5%, Fe = 10 mg/kg, Ti = 7–15 mg/kg, K = 40–60 mg/kg, Ca = 25–40 mg/kg, Mn = 10 mg/kg, Sr = 1–3 mg/kg, S = 150–300 mg/kg, Cr = 5–10 mg/kg, Zr = 1–3 mg/kg, Rb = 1–3 mg/kg, Ni = 10–20 mg/kg, Zn = 3–5 mg/kg, Cu = 5–7 mg/kg, Pb = 2–4 mg/kg, As = 1–3 mg/kg, Mo = 1–3 mg/kg, Th not declared.
Data processing
The results were processed using Microsoft Excel and QGIS 3.2.2. The spectra were evaluated using G2k and GENIE 3.2.3 (Mirion–Canberra). The results were corrected using MEFFTRAN (freeware).
Data normalisation was applied to the assessment of anomalous metal contribution using Al as a reference element. Data from the publication by Rudnick and Gao (2003) were used as the background values. The following formula was used for enrichment factor calculation (Zoller et al. 1974 and Sinex and Helz 1981):
A = the content of the element in the river sample. An = the content of the reference element in the river sample. B = the content of the element in the background samples. Bn = the content of the reference element in the background samples.
Hydrochemical modelling was conducted to study the solid–liquid interactions using PHREECQ software. This included the calculation of the mineral saturation index (SI) for both streams to compare the mineral stabilities in the water. The water composition (concentration of dissolved ions and anions), pH, and T values were used as input parameters for the SI calculation. SI is defined as an ion activity product in relation to solubility product on a logarithmic scale where SI value > 0 means saturation of mineral phases in the water, while SI values < 0 represent subsaturation.
Results and Discussion
Discharged water samples
The discharged mine waters have a stable temperature (23–25 °C) according to long-term measurements done by Diamo a.s. The pH of discharged waters was comparable (7.3–7.4), as was EC with the values of 20,700 μS/cm and 16,600 μS/cm for Karvinský potok and Stružka, respectively (Table 1). Both discharged waters (S1-W2-DIS and S2-W2-DIS) are classified as chloride types. In the cationic part, the proportion of alkali metals Na+ and K+ is more than 80 eq.%. It is generally assumed that the mine waters of USCB contain a substantial concentration of sulphates (Grmela and Jelínek 1999). These opinions are also supported by findings of sulphate minerals inside the mines (Matýsek et al. 2014) and the general assumption that coal contains an increased amount of sulphur (Deurbrouck 1972). However, the concentration of sulphates (SO4)2− in the studied mine water discharges was low (not exceeding 77 mg/l). This deficit of SO42− in mine water can be explained as either insufficient oxidation of sulphides in the mined bedrock or reached saturation limits of gypsum and/or jarosite and their precipitation (Banks 2006). A low content of mainly pyritic sulphur in coal-bearing strata of the Ostrava and Karvina Formations was found in boreholes in the Karvina region (Vöröš et al. 2022). Thus, low sulphates content will be conditioned by a lower pyrite content contained in the rock massif. A lower amount of Ba reaching 6.4 and 15.5 mg/l was found in the discharge water of Karvinský potok and Stružka, respectively (Table 1). However, the values are not as high as from the discharge of an active mine (up to 99 mg/l, Vöröš et al. 2021). Even higher content of Ba was measured in Polish parts of USCB, where in water samples the concentration was up to 2,000 mg/l (Lebecka et al. 1994). The measured activity of 226Ra in discharged waters was 0.70 Bq/l and 0.68 Bq/l for Karvinský potok and Stružka, respectively. Both mine waters has an extreme excess of 226Ra compared to 238U (Tab. 1).
Surface water samples
According to the ion composition the surface waters are classified as carbonate-sulphate, however, after the mine water discharge, it changes to chloride type. The pH values do not show a visible trend along both streams and are in the range of 6.8–8.1. The discharge of mine waters significantly increased the electrical conductivity values in both streams (Table 1). The subsequent development of conductivity is different for individual streams. In Karvinský potok, the EC decreases from 24,800 to 2030 μS/cm within a 5.7 km long section. In Stružka, the EC it does not fall immediately after discharge, but on the contrary rises and then falls. The activity ratio between 226Ra/238U is significantly lower (1.0–20.9) compared to its value in the discharged mine waters (93.7 in Karvinský potok and 67.3 in Struzka; Table 1).
River sediment samples
The field ADER measurement proved an increased content of radioactivity in both streams (Fig. 2). Highest measured radioactivity (up to 4000 nSv/h), with an average of 1,831 nSv/h was found directly after mine water discharge in the Karvinský potok. No place with ADER above 200 nSv/h has been found upstream of the mine water discharge. The highest ADER values exhibited in the Stružka exceeding 2000 nSv/h were measured 200 m after mine water discharge with an average of 898 nSv/h (Fig. 2). Laboratory gamma spectrometry of bottom sediments proved that the most active radionuclides in river sediments are 226Ra and 232Th in both studied streams (Table 2).
Stružka river sediments were composed of silt (10.5–75.4% w/w) and sand fraction (16.1–87.5% w/w). On the contrary, in the Karvinský potok sediments the granulometric distributions of the particles were less variable and the most abundant fraction is silt fraction (Median 80.5% w/w). Sample S1-3 is an exception with the prevailing sand fraction (85.1% w/w) and 1.2% w/w content of clay fraction (Fig. 3).
The SiO2/Al2O3 ratios of siliciclastic rocks can be used as a parameter sensitive to sediment recycling and the weathering process. With increasing sediment maturity, quartz is enriched compared to feldspars, mafic minerals and lithics (Roser et al. 1996). The average SiO2/Al2O3 ratios in unaltered igneous rocks ranged from 3.0 (basic rocks) to 5.0 (acidic rocks). The SiO2/Al2O3 ratio in the Karvinský potok ranges from 3.2 to 5.0, which confirms basic to intermediate maturity. Values of SiO2/Al2O3 ratio > 5.0 in Stružka sediments pointed to increased geochemical sediment maturity. There has been found no correlation between Al and Si (r = 0.14). It follows from the above that sediment material of both rivers is made up mainly of detrital components of weathered rocks with a minor portion of soil flushes from the stream surroundings.
A strong positive correlation was found for Al and K (r = 0.92) in both streams, and this is interpreted as the presence of clay minerals or feldspars. The sample S1-2 completely deviates from the evaluated set, as it contained corroded fragments of piping with a diameter of up to 2 cm (Fe content = 204 508 mg/kg). Samples S2-1, S2-2 and S2-11 are enriched in Fe content (over 48 720 mg/kg). The different element distribution was found in samples S2-3 and S2-4 where a low content of Fe (31 955 mg/kg and 23 321 mg/kg, respectively) and an increased content of Ca (average of 35 000 mg/kg) was measured (Appendix 1). The ratio of 226Ra and 238U in the river sediments of Karvinský potok is in the range 6.3–32.7 and in Stružka in the interval 3.3–43.1 (Table 2). All evaluated sediments contain 226Ra, which is not in secular equilibrium with its parent radionuclide 238U (Fig. 4). The ratio of 226Ra and 238U in sediments decreases downstream, but a systematic gradual decrease could not be traced. This is probably due to the natural movement of sediments within the stream. The most abundant radionuclide 226Ra correlates with 228Ra and 232Th in all evaluated samples, which reflects the different mobility of uranium, radium, and thorium in the environment (Fig. 5). Uranium is highly soluble under oxidising conditions, whereas radium is mobile regardless of oxidation–reduction environmental conditions. Thus, radium movement in the environment is controlled by precipitation or sorption.
To assess the mutual relationship between river sediment composition and radionuclides, it was necessary to eliminate the effect of grain size. Thus, the enrichment factor (EF) using Al as the normalising element was calculated. The EF for both streams reached extreme values for Sr, Cr, Mo, Th, Pb, Zn and Cu (Fig. 6).
A mutual correlation was found between the enrichment factor of Sr and the content of 226Ra (Fig. 5, Appendix 2). This is related to their oxidation states (+ 2) which do not change under typical groundwater conditions, thus their mobility in aquifers is indirectly affected by changes in geochemical conditions leading to precipitation or sorption onto negative mineral surfaces.
Saturation index and mineral dissolution
Two geochemical models applied for the results from discharge mine water (MW) and mixing discharge water with surface water (MIX) were evaluated in both Stružka and Karvinský potok (Table 3). The mineral saturation indices (SI) were calculated from the quarter-month monitoring data and revealed positive values for calcite and dolomite, averaged of 0.41 and 0.97 for Stružka; averaged of 0.49 and 0.93 for Karvinský potok (MW), respectively. This observation indicates supersaturation in respect to their solubilities (Table 3). Other hydrous carbonate phases such as aragonite showed lower SI values compared to aforementioned minerals, averaged of 0.27 for Stružka, and 0.35 for Karvinský potok.
The calculated internal partial pressure of CO2 showed negative log (pCO2) values in both streams, not exceeding log (pCO2) for the Earth’s atmosphere -3.4. It reflects on the potential degassing of CO2 from the solutions to the atmosphere (Boch et al. 2015). According to the results, both streams are supersaturated with respect to the amorphous and hydrous iron minerals, such as hematite, goethite and Fe-oxo hydroxides (FeOH)3. The SI values highly fluctuated and their values differed from each other. Amorphous (FeOH)3 exhibited the lowest SI values in Stružka and Karvinský potok, averaged of 1.71 and 2.25, respectively. Goethite and hematite showed much higher SI values in both streams with a slight difference between the two systems. This is in agreement with the observation of the first section of Stružka where Fe-hydroxide coatings were observed. The SI values for goethite and hematite were averaged of 7.56 and 17.12 in Stružka; averaged of 8.15 and 18.31 in Karvinský potok, respectively.
The variability of SI values for the mineral Jarosite-K was found in both streams. Only negative averaged SI values were observed in Stružka and Karvinský potok (− 3.99 and − 2.15, respectively). When the system does not reach the equilibrium with carbonate phases (calcite and dolomite), thus Jarosite-K remains undersaturated. Precipitation/dissolution of Jarosite-K is considered when the mine water reaches the equilibrium with carbonate phases or is mixed with surface water (positive and negative SI values, Table 3), which is controlled by various factors, such as temperature, alkalinity, content of sulphates and iron in the solution regardless of the equilibrium with these phases (Elwood Madden et al. 2012). Barium (Ba) solubility in the Stružka stream seems to be controlled with respect to barite that is found to be supersaturated (average SI > 1). According to the mineralisation, pH and the content of dominant anions, Ra can co-precipitate when incorporated in barite, gypsum or even calcite, but the co-precipitation with gypsum and calcite is negligible compared to barite (Bosbach et al. 2010; Yoshida et al. 2009; Vinson et al. 2012). A low content of sulphates is not favourable for Ra2+ precipitation as RaSO4. In water with dominant Cl− anion, Ra prefers to remain in solution (Beneš et al. 1982). Radium is readily and strongly adsorbed especially by the fine fraction of soils, but not in the Fe-oxide fraction. In addition, Mn solubility in the Karvinský potok is governed by the mineral phases of pyrolusite, manganite, and hausmannite that have been found supersaturated as well (average SI > 3 for manganite, and average SI > 6 for hausmannite and pyrolusite). The results of geochemical modelling lead to a conclusion that all presented supersaturated mineral phases are important for Fe, Ba, Ra and Mn removal from the discharged water in both streams.
Conclusion
The riverine environment in the Ostrava region has been influenced by discharged mine water for decades. The mine waters studied are of Na–Cl type with increased 226Ra activity. The study proved increased concentrations of Sr, Mo, Cr, Zn, Pb and Cu in river sediments and also an increased content of 226Ra. Detailed field ADER mapping and conductivity measurements showed that there is a relationship between both parameters. Isotopes of Ra and Th have exhibited strong correlations with the enrichment factor of Cr, Sr and Mo in sediments of both streams supporting their relationship to the mine water. The results of geochemical modelling proved that the geochemical fate of elements in two riverine systems is governed by the precipitation reactions forming Ca-minerals (calcite and aragonite), Fe-bearing minerals (hematite, goethite and amorphous Fe(OH)3) and Mn-bearing mineral hausmannite. Barium (Ba) was found in lower amounts in the mine water, which results in its instantaneous precipitation. The correlation of the 226Ra with the enrichment factor of Sr is explained as a result of the precipitation from solution in the Karvinský potok where mine water is the main component. In the case of the Stružka stream, mine waters are diluted with surface waters, which reduces the probability of precipitation from the solution, but rather leads to the spread of contaminants further down the stream. A weak correlation of Al and K (components of silicates) with the major contaminants has proved that the sorption to clay minerals is not the governing mechanism that removes radionuclides from the water.
Data Availability
Data available in appendix and from the corresponding author upon request.
References
Ahrland S, Bagnall KW, Brown, D, Dell RM, Eberle SH, Keller C, Lee J A, Liljenzin JO, Mardon PG, Marples JAC, Milner GWC, Phillips G, Potter PE, Rydberg J (1973) The chemistry of the actinides. Perg Texts Inorg Chem 10
Banks D (2006) Assessment of the impact of the mine flooding process on groundwater quality; chemical and mineralogical analysis of rock samples recovered from Janina Mine. In: Hydrogeological Modelling of Water Evolution, Final report of WaterNorm Project, European Union Grant No. MTKD-CT-2004–003163, 55
Beneš P, Obdržálek M, Cejchanová M (1982) The physico-chemical forms of traces of radium in aqueous solutions containing chlorides, sulfates and carbonates. Radiochem Radioanal Lett 50:227–242
Bondaruk J, Janson E, Wysocka M, Chalupnik S (2015) Identification of hazards for water environment in the Upper Silesian Coal Basin caused by the discharge of salt mine water containing particularly harmful substances and radionuclides. J Sustain Min 14(4):179–187
Bosbach D, Bottle M, Metz V (2010) Experimental study on Ra2+ uptake by barite (BaSO4). In: Kinetics of solid solution formation via BaSO4 dissolution and RaxBa1−xSO4 (re)precipitation. SKB Technical Report TR-10–43
Bourdon B, Turner S, Henderson GM, Lundstrom CC (2003) Introduction to U-series geochemistry. Rev Mineral Geochem 52:1–21. https://doi.org/10.2113/0520001
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, London
Boch R, Dietzel M, Reichl P, Leis A, Baldermann A, Mittermayr F (2015) Rapid ikaite (CaCO3·6H2O) crystallization in a man-made river bed: hydrogeochemical monitoring of a rarely documented mineral formation. Appl Geochem 63:366–379
Carmichael RS (1989) Practical handbook of physical properties of rocks and minerals. CRC Press. https://doi.org/10.1201/9780203710968
Chalupnik S, Michalik B, Wysocka M, Skubacz K, Mielnikow A (2001) Contamination of settling ponds and rivers as a result of discharge of radium-bearing waters from Polish coal mines. J Environ Radioact 54:85–98. https://doi.org/10.1016/S0265-931X(00)00168-5
Chalupnik S, Wysocka M, Janson E, Chmielewska I, Wiesner M (2017) Long term changes in the concentration of radium in discharge waters of coal mines and Upper Silesian rivers. J Environ Radioact 171:117–123. https://doi.org/10.1016/j.jenvrad.2017.02.007
Chevychelov A, Sobakin P, Gorokhov A, Kuznetsova L, Alekseev A (2021) Migration of 238U and 226Ra radionuclides in technogenic permafrost taiga landscapes of southern Yauktia, Russia. Water 13:966. https://doi.org/10.3390/w13070966
Cravotta CA (2008) Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part1: constituent concentrations and correlations. Appl Geochem 23:166–202. https://doi.org/10.1016/j.apgeochem.2007.10.011
Dahrazma B, Kharghani M (2012) The impacts of alkaline mine drainage on Ba, Cr, Ni, Pb and Zn concentration in the water resources of the Takht coal mine. Iran Earth Sci Res SJ 16(2):109–112
Dopita M, Kumpera O (1993) Geology of the Ostrava-Karviná coalfield, Upper Silesian Basin, Czech Republic, and its influence on mining. Int J Coal Geol 23:291–321. https://doi.org/10.1016/0166-5162(93)90053-D
Deurbrouck AW (1972) Sulfur reduction potential of the coals of the United States. In: U.S. Bureau of Mines. RI 7633.
Elwood Madden ME, Madden AS, Rimstidt JD, Zahrai S, Kendall MR, Miller MA (2012) Jarosite dissolution rates and nanoscale mineralogy. Geochim Cosmochim Acta 91:306–321
Geršlová E, Goldbach M, Geršl M, Skupien P (2016) Heat flow evolution, subsidence and erosion in Upper Silesian Coal Basin, Czech Republic. Int J Coal Geol 154–155:30–42. https://doi.org/10.1016/j.coal.2015.12.007
Gombert P, Sracek O, Koukouzas N, Gzyl G, Valladares ST, Frączek R, Klinger C, Bauerek A, Areces JEA, Chamberlain S, Paw K, Pierzchała L (2019) An overview of priority pollutants in selected coal mine discharges in Europe. Mine Water Environ 38:16–23. https://doi.org/10.1007/s10230-018-0547-8
Grmela A, Jelínek P (1999) Mine waters and their changes related to mines closure in the Czech part of the Upper Silesian Coal Basin. In: XXIX Congress IAH 1999 “Hydrology and land use management”. Proceeding, 751–757
Hanslík E, Kalinová E, Brtvová M, Ivanovová D, Sedlářová B, Svobodová J, Jedináková-Křížová V, Rieder M, Medek J, Forejt K, Vondrák L, Jahn K, Jusko J (2005) Radium isotopes in river sediments of Czech Republic. Limnologica 35:177–184. https://doi.org/10.1016/j.limno.2005.05.004
IAEA (2014) The environmental behavior of radium. In: Technical reports series no. 476. Vienna
IAEA (2022) Naturally occurring radioactive material. On-line: https://www.iaea.org/topics/radiation-safety-norm (seen 2022)
Iyengar MAR (1990) The natural distribution of radium. In: The environmental behaviour of radium, technical reports series no. 310, IAEA, Vienna
Janson E, Gzyl G, Banks D (2009) The occurrence and quality of mine water in the Upper Silesian Coal Basin, Poland. Mine Water Environ 28:232–244. https://doi.org/10.1007/s10230-009-0079-3
Jirásek J, Opluštil S, Sivek M, Schmitz MD, Abels HA (2018) Astronomical forcing of Carboniferous paralic sedimentary cycles in the Upper Silesian Basin, Czech Republic (Serpukhovian, latest Mississippian): new radiometric ages afford an astronomical age model for European biozonations and substages. Earth Sci Rev. https://doi.org/10.1016/j.earscirev.2017.12.005
Kelly J, Champagne P, Michel F (2006) Mitigation of alkaline mine drainage in a natural wetland system. Trans Ecol Environ 89:115–124
Langmuir D, Herman JS (1980) The mobility of thorium in natural waters at low temperatures. Geochim Cosmochim Acta 44:1753–1766
Lauer N, Vengosh A, Dai S (2017) Naturally occurring radioactive materials in uranium-rich coals and associated coal combustion residues from China. Environ Sci Technol 51(22):13487–13493. https://doi.org/10.1021/acs.est.7b03473
Lebecka J, Chalupnik S, Michalik B, Wysocka M, Skubacz K, Mielnikow A (1994) Radioactivity of mine waters in Upper Silesian coal basin and its influence on natural environment. In: Proceedings of 5th International Mine Water Association Congress, Nottingham. International Mine Water Association, pp 657–662
Matýsek D, Jirásek J, Osovský M, Skupien P (2014) Minerals formed by the weathering of sulfides in mines of the Czech part of the Upper Silesian Basin. Mineral Mag 78:1265–1286. https://doi.org/10.1180/minmag.2014.078.5.12
Papastefanou C (2010) Escaping radioactivity from coal-fired power plants (CPPs) due to coal burning and the associated hazards: a review. J Environ Radioact 101(3):191–200. https://doi.org/10.1016/j.jenvrad.2009.11.006
Paridaens J, Vanmarcke H (2001) Radium contamination of the banks of the river Laak as a consequence of the phosphate industry in Belgium. J Environ Radioact 54(1):53–60. https://doi.org/10.1016/S0265-931X(00)00165-X
Pujol L, Sanchez-Cabeza J (2000) Natural and artificial radioactivity in surface waters of the Ebro river basin (Northeast Spain). J Environ Radioact 51(2):181–210. https://doi.org/10.1016/S0265-931X(00)00076-X
Roser BP, Cooper RA, Nathan S, Tulloch AJ (1996) Reconnaissance sandstone geochemistry, provenance, and tectonic setting of the lower Paleozoic terraines of the West Coast and Nelson, New Zealand. N Zeal J Geol Geophys 39:1–16
Rudnick LR, Gao S (2003) Composition of the continental crust. Treat Geochem 3:1–64. https://doi.org/10.1016/B0-08-043751-6/03016-4
Sageman BB, Lyons TG (2003) Geochemistry of fine-grained sediments and sedimentary rocks. Treat Geochem. https://doi.org/10.1016/b0-08-043751-6/07157-7
Schmid S, Wiegand J (2003) Radionuclide contamination of surface waters, sediments, and soil caused by coal mining activities in the ruhr district (Germany). Mine Water Environ 22:130–140. https://doi.org/10.1007/s10230-003-0013-z
Shropshire Wildlife Trust (2019) River monitoring guidelines
Sinex SA, Helz GR (1981) Regional geochemistry of trace elements in chesapeake bay sediments. Environ Geol 3:315–323
Suárez-Ruíz I, Flores D, Marques MM, Martínez-Tarazona MR, Pis J, Rubiera F (2006) Geochemistry, mineralogy and technological properties of coals from Rio Maior (Portugal) and Penarroya (Spain) basins. Int J Coal Geol 67:171–190
Swaine DJ (1990) Trace Elements in Coal. Butterworth & Co. (Publishers) Ltd. London. https://doi.org/10.1016/c2013-0-00949-8 (ISBN: 978-0-408-03309)
Tomza I, Lebecka J (1981) Radium-bearing waters in coal mines: occurrence, methods of measurement and radiation hazard. In: American Institute of Mining, Metallurgical, and Petroleum Engineers, pp 945–948
USEPA (2004) Understanding variation in partition coefficient, Kd values volume III: Review of geochemistry and available Kd values for americium, arsenic, curium, iodine, neptunium, radium, and technetium. Office of air and radiation, EPA 402-R-04–002C
Vandenhove H, Verrezen F, Landa ER, Hurtgen C (2010) Radionuclides in the environment: radium. In: Radionuclides in the environment 1 ed. Wiley, West Sussex, pp 97–108
Vinson DS, Lundy JR, Dwyer GS, Vengosh A (2012) Implications of carbonate-like geochemical signatures in a sandstone aquifer: radium and strontium isotopes in the Cambrian Jordan aquifer (Minnesota, USA). Chem Geol 334:280–294. https://doi.org/10.1016/j.chemgeo.2012.10.030
Vöröš D, Řimnáčová D, Medvecká L, Geršlová E, Díaz-Somoano M (2021) The impact of saline mine water on fate of mineral elements and organic matter: The case study of the Upper Silesian Coal Basin. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.131397
Vöröš D, Geršlová E, Šimoníková L, Díaz-Somoano M (2022) Late Carboniferous palaeodepositional changes recorded by inorganic proxies and REE data from the coal-bearing strata: An example on the Czech part of the Upper Silesian Coal basin (USCB). Natl Gas Sci Eng 107:104789. https://doi.org/10.1016/j.jngse.2022.104789
Yoshida Y, Nakazawa T, Yoshikawa H, Nakanishi T (2009) Partition coefficient of Ra in gypsum. J Radioanal Nucl Chem 280:541–545. https://doi.org/10.1007/s10967-009-7470-1
Zoller WH, Gladney ES, Gordon GE, Bors JJ (1974) Emissions of trace elements from coal fired power plants. In: Conference: 8. annual conference on trace substances in environmental health, Vol 8
Acknowledgements
We thank technical staff from SURO and SUJCHBO organizations for their kind assistance in the sampling campaign.
Funding
Open access publishing supported by the National Technical Library in Prague. This research was funded by BETA2 Programme (project no. TITSSUJB703) and also received support from the long-term conceptual development of research organisation RVO: 67985891.
Author information
Authors and Affiliations
Contributions
DB: data curation, conceptualization, writing-original draft. EG: conceptualization, investigation, data curation, writing-original draft. PO: funding acquisition, project administration, data curation. DV: methodology, writing-original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bednář, D., Geršlová, E., Otáhal, P. et al. Effects of mine water discharge on river sediments: metal fate and behaviour, Upper Silesian Coal Basin. Environ Earth Sci 83, 71 (2024). https://doi.org/10.1007/s12665-023-11356-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-11356-6