Abstract
Herbaceous plants can form root systems by investing in one main taproot or many adventitious roots. While monocots have adventitious systems, eudicots can have either type in different species and even within a single species depending on its age, environment, or injury. Although clearly different, we know little about their relationship to ecological functions and response. We used Plantago lanceolata, a species that can have either root system and forms adventitious buds on roots, to test methods to form plants with a taproot, adventitious roots, or that grow from a root fragment, to obtain individuals of comparable size. We first evaluated injury response and root traits, then used selected models in a pilot study. For the pilot study, we selected an adventitious root model procedure with root removal from approximately 5-day-old seedlings at 1-2 mm below the stem base (hypocotyl) and rootsprout model of the topmost 4 cm of the taproot from 4-week-old plants. We planted adventitious and taprooted plants in three urban lawns and harvested them after three months. Adventitious and tap-rooted plants were similarly affected by competition, producing lower biomass of leaves and stem in more competitive lawns. Root and leaf traits were consistent regardless of architecture type. Plantago lanceolata fully compensated early loss of the taproot when injured at about 10 days old, and in mesic conditions both root architectures perform similarly. These model systems can be used for investigating the role of root architecture in a variety of ecological topics, for example, its function along a moisture gradient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can form root systems based on a large primary root (taproot) or many smaller roots that originate from the belowground stem (adventitious roots). Taproots are formed from the root pole of eudicot embryos and grow downward, forming branches (i.e. lateral roots). The taproot is topped by a transitional region between roots and stems (hypocotyl) and perennial belowground stems (caudex) bearing dormant buds from which new shoots may be formed (Bellini et al. 2014; Strock et al. 2019). The taproot can be lost at different stages of the plant’s life, after advancing age, after damage, or based on other environmental cues (Roeder et al. 2019). Some eudicots can have both a taproot and adventitious roots at the same time as adult plants (Gier 1940; Lynch and Brown 2001; Walk et al. 2006), while all monocots form root systems completely composed of adventitious roots (after a short period in early ontogeny with a seminal root). Although differences in root architecture because of presence or absence of the taproot are widely recognized (Jeník 1978; Kutschera and Lichtenegger 1992), understanding of their functional difference is limited.
Growth and development of both lateral roots from taproots and adventitious roots from stems is regulated via different signalling pathways but are both controlled by a variety of phytohormones (e.g. jasmonates and ethylene) that respond to cues from symbionts as well as abiotic cues such as flooding and the presence of heavy metals (Bellini et al. 2014; Betti et al. 2021). Taprooted systems form a single axis with potential for deep rooting which may be useful in arid ecosystems (Tumber-Dávila et al. 2022, Klimešová and Herben 2023) whereas root systems formed by adventitious roots are more decentralized and plastic in their response to environmental stimuli and in foraging for water and nutrients such as phosphorus (Walk et al. 2006; Fry et al. 2018). These differences in structure and architecture may have important consequences not only for foraging for nutrients (Walk et al. 2006, Weiser et al. 2016), but also for competition (Lynch and Brown 2001), or the response to different disturbances such as frost (Fry et al. 2018; Lubbe and Henry 2020; Lubbe et al. 2021).
The ability to form adventitious roots can enable the production of independent rooting units and therefore clonal multiplication (Groff and Kaplan 1988). In clonal plants, the oldest parts (the taproot, hypocotyl, and oldest part of the caudex) are lost (Klimešová et al. 2011; Klimešová 2018). The taproot (or seminal root in monocots) is replaced by adventitious roots and the caudex is replaced by specialized bud bearing belowground stems such as rhizomes, stem tubers, or bulbs (Klimešová et al. 2011; Klimešová 2018) that continuously produce new increments and lose the old (Bartušková et al. 2022). Alternatively, new rooting units can be produced by forming adventitious shoots from roots (rootsprouting) (Klimešová 2018). Clonal growth not only enables multiplication but also occupation of new areas. The renewal may provide adventitious root systems greater flexibility for contraction to pull the plant deeper into the protective soil layer than aging taproots of non-clonal plants (Lubbe et al. 2021). This lack of mobility may help explain why taprooted plants are frequently more damaged under freezing soil conditions compared to adventitious rooted plants (Perfect et al. 1987; Lubbe and Henry 2020); perhaps when pushed upward by frost heave, they are less flexible and thus unable to contract back to safety (Lubbe et al. 2021).
One limitation to our understanding of the effect of root architecture is the strong phylogenetic conservatism of the trait, especially between monocots and eudicots. Additionally, even though many eudicotyledonous genera can contain species that are either taprooted or forming adventitious root systems, there are other differences such as presence or absence of clonal growth organs that greatly alter the body and ecology of the plant (Martínková et al. 2020), making them difficult to compare. One way to overcome differences in form and phylogeny is the development of a model system wherein one species has specimens with either type of system without changing overall growth form. Here we propose the development of such a model using selective root removal from the seedlings of Plantago lanceolata. This species is an herbaceous perennial from the family Plantaginaceae, with a broad distribution, present across the globe and growing in a variety of anthropogenic habitats including meadows and disturbed habitats (Janeček et al. 2014). Plantago lanceolata plants initially form a taproot which can be lost over one year in wet habitats and replaced by adventitious roots while in dry habitats adventitious roots are absent and only the taproot persists, reaching a depth of nearly one meter (Kutschera and Lichtenegger 1992). Additionally, this species can form adventitious shoots/buds from injured roots (i.e. rootsprouting), creating another architectural type (i.e. rootsprouter) and also offering another trait to explore in terms of disturbance response (i.e. resprouting with adventitious shoots; Latzel et al. 2009).
To our knowledge, no model of root architecture for the comparison of taproot and adventitious root systems has been developed via the removal of roots from seedlings. Seedling root injury and manipulation has been conducted for the formation of split-root systems to assess response to intra-individual root competition (Maina et al. 2002) and resource heterogeneity for signalling and response within plants (Saiz-Fernández et al. 2021) but not for the formation and comparison of both adventitious and tap-rooted individuals. In the original method to investigate intra-individual root competition by Gersani and Sachs (1992), taproot formation and growth was halted with the removal of the radicle followed by selective removal of lateral roots to create two equal roots. These experiments were primarily performed on Pisum sativum and other species with relatively large seeds and seedlings (Gersani and Sachs 1992; Maina et al. 2002; Shemesh et al. 2010). Later studies have used a variety of methods ranging from full root removal to minor injury (Y grafting) to explore internal signalling and response to soil heterogeneity (and other topics) in a wide variety of species, especially Arabidopsis thaliana (Saiz-Fernández et al. 2021).
Taproot loss by injury and subsequent replacement by adventitious roots was described by Aeschimann and Bocquet (1980) but to our knowledge, this process was never described in detail. Nevertheless, loss of the main shoot has major consequences for plant fitness (e.g. Huhta et al. 2003; Martínková et al. 2008) and we can expect that the manipulation and initial injury necessary for creating a root architecture composed from adventitious roots will alter fine root traits in comparison with nonmanipulated taprooted individuals. Some changes might be ephemeral and caused by immediate injury response and compensation via root growth, whereas others may be caused by differences in root spatial arrangements and qualities of different root types such as the plasticity of adventitious roots (Walk et al. 2006; Lynch and Brown 2001) or inhibition of adventitious root formation during drought (Sebastian et al. 2016). Immediate injury response and trait compensation may be an important part of survival and recruitment for Plantago lanceolata because of its persistence in disturbed habitats (Latzel et al. 2009).
To analyze the response of different root architecture types to different conditions, we developed a protocol for the production of both taprooted and adventitious-rooted Plantago lanceolata specimens. To study resprouting, we tested the methods and response of plants to fragmentation to develop a rootsprout model. In our goal to develop these three models we address three questions: 1) what is the response of seedlings to root injury at different ages? 2) how do traits and biomass allocation differ between the three models? 3) how do adventitious and taprooted plants differ in their growth and traits in field sites? We first assessed the response of biomass and fine root traits to root injury at different locations on the root and times during early plant growth. Concurrently, we assessed the ability of young plants to regenerate from root fragments at different ages and locations along the root. To test our models of the two most common architecture types, adventitious and taprooted plants were grown and transplanted in urban lawns with different productivity levels, then harvested after one growing season.
Materials and Methods
Seeds of Plantago lanceolata were purchased from Planta naturalis (www.plantanaturalis.com), collected from plants of locally adapted central European ecotypes. Trials were all conducted during winter 2021 in a growth chamber with light from 6AM to 6PM. Seeds were cold stratified in petri dishes of damp sand at 5 °C for 3 weeks before being placed in the growth chamber.
Adventitious Root System Model Development
We created three sets of plants for development and testing of adventitious root systems. In the first set, we assessed injury time and type for plants between 8 and 14 days old (middle-aged). After growth and greening of the cotyledons (5 days), seedlings were moved to a tray of fertilized sand. After 3 days (8 days old), plants were removed and injured, and this was repeated twice, each after approximately an additional three days. Plants injured at 11 and 14 days already had distinct and demarcated stem regions. Plants of each injury time were harvested twice, one and two weeks after injury. At time of injury, average root length (cm; mean ± sd) was approximately 3.8±1.0 for plants at 8 day, 4.7±1.1 for 11 day, and 5.2±1.0 for 14 day injury age. Furthermore, there were two injury treatments intended to generate adventitious root systems for each of these three injury times: cut at 2 mm below the top of the base of the root (the white portion) and cut at 10 mm from the base. The adventitious model procedure tests were conducted alongside the formation of uninjured (control) plants for inclusion and comparison with taprooted plants. Plants were scanned, above and belowground structures were separated, dried at 60 °C for 24 hours, and weighed. We had 10 replicates for each treatment (cut at 2 mm/cut at 10 mm/control) for each injury time (8/11/14 days) and harvest (after 1/2 weeks).
In the second set (late), we assessed the response to the 2 mm injury on older plants. Additional plants were maintained in the petri dishes for 10 days before being planted in 12 x 12 cm pots of fertilized sand, four plants in each pot, watered from below. After one month, eight plants (2 pots) were removed, cut at one mm above the bottom of the stem to fully remove the root and only form adventitious roots, and planted. There was no control because plants were already too large for easy assessment, but original taproot length was recorded. The roots of these plants were collected for the root fragment model system development (see below). Plants were harvested 2 weeks after planting.
In the third set (early), we assessed the response of very young plants, the early treatment seeds were stratified two weeks and seedlings were removed immediately after the distinct greening of cotyledons (4 days). Injury treatments only had the 2 mm cut or control. Plants were harvested 2 weeks after planting. There were 5 replicates.
Root Fragment Model System Development
We formed fragments from the taproots of plants after approximately 4, 6, and 8 weeks of growth. Taproots of 4-week-old plants were approximately 0.5 mm or less in diameter. The taproot was cut from one centimetre below the stem region and then cut into approximately 4 cm long pieces. Some fragments were 3.5 cm if roots were too short, or 5 cm if roots became thinner than the rest. The fragments were planted in fertilized sand, as above, but at an incline (approximately 1.5-2 cm at its deepest) with a deeper hole at the bottom of this incline for excess roots. Approximately 1-1.5 cm of the root was left exposed on the surface of the soil with the rest buried in the sand. Plants were monitored daily for bud emergence from the exposed portion of the root fragment and harvested approximately 2 weeks after the presence of visible buds. Sprout number was measured upon harvest. Fragmentation tests to produce the rootsprout model did not include taprooted plants because of differences in generation time.
Field Study
Based on observation of root growth and architecture from harvest scans, and logistic concerns regarding plant handling and the amount of time to produce viable plants for use in experiments, we selected the 2 mm cut and 8-day injury time (discussed in greater detail in results). Plants were stratified for 10 days as above before movement to the growth chamber. We removed the movement to a tray for immediate growth to avoid the stress of transplantation and possible damage during additional handling. Root fragments were not included in the field experiment because of delays in cultivation caused by sudden and intensely hot weather.
Because of an initial disparity in weight and growth of plants between the adventitious and taprooted seedlings, adventitious plants were grown and prepared a week earlier than taprooted plants. Adventitious plants were removed from stratification on 6 April 2021 and injured and planted 6 days later on 12 April 2021. Taprooted plants were removed from stratification on 12 April 2021 and planted 7 days later on 19 April 2021.
For initial growth and establishment, seedlings were grown in 12x12x15 cm pots containing a 1:2 sand and soil mixture. Soil was fine peat mixed with compost and silicate sand (Lawn Substrate; Agro: Říkov, CZ). 16 plants of each architecture type (taproot and adventitious) were planted in one of three locations in the South Bohemia region on 14 and 15 May 2021. Site flora and productivity were characterized on 17 June 2021 via the collection of aboveground biomass for 3 15x15 cm squares near the planted areas from which species number was counted. Site biomass samples were identified and sorted into functional groups (graminoid, forb, and legume), dried at 70 °C for 48 hours, and weighed. The three sites varied in productivity and flora: Jindřichův Hradec (J) – graminoid dominated with scattered eudicot herbs of various species, Písek (P) – very few species and almost entirely graminoids, and Vyšší Brod (V) – eudicot herb dominated and diverse.
Leaf number, longest leaf length, stem number, and flowering time were assessed weekly for each plant. Plants were harvested from the sites between 9 and 11 August 2021. Upon harvest, above and belowground organs were separated. Aboveground organs were sorted into leaves and stems, and one leaf from each plant was scanned for leaf traits. Belowground structures were washed, and fine roots were collected and scanned for root traits. Only fine root traits were assessed because of a lack in confidence in collection of full and intact root systems from the field. Leaves, roots, and stems were then dried at 50 °C for 48 hours before being weighed. Root samples were milled for carbon and nitrogen analysis.
Trait Assessment
Root scans were analyzed with WinRHIZO (Regent Instruments Inc. 2013), these assessments were used to acquire specific root length (SRL: root length divided by dry weight; mm/mg), root tissue density (RTD: root dry weight divided by volume; mg/mm3), average root diameter (mm), and number of root tips (for model trials only). Leaves were also scanned in the same program to collect specific leaf area (SLA: leaf area divided by dry weight; cm2/g).
For carbon and nitrogen analysis of field plants, ground roots were put into the Flash 2000 analyzer, wherein the sample was burned in a stream of pure oxygen at a temperature of 1000°C. The resulting oxides of carbon and nitrogen were led by a reducing Cu charge into a separation column, where the moisture was separated with helium as a carrier gas. The contents of separated oxides were determined by a conductivity detector and the signal evaluated with Eager Xperience software (Thermo Fisher Scientific Gmbh 2016). From these measures we acquired root nitrogen (N; %), root carbon (C, %) and root C:N (carbon % divided by nitrogen %).
Data Analysis
The data from the model system development experiments were analysed in three groups: (a) all middle-aged adventitious root system model plants (age at injury time 8, 11, and 14 days); (b) all early and late treatments in combination with final root system models – adventitious 2mm, taproot, and root fragment; and (c) root fragments (age at injury time 4, 6, and 8 weeks) only. After data exploration and visualisation, redundancy analysis (RDA) was carried out (function rda, package vegan; Oksanen et al. 2022) in each group. The effect of predictors in RDA was tested by permutation test with 999 permutations. Following the RDA, we conducted separate one-dimensional analyses of variance (ANOVA) for each response variable to provide perspective on the specific response each variable; effects of predictors were tested using F tests. The response variables were transformed (ln – natural logarithm, sqrt – square root or 1/x - inverse) if necessary to meet the assumptions of the model. In groups (a) and (c), sums of squares type II were used because the models contained multiple predictors (function Anova, package car; Fox and Weisberg 2019). In a few cases, outliers were omitted from a model based on high probability of measurement error (judging by extreme values in at least two variables and/or an unusual ratio of these variables). In case of a single predictor, Tukey’s post-hoc test was used to compare predictor levels (Tukey 1949).
The field data were used to perform RDA, and effect of each predictor was tested using permutation test. Initial number of leaves was always used as a covariate to account for possible differences arising from initial size variation, although these measures only began two weeks after planting. Again, the response variables were transformed (ln, sqrt or 1/x) if necessary to meet the assumptions of the model. No follow-up analyses were conducted because of the lack of significance for root system type effect. All analyses were performed using R software (version 4.2.1; R Core Team 2022) within RStudio (version 2022.07.2; RStudio Team 2022).
Results
Adventitious Root System Model Development
Seedlings from all stages (including dishes, trays, and other movements and manipulations) were generally healthy and survived these procedures. The 10 mm cut caused the formation of root systems dominated by lateral branches from the original root and the 2 mm cut formed adventitious roots from the belowground stem tissue. Redundancy analysis revealed significant effect of all three predictors (age, treatment, and harvest; all predictors p = 0.001; Fig. 1) together with interaction between age and harvest time (p = 0.001). Harvest time generally explained the most variation (Table 1), indicating a relatively fast shift in traits during the second week after injury. Early injured plants (8 days old) were less affected by injury in the first harvest, later injured plants generally compensated and re-grew better and faster and were more similar to controls (Fig. 2). Treatment type had effects on all measured parameters, the stronger disturbance (2 mm cut), the more delayed the development of the root system in all measured parameters.
Redundancy analysis graph of adventitious root system model development. Predictors are represented by data centroids shown as points (age), crosses (harvest), or circles with number or letter (treatment); arrows represent direction of increasing values of a given response variable. Abbreviations used: (2) – 2 mm cut, (10) – 10 mm cut, (T) – taproot, SLA – specific leaf area, SRL – specific root length, RTD – root tissue density, tips – number of root tips. Axis label shows percentage of variation explained by axis. Axis RDA 1 accounted for 70.72 % of maximum possible explained variation (i.e. by the first axis of an unconstrained analysis)
Trait variation in response to injury treatment (T; 2 mm, 10 mm, or control (taproot)), age at the time of injury (A; 8, 11, or 14 days), and harvest (H; 1 or 2 weeks after injury). Abbreviations in labels: SLA – specific leaf area, SRL – specific root length, RTD – root tissue density. The upper and lower ends of boxes indicate 1st and 3rd quartiles, respectively; the horizontal line inside the boxes represents the median and the ends of whiskers show minimum and maximum values apart from outliers (full / open circles; outliers = points farther than 1.5 × interquartile range from box end). Graphs in the figure are scaled according to the transformation used for the analysis (in italics) with the exception of inverse-transposed data; in these cases scaling was chosen arbitrarily so as to optimize graph readability. P values shown indicate significant effect of a given factor (A, T or H) or their interaction (represented by colon symbol (:)). See Table 1 for detailed information on model results
Root Fragment Trials
Plants injured at 4 weeks regularly sprouted from fragment 1 (most proximal fragment) within 2 weeks. Plants for fragmentation at the 6-week injury time had longer taproots and more viable fragments but were otherwise not considerably larger or healthier in appearance than plants for the 4-week injury. Plants kept growing longer before fragmentation were more likely to have damage to the lower portion of the taproot as altered growth along the bottom of the pot or through holes in the pot, or loss because of anoxic conditions in the bottom of the pot. Redundancy analysis (Fig. 3) and ANOVAs for individual traits (Fig. 4 & Table 2) revealed significant effects of both age and fragment position (both p = 0.001), but no conclusions can be made for trait differences with fragment position because of the limited number of observations for fragment positions 2 and 3 (Fig. 4). With later injury time (older plants), the resprouts had greater SLA, SRL, number of tips, and root mass; root diameter remained consistent. Fragment survival was high, but some fragments died after injury, and mortality increased for more distal fragments (as indicated by variable box widths in Fig. 4). Sprout number varied between age groups, with greater numbers of sprouts from older plants (4 weeks [mean ± sd] 1.22 ± 0.44; 6 weeks 2.21 ± 1.19; 8 weeks 3.5 ± 2.17).
Redundancy analysis graph of root fragment trials. Predictors are represented by data centroids shown as full circles (age) or open circles (fragment position); arrows represent direction of increasing values of a given response variable. Ellipses around centroids show standard deviation of these centroid points on two principal axes capturing most variation in a respective group (represented by such centroid and ellipse). Abbreviations used: SLA – specific leaf area, SRL – specific root length, RTD – root tissue density, tips – number of root tips, frag – fragment. Axis label shows percentage of variation explained by axis. Axis RDA 1 accounted for 69.61 % of maximum possible explained variation (i.e. by the first axis of an unconstrained analysis)
Trait response of root fragments to time of fragmentation and origin along length of root (fragment position). Abbreviations in labels: SLA – specific leaf area, SRL – specific root length, RTD – root tissue density. Box widths represent relative sample size of groups – wider boxes indicate larger number of observations. Graphs in the figure are scaled according to the transformation used for the analysis (in italics) with the exception of inverse-transposed data; in these cases scaling was chosen arbitrarily so as to optimize graph readability. P values shown indicate significant effect of a given factor (age – age at the time of injury, frag – fragment position). See Fig. 2 for box and whiskers explanation. See Table 2 for detailed information on model results
Model Comparison
We selected the 2 mm cut and taproot treatments from the 8-day injury time as the adventitious and taproot models, respectively (Fig. 5). For step-by-step protocols, see text boxes 1 & 2. Plants injured or transplanted at the early time point (3 days old) were more likely to have damage from handling because of more sensitive cotyledon or hypocotyl tissue and especially the tip of the taproot. Plants injured later than 8 days were more likely to be damaged by additional handling steps if transferred to a substrate after germination but before injury (as initial methods), but if kept in the petri dish for this time they were more likely to have altered shape from constraint within the dish and among one another or exposure of the root to air and light (possibly causing differences in root hair growth). Late injury plants (4 weeks old) were most likely to have taproot injury from extended growth in an intermediate location and taproot damage during handling. In addition to these adventitious and taproot models, we selected fragment 1 of roots injured at 4 weeks age as our rootsprout model for greater consistency, and because of time spent to produce plants ready for fragmentation to endure less damage from handling and prolonged growth before fragmentation.
Box 1 Adventitious model protocol
Adventitious model preparation |
|---|
Germination |
Sprinkle Plantago lanceolata seeds into petri dishes containing damp sand. Dishes should contain enough seeds to overcome any seeds that do not germinate but also so that plants will not be too cramped after growing for several days. |
Set to stratify at approximately 5 °C for a 7 to 14 days. |
Remove from the cold, re-wet sand, and place in the desired location, with light but not too bright. |
Leave to germinate and grow, there will be a few days until clearly evident cotyledon growth and a few more days after that for the plants to be more manageable to handle and to identify regions. |
Injury |
Remove plants when they are of an appropriate size (cotyledons greater than 1 cm long but not much over, root approximately 4 cm long but anywhere between 3 and 6 cm is fine). This is generally at 5 to 8 days after germination, but size and exact germination time can vary, thus plant size is often a better method to ensure similar size and condition of plants. |
For adventitious system plants, remove from dish and cut the root at approximately 2 mm from the green portion of the plant. |
Planting |
Plant both cut and uncut plants into fertilized substrate. The green of cut plants should be partially within the substrate. Water thoroughly and regularly as plants establish. |
Box 2 Rootsprout protocol
Root fragment model preparation: |
|---|
Germination |
For germination instructions, follow as for adventitious model (Box 1). Grow plants in chosen substrate for 4 weeks, preferably in pots of 10 cm or deeper pots for adequate room for growth. |
Injury |
Remove from substrate, rinse for better visibility of structures if necessary, place on a tray, and add some water to allow better ability to see and manipulate roots. |
Spread the plant, carefully extending the taproot, and measure its length (from the base of the green/brown stem region). |
Cut the plant from just below this region, where there is certainty it is no longer stem tissue, and cut again at 4 cm from this point. Note: only cut the taproot, spread the roots away for more visibility, keep all branches of the fragment intact if possible, and take care when separating the fragment from the rest of the root system. |
Planting fragments |
Make an indent in the substrate at an incline (approximately 1.5-2 cm at its deepest) and a deeper hole at the base of this indent. |
Place the root along this indent, have the top 1-1.5 cm of the fragment lay along the sand surface with the rest following down this incline. |
If possible, have the branches somewhat separated from one another (a good method for this is to spread them from the taproot fragment, lift by placing your thumb against the fragment, and then place it on the indent in this way. Let the long roots fall down through the hole at the base of the indent. |
If growing in greenhouse or under especially warm and dry conditions, cover fragments with a petri dish to retain moisture. Monitor and wet frequently during initial growth as plant establishes. |
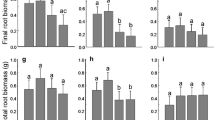
SRL, RTD, and root diameter was consistent across model types (Figs. 6 & 7; Table 3). Model taproots had greater root mass, shoot mass, and number of root tips than the adventitious and root fragment models. Fragments had lower shoot mass than both taproot and adventitious models. Early treatment plants generally were intermediates between the model taproot and adventitious rooted plants. Late treatment plants had the lowest root mass and number of root tips.
Redundancy analysis graph of model comparison. Treatment (see Fig. 7 for a list with explanations) is represented by data centroids shown as full circles; arrows represent direction of increasing values of a given response variable. Ellipses around centroids show standard deviation of these centroid points on two principal axes capturing most variation in a respective group (represented by such centroid and ellipse). Abbreviations used: SLA – specific leaf area, SRL – specific root length, RTD – root tissue density, tips – number of root tips. Axis label shows percentage of variation explained by axis. Axis RDA 1 accounted for 54.71 % of maximum possible explained variation (i.e. by the first axis of an unconstrained analysis)
Trait variation of early, model, late and root fragment plants. Horizontal axis stands for treatment: EA – early adventitious and ET – early taproot, 3 days old; MT – model taproot and MA – model adventitious, 8 days old; LA – late adventitious, 4 weeks old; RF – root fragment, 4 weeks old. Abbreviations in labels: SLA – specific leaf area, SRL – specific root length, RTD – root tissue density. Treatments with no common letter above the box have significantly different trait mean. Graphs in the figure are scaled according to the transformation used for the analysis (in italics). P values shown indicate significant overall effect of treatment. See Fig. 2 for box and whiskers explanation. See Table 3 for detailed information on model results
Field Study
The sites varied in productivity with the greatest biomass in Jindřichův Hradec (J; average biomass (± standard error of mean, SEM) 17.79 g ± 3.34 g) distantly followed by Vyšší Brod (V; average biomass 5.18 g ± 0.82 g) and Písek (P; average biomass 3.21 g ± 0.4 g). After correction for initial size differences, RDA confirmed a significant effect of locality on plant traits (p = 0.001, adj. R2 = 0.16; Fig. 8). In contrast, there was no effect of root system type (p = 0.106, adj. R2 = 0.01) on any leaf, root, or growing season measurements. Some harvested plants exhibited variation from their original architecture, with some taprooted plants producing numerous adventitious roots while some plants with manipulated root systems (adventitious model) had only few thick adventitious roots (Fig. 9).
Redundancy analysis graph of field study. Locality is represented by data centroids shown as full circles (Locality J, P, and V); arrows represent direction of increasing values of a given response variable. Ellipses around centroids show standard deviation of these centroid points on two principal axes capturing most variation in a respective group (represented by such centroid and ellipse). Abbreviations used: SLA – specific leaf area, SRL – specific root length, RTD – root tissue density, tips – number of root tips. Axis label shows percentage of variation explained by axis. Axis RDA 1 accounted for 39.02 % of maximum possible explained variation (i.e. by the first axis of an unconstrained analysis).
Discussion
We developed 3 models of root system architectures within a single species for assessment of response to environment and competition. Plant traits and growth varied among the different treatments and ages but generally the seedlings were quite resilient and were able to compensate taproot loss two weeks after injury for most traits. Although plants injured at 8 days old had delayed development in comparison with plants injured at 11 or 14 days old, they were easier to handle and replant. Plants regenerating from root fragments originating from older plants or from more proximal root fragments generally recovered better than younger plants or more distal fragments but were similarly healthy to the most proximal fragments of younger plants (injured at 4 weeks old). Additionally, the 4 week injury age reduced wait time and root damage with prolonged growth in a pot. Therefore, we selected injury at 5-10 days old for the adventitious root model and injury at 4 weeks old for the root fragment model. Plants of the two new models were able to be formed with relative ease and speed, and they were healthy and of comparable size and fine root traits as intact (taproot) plants. When plants grew in urban lawns with various productivity levels, the two tested architectural models (adventitious roots and taproot model) responded similarly in measured parameters. The methodology to produce these two models provides plants of different root architecture types that have similar traits under relatively mild conditions and provide a good starting point to test the role of root architecture type in response to more severe conditions such as strong drought stress or competition.
Seedling Injury Response
From our experimental manipulation, plants growing in favorable conditions with sufficient light, water, and nutrients survived severe injury and quickly recovered from total loss of the taproot or root system fragmentation. Root biomass and root tip number were most affected and decreased greatly in injured plants. Root traits were less affected in younger plants (8 days) but recovery was faster when plants were injured later (11 or 14 days old). While differences were most conspicuous after one week from injury, after two weeks they disappeared except for the youngest cohort of plants. Plantago lanceolata frequently grows in disturbed areas where seedlings may experience severe disturbance, including root fragmentation (Latzel and Klimešová 2009; Schnoor et al. 2011), but in much less favourable growing conditions than those experienced during these experiments. Although compensation for the loss of the taproot is fast and complete in the favorable conditions of our experiment, the differences in early plant growth and development may decrease survival and establishment of injured plants with greater pressures to acquire sufficient resources such as under competition for light or nutrients (Mašková and Herben 2018). Root tips are necessary for nutrient acquisition and mycorrhizal colonization (Freschet et al. 2021a) and in dry and nutrient poor soil, plants with an injured taproot may be in disadvantage over non-disturbed plants. The advantage of greater initial size was also observed in the field experiment, where plants that were larger at the beginning of the experiment remained larger.
Disturbance Response and Rootsprouting
Plantago lanceolata is known for its ability to form adventitious buds on roots (rootsprouting; Klimešová 2018) that enable the plant to survive a wide range of disturbance types of high severity (e.g. soil slides, water erosion, or human activities). Plants as young as 4 weeks old, and with very thin taproots (approximately 0.5 mm), survived and resprouted after fragmentation, and this ability appears to increase with age (with greater sprouting and potentially survival of more distal fragments). This is similar to observations of other root-sprouting species that were able to survive root fragmentation early in their life (Martínková et al. 2004). Although the root fragment specimens developed through this procedure have strong sensitivity to drying, this shortcoming can be overcome by preparing a larger number of specimens than needed, as well as consistent watering, careful monitoring, and covering with a petri dish to retain moisture in intense conditions in greenhouses.
Plantago lanceolata as a Model of Root Architecture
Our results are in accord with previous observations that Plantago lanceolata is a very flexible plant capable of surviving severe injuries and thriving in many different types of habitats, especially with higher levels of disturbance (Latzel et al., 2009). We also confirmed its sensitivity to competition with grasses (Šmilauerová et al. 2012) as indicated by the lower plant performance in locality J, which had the highest productivity. We observed adventitious roots growing from some uninjured (i.e. taprooted) individuals and variation in investment (i.e. diameter, length, and number when applicable) in the taproot, adventitious roots, and lateral branches of both seedlings and mature plants from the field, in accordance with previous observations (Kutschera and Lichtenegger, 1992; Torres et al. 2022). Additionally, previous observations of young Plantago lanceolata specimens in the field indicate an early investment and reliance on lateral roots and branching in the shallowest regions of the soil (Torres et al. 2022). These shallow lateral roots are frequently replaced over time by adventitious roots in fertile and moist growing conditions, while in dry conditions, the taproot generally dominates the root system (Kutschera and Lichtenegger, 1992; Torres et al. 2022). Counting of roots of different types and other measurements may aid in the understanding of model response to gradients of drought, competition, and other treatments.
We believe that our model system will be especially suited for questions regarding how plants develop and invest into one skeletal root (taprooted architecture) versus many (adventitious rooted architecture), which could affect exploitation of the soil for limited resources in the horizontal versus vertical direction, under different levels of competition or under permanent versus periodical drought conditions (Fry et al., 2018; Orman-Ligeza et al. 2018; Walk et al. 2006; Lynch and Brown 2001; Kutschera and Lichtenegger 1992). Additionally, these models have a great advantage for the investigation of plant response to soil disturbance (e.g. soil slides, water erosion, or human activities) because of the capacity of Plantago lanceolata to form adventitious shoots (rootsprouting) which may vary in response depending on architecture type and severity of soil disturbance. We could expect that plants with numerous thin skeletal roots (adventitious model) will be more prone to root system fragmentation, but the new plants produced from these fragments may be sensitive to drought or burial. In contrast, plants investing in a single thick taproot may be less easily broken and thus more resistant but with sufficient damage for fragmentation would create fewer but larger root fragments that therefore may have a higher probability of survival (Klimešová et al. 2008; Martínková and Klimešová 2016). The different root architectures might also differ in their ability to protect soil from erosion because of the level of spread and branching of the root system (Freschet et al. 2021b). The response to freezing temperatures and frost heave may differ between architecture types because of potential differences in the ability of root contraction between a persistent taproot and more frequently renewed adventitious roots (Lubbe and Henry 2020; Lubbe et al. 2021).
Conclusion
No single species can accurately capture the traits and responses of all plants, but the diversity of traits and relative resilience of this species makes this model a strong step in elucidating the role of coarse root traits and root architecture type in plant ecology. Plantago lanceolata is easily grown and can survive a wide variety of conditions and stresses, thus it can be used to explore the relationship of architecture type to a gradient of severity for competition, drought, frost, mycorrhizal association, disturbance, and nutrient availability and heterogeneity. These conditions can be explored in the field as in our field study example, either with further experimental manipulation or purely observational, or in greenhouse conditions. The plants of all three types grow large and robust in greenhouse conditions (observations from concurrent explorations) and can be grown in different pots and arrangements. With these three models, we can use the versatility of this cosmopolitan weed to explore and better understand a wide array of ecological questions concerning belowground organs’ functions.
Data Availability
Should the manuscript be accepted, all data supporting the results will be archived in the Mendeley Data depository (https://data.mendeley.com) and the data DOI will be included in the paper.
References
Aeschimann D, Bocquet G (1980) Allorhizie et homorhizie, une reconside ́ration des definitions et de la terminologie. Candollea 35:20–35.
Bartušková A, Lubbe FC, Qian J, Herben T, Klimešová J (2022) The effect of moisture, nutrients and disturbance on storage organ size and persistence in temperate herbs. Funct Ecol 36(2):314–25
Bellini C, Pacurar DI, Perrone I (2014) Adventitious Roots and Lateral Roots: Similarities and Differences. Annu Rev Plant Biol 65:639–666
Betti C, Rovere FD, Piacentini D, Fattorini L, Falasca G, Altamura M (2021) Jasmonates, Ethylene and Brassinosteroids Control Adventitious and Lateral Rooting as Stress Avoidance Responses to Heavy Metals and Metalloids. Biomolecules 11:77
Fox J, Weisberg S (2019) An {R} Companion to Applied Regression, Third Edition. Thousand Oaks CA: Sage. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed 20 Dec 2022
Freschet GT, Pagès L, Iversen CM, Comas LH, Rewald B, … McCormack ML (2021a). A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytologist 232:973-1122
Freschet GT, Roumet C, Comas LH, Weemstra M, Bengough AG, … Stokes A (2021b) Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytologist 232:1123-1158
Fry EL, Evans AL, Sturrock CJ, Bullock JM, Bardgett RD (2018) Root architecture governs plasticity in response to drought. Plant Soil 433:189–200
Gersani M, Sachs T (1992) Development correlations between roots in heterogeneous environments. Plant Cell Environ 15:463–469
Gier LJ (1940) Root Systems of Bright Belt Tobacco. Amer J Bot 27(9):780–787
Groff PA, Kaplan DR (1988) The relation of root systems to shoot systems in vascular plants. Bot Rev 54:387-422
Huhta AP, Hellström K, Rautio P, Tuomi J (2003) Grazing tolerance of Gentianella amarella and other monocarpic herbs: why is tolerance highest at low damage levels? Plant Ecol 166:49–61.
Janeček Š, Patáčová E, Klimešová J. (2014) Effects of fertilization and competition on plant biomass allocation and internal resources: does Plantago lanceolata follow the rules of economic theory? Folia Geobotanica, 49:49-64.
Jeník J (1978) Roots and root systems in tropical trees: morphologic and ecologic aspects. In: Tomlinson PB, Zimmerman M [eds.] Tropical trees as living systems. Cambridge University Press pp. 323–350.
Klimešová J, Doležal J, Sammul M (2011) Evolutionary and organismic constraints on the relationship between spacer length and environmental conditions in clonal plants. Oikos 120(7):1110–1120
Klimešová J, Herben T (2023) The hidden half of the fine root differentiation in herbs: nonacquisitive belowground organs determine fine-root traits. Oikos https://doi.org/https://doi.org/10.1111/oik.08794
Klimešová J, Kociánová A, Martínková J (2008) Weeds that can do both tricks: vegetative versus generative regeneration of the short-lived root-sprouting herbs Rorippa palustris and Barbarea vulgaris. Weed Research 48: 131-135.
Klimešová J (2018) Temperate herbs: an architectural analysis. Academia, Praha
Kutschera L, Lichtenegger E (1992) Wurzelatlas mitteleuropäischer Grünlandpflanzen. Band 2 Pteridophyta und Dicotyledoneae (Magnoliopsida), Teil 2 Morphologie, Anatomie, Ökologie, Verbreitung, Soziologie, Wirtschaft. Gustav Fischer Verlag.
Latzel V, Hájek T, Klimešová J, Gomez S (2009) Nutrients and disturbance history in two Plantago species: maternal effects as a clue for observed dichotomy between resprouting and seeding strategies. Oikos 118:1669–1678
Latzel V, Klimešová J (2009) Fitness of resprouters versus seeders in relation to nutrient availability in two Plantago species. Acta Oecologica 35 (4):541–547
Lubbe FC, Henry HAL (2020) The role of perennation traits in plant community soil frost stress responses. Ann Bot 126(5):873–881
Lubbe FC, Klimešová J, Henry HAL (2021) Winter belowground: changing winters and the perennating organs of herbaceous plants. Funct Ecol 35(8):1627–1639
Lynch JP, Brown KM (2001) Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant Soil 237:225–237
Maina GG, Brown JS, Gersani M (2002) Intra-plant versus inter-plant root competition inbeans: Avoidance, resource matching or tragedy of the commons. Plant Ecol 160:235–247
Martínková J, Klimeš A, Puy J, Klimešová J (2020) Response of clonal versus non-clonal herbs to disturbance: Different strategies revealed. Perspectives Plant Ecol Evol Systematics 44:124429
Martínková J, Klimešová J (2016) Enforced clonality confers a fitness advantage Front Plant Sci 7:Article 2. https://doi.org/10.3389/fpls.2016.00002
Martínková J, J Klimešová, S Mihulka (2008) Compensation of seed production after severe injury in the short-lived herb Barbarea vulgaris. Basic Applied Ecol 9(1):44–54
Martínková J, Kočvarová M, Klimešová J (2004) Resprouting after disturbance in the short-lived herb Rorippa palustris (Brassicaceae): an experiment with juveniles. Acta Oecologica 25(3):143–150
Mašková T, Herben T (2018) Root: shoot ratio in developing seedlings: How seedlings change their allocation in response to seed mass and ambient nutrient supply. Ecol Evol 8(14):7143–7150
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2022). vegan: Community Ecology Package. R package version 2.6-2. URL: https://CRAN.R-project.org/package=vegan. Accessed 20 Dec 2022
Orman-Ligeza B, Morris EC, Parizot B, Lavigne T, Babé A, Ligeza A, Klein S, Sturrock C, Xuan W, Novák O, Ljung K, Fernandez MA, Rodriguez PL, Dodd IC, De Smet I,Chaumont F, Batoko H, Périlleux C, Lynch JP, Bennett MJ, Beeckman T, Draye X (2018) The xerobranching response represses lateral root formation when roots are not in contact with water. Current Biology 28(19):3165–3173
Perfect E, Miller R D, Burton B. (1987). Root morphology and vigor effects on winter heaving of established alfalfa. Agron Jo 79(6):1061–1067
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Regent Instruments Inc. (2013) WinRHIZO 2013 Basic, Reg, Pro & Arabidopsis for root measurement user manual
RStudio Team (2022) RStudio: Integrated development environment for R. RStudio, PBC, Boston, MA. URL: http://www.rstudio.com/. Accessed 20 Dec 2022
Roeder A, Schweingruber FH, Fischer M, Roscher C (2019) Increasing plant diversity of experimental grasslands alters the age and growth of Plantago lanceolata from youngerand faster to older and slower. Oikos 128:1182–1193
Saiz-Fernández I, Černý M, Skalák J, Brzobohatý B (2021) Split-root systems: detailed methodology, alternative applications, and implications at leaf proteome level. Plant Methods 17:7
Schnoor TK, Lekberg Y, Rosendahl S, Olsson PA (2011) Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza 21:211–220
Sebastian J, Yee M-C, Goudinho Viana W, Rellán-Álvarez R, Feldman M, Priest HD, Trontin C, Lee T, Jiang H, Baxter I, Mockler TC, Hochholdinger F, Brutnell TP, Dinneny JR (2016) Grasses suppress shoot-borne roots to conserve water during drought. Proc Natl Acad SciUSA 113:8861–8866
Shemesh H, Arbiv A, Gersani M, Ovadia O, Novoplansky A (2010) The effects of nutrient dynamics on root patch choice. PLoS ONE 5: e10824
Šmilauerová M, Lokvencová M, Šmilauer P (2012) Fertilization and forb:graminoid ratio affect arbuscular mycorrhiza in seedlings but not adult plants of Plantago lanceolata. Plant Soil 351:309–324.
Strock CF, Burridge J, Massas ASF, Beaver J, Beebe S, Camilo SA, Fourie D, Jochua C, Miguel M, Miklas PN, Endola E, Nchimbi-Msolla S, Polania J, Porch TG, Rosas JC, Trapp JJ,Lynch JP (2019) Seedling root architecture and its relationship with seed yield acrossdiverse environments in Phaseolus vulgaris. Field Crops Research 237:53–64
Thermo Fisher Scientific Gmbh. (2016) EagerSmart – Data Handling Software for FLASH Elemental Analyzers, Software Version 2.0, Bremen, Germany
Torres CD, Magnin A, Sabatier S, Puntieri JG, Caraglio Y (2022) Assessing coordinated intra-specific variation in root/shoot traits in two herbaceous species based onarchitecture and ontogeny. Folia Geobotanica 57:167–180
Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5(2):99–114
Tumber-Dávila SJ, Schenk HJ, Du E, Jackson RB (2022) Plant sizes and shapes above and belowground and their interactions with climate. New Phytol 235:1032-1056
Walk TC, Jaramillo R, Lynch JP (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279:347–366
Weiser M, Koubek T, Herben T (2016) Root foraging performance and life-history traits. Frontiers Plant Sci 7:779
Acknowledgements and Funding
The study was supported by Open Science project supporting participation of students in research, by long-term research development project of the Czech Academy of Sciences [No. RVO 67985939] and by the Praemium Academiae award from the Czech Academy of Sciences of the Czech Republic to Jitka Klimešová.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
JK and FCL developed concept and study design.
FCL developed and tested injury protocols with assistance from MB.
KM, JV, and NK monitored plants in field.
FCL, MB, AH, KM, JV, and NK harvested and processed field plants.
MB and AH scanned roots and assessed root traits.
AH analyzed data with assistance from AK.
FCL led writing of manuscript.
All authors contributed to writing of manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no conflicts of interest or relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lubbe, F.C., Hrouda, A., Bartoš, M. et al. Creating a root architecture model: taprooted or adventitious-rooted Plantago lanceolata. Folia Geobot 58, 293–309 (2024). https://doi.org/10.1007/s12224-023-09429-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-023-09429-2