-
PDF
- Split View
-
Views
-
Cite
Cite
Eva Vaňková, Petra Kašparová, Nikola Dulíčková, Václav Čeřovský, Combined effect of lasioglossin LL-III derivative with azoles against Candida albicans virulence factors: biofilm formation, phospholipases, proteases and hemolytic activity, FEMS Yeast Research, Volume 20, Issue 3, May 2020, foaa020, https://doi.org/10.1093/femsyr/foaa020
Close - Share Icon Share
ABSTRACT
Candida albicans has several virulence factors at its disposal, including yeast–hyphal transition associated with biofilm formation, phospholipases, proteases and hemolytic activity, all of which contribute to its pathogenesis. We used synthetic derivative LL-III/43 of antimicrobial peptide lasioglossin LL-III to enhance effect of azoles on attenuation of C. albicans virulence factors. LL-III/43 was able to inhibit initial adhesion or biofilm formation of C. albicans strains at 50 µM. Azoles, however, were ineffective at this concentration. Using fluorescently labeled LL-III/43, we observed that peptide covered C. albicans cells, partially penetrated through their membranes and then accumulated inside cells. LL-III/43 (25 µM) in combination with clotrimazole prevented biofilm formation already at 3.1 µM clotrimazole. Neither LL-III/43 nor azoles were able to significantly inhibit phospholipases, proteases, or hemolytic activity of C. albicans. LL-III/43 (25 µM) and clotrimazole (50 µM) in combination decreased production of these virulence factors, and it completely attenuated its hemolytic activity. Scanning electron microscopy showed that LL-III/43 (50 µM) prevented C. albicans biofilm formation on Ti-6Al-4 V alloy used in orthopedic surgeries and combination of LL-III/43 (25 µM) with clotrimazole (3.1 µM) prevented biofilm formation on urinary catheters. Therefore, mixture of LL-III/43 and clotrimazole is suitable candidate for future pharmaceutical research.
INTRODUCTION
Candida albicans is considered to be the most prevalent fungal species present in the human body, where it asymptomatically colonizes gastrointestinal and genitourinary tracts and the skin of healthy individuals. It can, however, switch into a virulent form with high resistance to antimycotics and thereby infect almost every host tissue.
Immunodeficiency in patients, stress and other external influences can lead to overgrowth of C. albicans and its morphological change (as a dimorphic fungus) between yeast-like round cells and hyphae. This morphological switching is known to increase the actual virulence of Candida spp. in their hosts (Ma et al. 2015; Zuza-Alves et al. 2016; Yang et al. 2018). The round cells are more susceptible to antimicrobials than are hyphae, and they are less capable of adhering to surfaces (Cui et al. 2015). Others of the fungus's virulence factors include host recognition biomolecules (adhesins), phospholipases, secreted aspartyl proteases and hemolysins that are related to active invasion of host tissue (Niewerth and Korting 2001). C. albicans thus causes infections ranging from superficial mucosal to hematogenously disseminated systematic candidiasis, e.g. oropharyngeal candidiasis or vulvovaginitis (Mathé and Van Dijck 2013; Nobile and Johnson 2015). Most of these infections are strongly related to the great ability of this pathogen to form biofilms, i.e. on medical implants, such as indwelling catheters, artificial joints, pacemakers and central nervous system shunts. Candidiasis today constitute one of the most frequently occurring nosocomial infections and contribute to almost 40% mortality. The formation of Candida spp. biofilms on these prosthetic devices is one of the major predisposing conditions for the development of systemic candidiasis. Antifungal resistance is an intrinsic characteristic of biofilms and its emergence can be detected within minutes to hours of adhesion to a surface. Echinocandins and lipid formulations of amphotericin B are possible alternative drugs because fungal biofilms remain susceptible to them even after their formation (Mukherjee and Chandra 2004). However, it should be noted that antifungal azoles are still the drugs of choice for the treatment of Candida spp. infections rather than echinocandins, and the phenomenon of biofilm resistance represents a major threat for their prescription (Bondaryk, Kurzątkowski and Staniszewska 2013; Marcos-Zambrano et al. 2016; Bersani et al. 2019).
In general, C. albicans biofilms display an innate resistance to available drug classes at concentrations up to 1000-fold greater than those which are effective against non-biofilm planktonic cells (Chandra et al. 2001; Donlan and Costerton 2002; Douglas 2002). Antifungal azoles (e.g. fluconazole, clotrimazole, miconazole, itraconazole and voriconazole) represent one of the most commonly used classes of antifungal agents. Their mechanism of action is the inhibition of ergosterol synthesis by targeting the enzyme lanosterol 14 α-demethylase that converts lanosterol to ergosterol. This leads to the accumulation of methylated sterols in the cell membrane and depletion of ergosterol, which affects the structure and function of the cell membrane, and subsequently stops the fungal growth. Although frequent overuse of these antimycotics results in drug resistance generally, antimycotic resistance acquired by Candida spp. planktonic cells is relatively uncommon (Cleveland et al. 2012). Conversely, intrinsic resistance during biofilm growth is universal among all Candida spp. (Uppuluri and Ribot 2017).
An attractive alternative to common antibiotics consists in antimicrobial peptides (AMP) which take part in a first line of defense in almost all living organisms. Typically, relatively short (10–50 amino acids) AMP act on the microorganisms by destabilizing or damaging the bacterial or fungal cell membrane, thereby leading to the leakage of cytoplasmic components and cell death (Jenssen, Hamill and Hancock 2006; Brogden and Brogden 2011; Nguyen, Haney and Vogel 2011; Mishra et al. 2017; Koehbach and Craik 2019). Some of them may kill microorganisms also through interaction with their intracellular targets. The most studied AMP are those whose activity is linked to their propensity to adopt an amphipathic α-helical structure within the environment of bacterial cell membrane (Tossi, Sandri and Giangaspero 2000). A number of AMP within this category have been isolated from the venom of hymenoptera insects in our laboratory (Čeřovský et al. 2009; Monincová et al. 2010; Nešuta et al. 2016; Volejníková et al. 2019). In addition to potent antibacterial activity, they also exhibit promising antifungal activity against planktonic cells of C. albicans (Slaninová et al. 2011; Vráblíková et al. 2017). Along with our findings, knowledge about the antifungal properties of AMP has been substantially expanded in recent decades (Lee et al. 2003; Lee, Hahm and Shin 2004; Viejo-Díaz, Andrés and Fierro 2005; Park and Lee 2009; Lee and Lee 2015; Slaninová et al. 2015). Only limited information has been obtained in recent years regarding the antibiofilm activities of AMP in relation to Candida spp. (Scarsini et al. 2015; Guilhelmelli et al. 2016; Fais et al. 2017; Vráblíková et al. 2017; Tan et al. 2018; Kočendová et al. 2019). Moreover, possibilities for combining AMP with commonly used antimycotics for the treatment of Candida spp. biofilm-associated infections, which is an innovative idea for antifungal therapy, are very poorly explored (Mora‐Navarro et al. 2015; Vriens et al. 2015; Cools et al. 2017).
Based on these facts, we investigated the ability of the peptide LL-III/43 (VNWKKILGKIIKVVK-NH2) derived from lasioglossin LL-III by replacing the Lys in position 9 with its D-Lys isomer to enhance the antibiofilm activity of antifungal azoles (i.e. fluconazole, clotrimazole and voriconazole) and its activity against C. albicans virulence factors including biofilm formation, phospholipases, proteases and hemolytic activity, as well as the change of biofilm cell surface hydrophobicity.
MATERIALS AND METHODS
Fungal strains
A total of two strains that had originated from the American Type Culture Collection (ATCC) were obtained from the Institute of Physiology of the Czech Academy of Sciences, Prague (Candida albicans ATCC MYA-2876 [C. albicans SC5314] and C. albicans ATCC 10231) and one strain from the Czech National Collection of Type Cultures (C. albicans ATCC 2091). A clinical isolate from a patient with oropharyngeal candidiasis (C. albicans F7–39/IDE99) was received from Palacký University Olomouc. The second clinical isolate, C. albicans N-873, obtained from the Department of Medical Microbiology, 2nd Faculty of Medicine, Charles University in Prague and Motol University Hospital, had been isolated from a patient with clinically manifested vulvovaginal candidiasis. All these five strains were susceptible to fluconazole in planktonic form. It means that their MIC values were lower than 1.6 µM as described in our previous study (Kočendová et al. 2019). They were stored in glycerol stock at −80°C prior to use, then precultured prior to each experiment in yeast extract peptone dextrose (YPD) medium (Carl Roth, Karlsruhe, Germany) until the exponential phase of growth. For biofilm formation, we used Roswell Park Memorial Institute 1640 (RPMI-1640) medium (BioSera, Nuaille, France) with L-glutamine, without phenol red, and supplemented with 3-morpholinopropane-1-sulfonic acid (MOPS) (0.165 M) and NaHCO3 (0.024 M).
Antifungal agents
Peptide LL-III/43 (VNWKKILGKIIKVVK-NH2, Mw = 1764.19) was synthesized by solid phase peptide synthesis on an Fmoc-Rink Amide MBHA resin in 5 mL polypropylene syringes according to the standard Nα-Fmoc protocol and then purified by reversed-phase high-performance liquid chromatography (RP-HPLC) as described earlier (Čeřovský et al. 2009). Its purity was checked by analytical RP-HPLC and the identity was verified by mass spectrometry. Basic antifungal properties, early formed biofilm inhibition concentration (BIC50 and BIC90) as well as mature biofilm eradication concentration (BEC50 and BEC90) of LL-III/43 against different Candida spp. had been determined earlier (Kočendová et al. 2019). Cytotoxicity of this peptide against human red blood cells and human umbilical vein endothelial cells had been also described (Kočendová et al. 2019). In present study, we followed the time course of LL-III/43 degradation in human plasma and determined its half-life to be 160 min (see Supplementary Fig. 1). The analogous peptide LL-III/Nal containing 1-naphthylalanine (Nal) in position 3 (substituting for tryptophan—Trp) was prepared similarly (VNXKKILGKIIKVVK-NH2, X = Nal, Mw = 1775.19). Fluorescein-labelled peptide Fl-LL-III/43 (Mw = 2122.23) was prepared by coupling of 5(6)-carboxyfluorescein to the α-amino terminal group of resin-bound side chain-protected LL-III/43 peptide using N,N′-diisopropylcarbodiimide/1-hydroxy-benzotrialzole in dimethylformamide, as described by Weber et al. 1998. The antimycotics used from the group of azoles (fluconazole—FLC, clotrimazole—CLT and voriconazole—VOR) were obtained from Sigma-Aldrich, Czech Republic. Stock solutions of antifungal agents (for peptide LL-III/43 and FLC at concentration 1 mM; for CLT and VOR at concentrations 434, 822, 1820, 3500 µM and 7 mM) were prepared according to Clinical and Laboratory Standards Institute (CLSI) guidelines using water (LL-III/43 and FLC) or dimethyl-sulfoxide (DMSO; CLT and VOR) (Clinical and Institute 2017) and then stored at −20°C. The maximum concentration of DMSO used in experiments was 1% in all cases. A DMSO control was included into each experiment.
Antiadhesive and antibiofilm properties of LL-III/43 and azoles alone and in their combination
The inoculum precultured in YPD medium was centrifuged and resuspended in RPMI-1640 medium. The cell suspension, adjusted to a specific number of colony forming units per 1 mL (1 × 108 CFU/mL), was added into 96-well polystyrene microtiter plates (TPP, Sigma-Aldrich, Czech Republic) in a volume of 210 μL and topped up to 280 μL of total volume with medium and the stock solution of antifungal agents (LL-III/43 or one of the azoles). Based on the preliminary results for the LL-III/43 action on C. albicans biofilm formation, the concentrations of each antifungal agent ranged from 3.1 to 50 µM. The cells were then incubated under two experimental arrangements differing in their cultivation times. The effect of antifungal agents on initial microbial adhesion was investigated after 2 h of cultivation (at 37°C, without shaking). The effect of antifungal agents on biofilm formation was observed after 24 h of cultivation (at the same culture conditions). Before the process for quantifying the adhered cells or biofilm, each of the wells was twice washed with saline to remove non-adhered cells. The adhered cells or biofilm formed on the bottom of the wells was quantified by resazurin viability assay according to Mariscal et al. 2009, with minor modifications. An aliquot of 25 µL of resazurin (0.15 mg/L), 25 µL of glucose (180 g/L) and 100 µL of saline was added into each well. After 1 h of cultivation (at 37°C in darkness), the fluorescence intensity of resorufin was measured using a spectrophotometry reader (Tecan, Männedorf, Switzerland) at excitation/emission wavelengths 545/575 nm. The results were expressed in relative percentages of metabolic activity of remaining cells (the control serving as 100%) with the fluorescence intensity of control samples 30 000 ± 2 800 (standard deviation—SD) in average. The effect of combining azoles (3.1 to 50 µM) with the peptide LL-III/43 at 13 or 25 µM concentration on initial adhesion and biofilm formation was tested similarly as described above for single antifungal agents using resazurin assay. The data obtained were compared to the results for antiadhesive and antibiofilm activity of azoles (3.1 to 50 µM). All experiments were performed in triplicate in at least three independent repetitions and the data obtained were averaged. For a clear view as to the overall effect of the combination of antifungal agents on C. albicans initial adhesion or biofilm formation, the results obtained were averaged for all those strains of C. albicans used. The results are shown in 3D graphs for a better overview, but without the SD given (SD ranged from 5 to 20%).
Effect of antifungal agents on culturability of biofilm cells
The biofilm cells were cultured in both the presence and absence of antifungal agents as described above. The only difference was in using 24-well polystyrene plates (Nunclon Delta Surface, Thermo Scientific, Denmark) with 1 mL of cell suspension (1 × 108 CFU/mL, 24 h). The C. albicans ATCC MYA-2876 biofilm cells obtained in this experimental setup were used for determination of their culturability (Orlandi, Martegani and Bolognese 2018). The washed biofilm cells were mechanically scraped from the well bottom with a pipette tip and resuspended in saline. An aliquot of 10 µL of resuspended biofilm cells was spotted into the YPD agar and spilled onto its surface. After 48 h of cultivation at 37°C, the culturability was evaluated. Experiments were performed in triplicate in at least three independent repetitions and the data obtained were averaged. The extent of cell culturability was expressed as CFU/mL. Values greater than 1 × 106 CFU/mL indicate high culturability (+++), greater than 1 × 104 CFU/mL mild culturability (++) and greater than 1 × 102 CFU/mL low culturability (+). Samples were designated as non-culturable (−) when there was no visible growth.
Statistical analysis
The results representing antibiofilm effect of antifungal agents alone and in their combinations in relation to C. albicans strains were compared with the results of biofilm cells grown in the absence of antifungal agents using one-way analysis of variance (anova). Differences were considered statistically significant at P ˂ 0.05.
Effect of antifungal agents on hydrophobicity of biofilm cells surface
The hydrophobicity of cell surface in C. albicans ATCC MYA-2876 biofilm cells was measured using the MATH method modified according to Polaquini et al. 2006. The biofilm cells were prepared in 24-well polystyrene plates (with and without antifungal agents) as describe above. These cells were washed twice with saline, removed from the bottom of four parallel wells by mechanical scraping with a pipette tip, resuspended in saline (1 mL) and centrifuged. The precipitated cells were adjusted to optical density OD600nm = 0.300 ± 0.020 (ODinitial). An aliquot of 2.5 mL of this suspension was mixed with 0.25 mL of hexane and transferred into a 5 mL polypropylene tube (VWR International, Radnor, PA). The mixture was shaken intensively by hand for 1 min and then incubated for 10 min at laboratory temperature without shaking. Afterwards, OD600nm of aqueous phase was measured again (ODfinal) and the cell surface hydrophobicity (CSH) index was calculated in percentage terms as CSH = (ODinitial − ODfinal)/ODinitial × 100.
Stability of LL-III/43 and LL-III/Nal during their interaction with C. albicans biofilm cells followed by HPLC
Biofilm cells of C. albicans ATCC MYA-2876 were cultured as described above in the presence of LL-III/43 (50 µM) or its analogue LL-III/Nal (50 µM) in RPMI-1640 medium. At selected times (0, 1, 2, 4, 8 and 24 h) during cultivation, aliquots (50 μL) of cell culture surrounding the biofilm were taken and quenched using a stop solution of 50% aqueous acetonitrile containing 1% trifluoroacetic acid (TFA) (50 μL). These samples (50 μL) were analyzed by RP-HPLC using an Agilent Technologies 1200 Series module with a Supelco C-18, 250 × 4.6 mm; 5 μm, column (Sigma-Aldrich, Czech Republic) at 1 mL/min flow rate and with a solvent gradient ranging from 5% to 70% acetonitrile/water/0.1% TFA over 60 min. The observed decrease of peptides concentration over time due to their degradation by proteases secreted from C. albicans biofilm cells was evaluated based on their peak area at 280 nm. We also followed the increment in the peak area of Trp, which correspondingly accompanied the decrease of LL-III/43. The degradation of LL-III/Nal was analogously accompanied by the formation of Nal. Each data point was measured in triplicates. At the end, the remaining biofilm was gently rinsed, suspended in the stop solution (100 μL), sonicated, centrifuged and the supernatant was then analyzed using RP-HPLC. This was done to refute the possibility for adsorption of the LL-III/43 on the biofilm surface. Peptide LL-III/43, at concentration 50 µM, dissolved in RPMI-1640 medium was used as a control.
Effect of antifungal agents on phospholipases, proteases and hemolytic activity
For these experiments, we used C. albicans ATCC MYA-2876 biofilm cells that had been prepared similarly as described above (in 24-well polystyrene plates). Phospholipases activity of C. albicans ATCC MYA-2876 biofilm cells was determined following the method described in detail by Kadir, Gümrü and Uygun-Can 2007. Briefly, aliquots of 5 µL of biofilm cells (treated and untreated with antifungal agents) mechanically scraped from the well bottom with a pipette tip and resuspended in saline were spotted onto Sabouraud dextrose agar supplemented with 10% egg yolk emulsion (Sigma-Aldrich, Czech Republic). The agar plates with the spotted cells were incubated at 37°C for 72 h to observe the precipitation zones. Phospholipases activity expressed as Pz value was calculated as the ratio of colony diameter (mm) to the diameter of colony plus precipitation zone (mm). Thus, the higher the Pz value, the lower the phospholipases activity of C. albicans exhibited. Experiments were performed in triplicate in at least three independent repetitions and the data obtained were averaged.
Proteases activity of C. albicans ATCC MYA-2876 biofilm cells was determined by the method described in detail by Galan-Ladero et al. 2010. Briefly, aliquots of 5 µL of biofilm cells (treated and untreated), prepared as described in the first paragraph above, were spotted onto 15 mg/mL of bacteriological agar (Oxoid, Germany) supplemented with 1 mg/mL of bovine serum albumin Fraction V (Roche, Germany), 0.1 mg/mL of yeast extract (Difco, Detroit, MI, USA) and 11.7 mg/mL of yeast carbon base (Sigma-Aldrich, Czech Republic). The agar plates with the spotted cells were incubated at 37°C for 5 days to observe white zones around the formed colonies and indicating proteases activity. Proteases activity was expressed as Pz value, which means the ratio of the diameter of the colony to the diameter of the colony plus precipitation zone. The higher the Pz value, therefore, the lesser the exhibited proteases activity of C. albicans. Experiments were performed in triplicate in at least three independent repetitions and the data obtained were averaged.
Hemolytic activity of C. albicans ATCC MYA-2876 biofilm cells was determined following the method described by Luo, Samaranayake and Yau 2001. Aliquots of 5 µL of biofilm cells (treated and untreated), prepared as described in the first paragraph above, were spotted onto Sabouraud agar supplemented with 5% of defibrinated sheep blood (LabMediaServis, Jaroměř, Czech Republic). The agar plates with the spotted cells were incubated for 48 h. The formation of clear zones indicated β-hemolytic activity, meaning that the cells produced β-hemolysin (a toxin digesting erythrocytes). Experiments were performed in triplicate in at least three independent repetitions and the data obtained were averaged. The activity was evaluated similarly to that of other hydrolytic enzymes by determining their Pz values.
As described by Paula-Mattiello, Oliveira and Medina-Silva 2017, the extent of enzymatic activity was categorized as: Pz = 1.0: no activity; Pz = 0.90 − 0.99: weak activity; Pz = 0.80 − 0.89: mild activity; Pz = 0.70 − 0.79: strong activity; Pz < 0.69: very strong activity.
Fl-LL-III/43 interaction with adhered C. albicans cells visualized using spinning disk confocal microscopy
The localization of peptide Fl-LL-III/43 in adhered cells was visualized using spinning disk confocal microscopy (SDCM; Olympus/Andor, Japan/Ireland) according to a modified method of Slaninová et al. 2011. The adhered C. albicans ATCC MYA-2876 cells in 96-well microtiter plates were prepared in the same way as described above (for 2 h, in the absence of Fl-LL-III/43), the difference being in the use of plates with thin polystyrene bottom suitable for microscopy (Greiner Bio-One, Frickenhausen, Germany). Fl-LL-III/43 (2 µM) was added to the adhered cells and the location of the peptide was visualized during 5.5 min of incubation in darkness at room temperature. Double staining of Fl-LL-III/43 (488 nm) with DAPI (405 nm; Sigma-Aldrich, Czech Republic) was used to distinguish the location of Fl-LL-III/43 in C. albicans cells.
Effect of LL-III/43 or its combination with CLT on biofilm formation on Ti-6Al-4 V alloys and on silicone catheters visualized using SEM
The biofilm formation of C. albicans ATCC MYA-2876 was visualized on two types of medical materials, one used for joint implants and the other in catheter manufacturing. The Ti-6Al-4 V alloy coupons 15 mm in diameter introduced to our methodology in a previous study (Vaňková et al. 2019) were provided by Prospon, Czech Republic. Medical grade sterile urinary silicone catheters (All Silicone Foley Catheter, Well Lead Medical, China) were aseptically cut into approximately 1 cm long pieces. The inoculum of C. albicans ATCC MYA-2876 was prepared as described above.
The biofilm formation of C. albicans ATCC MYA-2876 on Ti-6Al-4 V alloy coupons and its yeast-to-hyphae switch affected by LL-III/43 alone (25 and 50 µM) were studied according to a modified method of Romo et al. 2017. The experiments were performed in sterile plastic containers containing 3 mL of inoculum in YPD (control, a hyphae-noninducing medium) or RPMI-1640 medium (hyphae-inducing medium) and 300 µL of LL-III/43 dissolved in appropriate medium to desired concentrations from the stock solutions (1 mM) at 37°C for 24 h, with shaking at 150 rpm.
The biofilm of C. albicans ATCC MYA-2876 was formed on silicone pieces affected by LL-III/43 (25 µM) and CLT (3.1 µM) alone or in their combination. Experiments were performed in sterile polystyrene microtubes containing 1 mL of inoculum in RPMI-1640 medium and 100 µL of antifungal agents dissolved in the same medium to desired concentrations from the stock solutions (1 mM for LL-III/43 or 434 µM for CLT) at 37°C for 24 h, with shaking at 150 rpm.
After cultivations, the colonized materials were gently rinsed with saline and let to dry by laminar flow for several hours. They were then dried out completely in a desiccator under vacuum before visualizing by scanning electron microscope (SEM). The biofilm formation and cells morphology were examined using a Nova NanoSEM 450 (FEI) electron microscope (USA) operating at 5 kV. The samples were imaged under low vacuum condition using the low vacuum detector (LVD) at 1000 × magnification with dwell time of 20 µs.
RESULTS
Antiadhesive and antibiofilm properties of LL-III/43 and azoles alone or in their combination
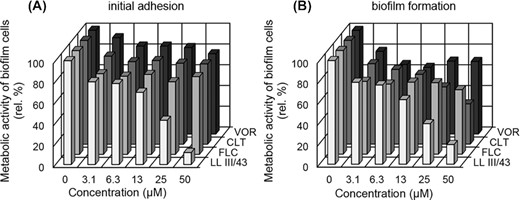
Despite that we used C. albicans strains susceptible to FLC in planktonic form, the initial adhesion as well as biofilm formation of these yeast strains were not effectively inhibited by this antifungal azole even at its highest tested concentration of 50 µM (Fig. 1). Similar results were obtained for the other tested azoles (CLT and VOR) in the case of initial adhesion (Fig. 1A). Biofilm formation of C. albicans was inhibited by at most 61%, but only when using 50 µM CLT (Fig. 1B). Assuming that the maximum inhibition should be optimally achieved, we considered the ability of azoles to prevent biofilm formation of C. albicans to be insufficient. In contrast, the LL-III/43 exhibited significant antiadhesive and antibiofilm properties, inhibiting C. albicans strains’ initial adhesion by 57% at 25 µM and by 88% at 50 µM (Fig. 1A), as confirmed by anova (P ˂ 0.05). Biofilm formation was inhibited by 61% and 81% (P ˂ 0.05) at 25 µM and 50 µM, respectively (Fig. 1B).
Effect of LL-III/43, fluconazole (FLC), clotrimazole (CLT) and voriconazole (VOR) on initial adhesion (A) and biofilm formation (B) of Candida albicans strains. Results are given in relative percentages (based on sample of cells cultured in the absence of any antifungal agent; 100%). SD (±) ranged from 5 to 20%.
Moreover, LL-III/43 (50 µM) was able to inhibit culturability of C. albicans ATCC MYA-2876 biofilm cells to only low culturability (+). That was in contrast to the azoles, which were ineffective even at their highest tested concentration (high culturability; +++) (Table 1). Thus LL-III/43 at 50 µM significantly inhibited metabolic activity of biofilm cells and also suppressed their culturability such that most of the cells can therefore be considered dead. These results were significant by anova (P ˂ 0.05).
Effect of antifungal agents on culturability of Candida albicans ATCC MYA-2876 biofilm cells.
| . | Culturability of biofilm cells affected by antifungal agents . | |||||
|---|---|---|---|---|---|---|
| . | LL-III/43 (µM) . | FLC (µM) . | VOR (µM) . | CLT (µM) . | CLT (µM) + 13 µM LL III/43 . | CLT (µM) + 25 µM LL III/43 . |
| 0 | +++ | +++ | +++ | +++ | +++ | +++ |
| 3.1 | +++ | +++ | +++ | +++ | +++ | +++ |
| 6.3 | +++ | +++ | +++ | +++ | +++ | ++ |
| 13 | +++ | +++ | +++ | +++ | +++ | ++ |
| 25 | +++ | +++ | +++ | +++ | +++ | + |
| 50 | + | +++ | +++ | +++ | ++ | − |
| . | Culturability of biofilm cells affected by antifungal agents . | |||||
|---|---|---|---|---|---|---|
| . | LL-III/43 (µM) . | FLC (µM) . | VOR (µM) . | CLT (µM) . | CLT (µM) + 13 µM LL III/43 . | CLT (µM) + 25 µM LL III/43 . |
| 0 | +++ | +++ | +++ | +++ | +++ | +++ |
| 3.1 | +++ | +++ | +++ | +++ | +++ | +++ |
| 6.3 | +++ | +++ | +++ | +++ | +++ | ++ |
| 13 | +++ | +++ | +++ | +++ | +++ | ++ |
| 25 | +++ | +++ | +++ | +++ | +++ | + |
| 50 | + | +++ | +++ | +++ | ++ | − |
FLC—fluconazole; VOR—voriconazole, CLT—clotrimazole; +++ high culturability (> 1 × 106 CFU mL−1); ++ mild culturability (> 1 × 104 CFU mL−1); + low culturability (> 1 × 102 CFU mL−1); − non-culturable cells.
Effect of antifungal agents on culturability of Candida albicans ATCC MYA-2876 biofilm cells.
| . | Culturability of biofilm cells affected by antifungal agents . | |||||
|---|---|---|---|---|---|---|
| . | LL-III/43 (µM) . | FLC (µM) . | VOR (µM) . | CLT (µM) . | CLT (µM) + 13 µM LL III/43 . | CLT (µM) + 25 µM LL III/43 . |
| 0 | +++ | +++ | +++ | +++ | +++ | +++ |
| 3.1 | +++ | +++ | +++ | +++ | +++ | +++ |
| 6.3 | +++ | +++ | +++ | +++ | +++ | ++ |
| 13 | +++ | +++ | +++ | +++ | +++ | ++ |
| 25 | +++ | +++ | +++ | +++ | +++ | + |
| 50 | + | +++ | +++ | +++ | ++ | − |
| . | Culturability of biofilm cells affected by antifungal agents . | |||||
|---|---|---|---|---|---|---|
| . | LL-III/43 (µM) . | FLC (µM) . | VOR (µM) . | CLT (µM) . | CLT (µM) + 13 µM LL III/43 . | CLT (µM) + 25 µM LL III/43 . |
| 0 | +++ | +++ | +++ | +++ | +++ | +++ |
| 3.1 | +++ | +++ | +++ | +++ | +++ | +++ |
| 6.3 | +++ | +++ | +++ | +++ | +++ | ++ |
| 13 | +++ | +++ | +++ | +++ | +++ | ++ |
| 25 | +++ | +++ | +++ | +++ | +++ | + |
| 50 | + | +++ | +++ | +++ | ++ | − |
FLC—fluconazole; VOR—voriconazole, CLT—clotrimazole; +++ high culturability (> 1 × 106 CFU mL−1); ++ mild culturability (> 1 × 104 CFU mL−1); + low culturability (> 1 × 102 CFU mL−1); − non-culturable cells.
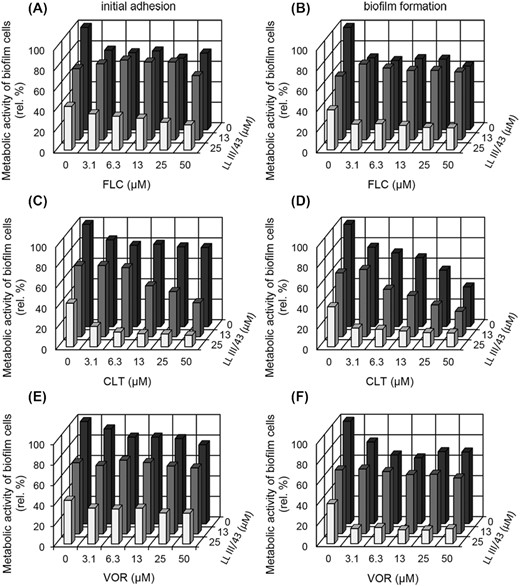
Since LL-III/43 was proven to have potent antiadhesive and antibiofilm activity even as the azoles were almost inefficient, we combined the azoles (at concentration series 3.1 to 50 µM) with LL-III/43 (13 or 25 µM) to enhance their effect against initial adhesion and biofilm formation of C. albicans strains (Fig. 2).
Effect of fluconazole (FLC), clotrimazole (CLT) and voriconazole (VOR) alone and in combination with 13 or 25 µM LL-III/43 on initial adhesion and biofilm formation of Candida albicans strains. Initial adhesion affected by FLC (A), biofilm formation affected by FLC (B), initial adhesion affected by CLT (C), biofilm formation affected by CLT (D), initial adhesion affected by VOR (E) and biofilm formation affected by VOR (F). Results are given in relative percentages (based on sample of cells cultured in the absence of any antifungal agent; 100%). SD (±) ranged from 5 to 20%.
The initial adhesion of C. albicans strains was practically unaffected by the combination of FLC and 13 µM LL-III/43 (Fig. 2A), and only weak enhancement was observed in the case of VOR when using the same concentration of peptide (Fig. 2E). On the other hand, the combination of CLT and LL-III/43 resulted in apparent inhibition of the cell adhesion (P ˂ 0.05). At 50 µM CLT concentration with 13 µM LL-III/43, only 33% of cells were metabolically active (compared to 77% at 50 µM CLT alone) (Fig. 2C).
The addition of 25 µM LL-III/43 to azoles during initial adhesion of C. albicans strains resulted in more efficient inhibition. Nonetheless, in the cases of FLC and VOR the metabolic activity of adhered cells did not fall below 20% even at their highest tested concentration (50 µM) (Fig. 2A and C). In contrast, only 19% of metabolically active cells adhered when 3.1 µM CLT was used in combination with 25 µM LL-III/43 (compared to 85% at 3.1 µM CLT alone) (Fig. 2B).
In general, we observed no significant difference between the inhibitory effect of azoles in combination with LL-III/43 on the initial adhesion and biofilm formation of C. albicans strains (compare Fig. 2A, C and E to Fig. 2B, D and F). In summary, the most significant enhancement of azoles activity by LL-III/43 was obtained by its combination with CLT across the whole range of concentrations with 25 µM LL-III/43, which by comparison inhibited both initial adhesion and biofilm formation of C. albicans strains by approximately 80% (Fig. 2C and D).
Moreover, the combination of 50 µM CLT with 25 µM LL III/43 completely suppressed the culturability of C. albicans ATCC MYA-2876 biofilm cells (Table 1). The biofilm cells cultivated in the presence of these concentrations of antifungal agents were non-culturable (compared to high culturability at 50 µM CLT alone) and can therefore be considered dead.
Effect of LL-III/43 and azoles and combination of LL-III/43 with CLT on hydrophobicity of biofilm cells surface
The cell surface hydrophobicity of C. albicans biofilm cells, measured as a CSH index, is one of its virulence factors affecting the ability of cells to adhere to adhesive surfaces, such as medical implants and catheters. To clarify the possible mechanism of LL-III/43 antibiofilm action, we examined the changes in hydrophobicity of C. albicans ATCC MYA-2876 biofilm cells surface affected by LL-III/43 and compared it with the effect of azoles. The CSH index of C. albicans biofilm cells measured in the absence of antifungal agents was approximately 80%, thus indicating high hydrophobicity of their surface (Tables 2 and 3). LL-III/43 alone at 50 μM reduced the CSH index to 38%, thus reflecting its high antibiofilm activity. Among the azoles, CLT exhibited the strongest antibiofilm activity, reducing the CSH index to 52% at 50 μM concentration. The other two azoles (FLC, VOR) had no significant effect on the CSH (Table 2). Interestingly, the combination of antifungal agents with the most significant antibiofilm activity (50 µM CLT and 25 µM LL-III/43) did not reduce the hydrophobicity of C. albicans biofilm cells (CSH index 57%) by more did the individual agents (52% by 50 µM CLT, 64% and 38% by 25 µM and 50 µM LL-III/43, respectively) (Table 3).
Effect of antifungal agents on phospholipases, proteases, hemolytic activity and hydrophobicity of Candida albicans ATCC MYA-2876 biofilm cells surface.
| . | Effect of LL-III/43 and azoles on biofilm properties . | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||||
| . | LL-III/43 . | FLC . | CLT . | VOR . | LL-III/43 . | FLC . | CLT . | VOR . |
| 0 | 0.61 | 0.74 | 0.67 | 0.72 | 0.45 | 0.41 | 0.46 | 0.40 |
| 3.1 | 0.60 | 0.74 | 0.72 | 0.77 | 0.47 | 0.40 | 0.47 | 0.43 |
| 6.3 | 0.64 | 0.77 | 0.72 | 0.74 | 0.48 | 0.42 | 0.49 | 0.47 |
| 13 | 0.64 | 0.79 | 0.73 | 0.78 | 0.50 | 0.42 | 0.46 | 0.46 |
| 25 | 0.68 | 0.76 | 0.76 | 0.79 | 0.46 | 0.41 | 0.48 | 0.46 |
| 50 | 0.73 | 0.77 | 0.73 | 0.73 | 0.46 | 0.40 | 0.42 | 0.47 |
| Concentration (μM) | Hemolytic activity (Pz) | CSH index (%) | ||||||
| LL-III/43 | FLC | CLT | VOR | LL-III/43 | FLC | CLT | VOR | |
| 0 | 0.26 | 0.22 | 0.23 | 0.27 | 76 | 88 | 82 | 72 |
| 3.1 | 0.23 | 0.24 | 0.24 | 0.25 | 83 | 90 | 69 | 81 |
| 6.3 | 0.30 | 0.23 | 0.23 | 0.26 | 78 | 89 | 59 | 84 |
| 13 | 0.37 | 0.24 | 0.25 | 0.27 | 80 | 91 | 67 | 85 |
| 25 | 0.42 | 0.23 | 0.26 | 0.26 | 64 | 86 | 60 | 88 |
| 50 | 0.55 | 0.22 | 0.72 | 0.27 | 38 | 90 | 52 | 85 |
| . | Effect of LL-III/43 and azoles on biofilm properties . | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||||
| . | LL-III/43 . | FLC . | CLT . | VOR . | LL-III/43 . | FLC . | CLT . | VOR . |
| 0 | 0.61 | 0.74 | 0.67 | 0.72 | 0.45 | 0.41 | 0.46 | 0.40 |
| 3.1 | 0.60 | 0.74 | 0.72 | 0.77 | 0.47 | 0.40 | 0.47 | 0.43 |
| 6.3 | 0.64 | 0.77 | 0.72 | 0.74 | 0.48 | 0.42 | 0.49 | 0.47 |
| 13 | 0.64 | 0.79 | 0.73 | 0.78 | 0.50 | 0.42 | 0.46 | 0.46 |
| 25 | 0.68 | 0.76 | 0.76 | 0.79 | 0.46 | 0.41 | 0.48 | 0.46 |
| 50 | 0.73 | 0.77 | 0.73 | 0.73 | 0.46 | 0.40 | 0.42 | 0.47 |
| Concentration (μM) | Hemolytic activity (Pz) | CSH index (%) | ||||||
| LL-III/43 | FLC | CLT | VOR | LL-III/43 | FLC | CLT | VOR | |
| 0 | 0.26 | 0.22 | 0.23 | 0.27 | 76 | 88 | 82 | 72 |
| 3.1 | 0.23 | 0.24 | 0.24 | 0.25 | 83 | 90 | 69 | 81 |
| 6.3 | 0.30 | 0.23 | 0.23 | 0.26 | 78 | 89 | 59 | 84 |
| 13 | 0.37 | 0.24 | 0.25 | 0.27 | 80 | 91 | 67 | 85 |
| 25 | 0.42 | 0.23 | 0.26 | 0.26 | 64 | 86 | 60 | 88 |
| 50 | 0.55 | 0.22 | 0.72 | 0.27 | 38 | 90 | 52 | 85 |
FLC—fluconazole; CLT—clotrimazole; VOR—voriconazole; Pz = 1.0: no activity; Pz = 0.90–0.99: weak activity; Pz = 0.80–0.89: mild activity; Pz 0.70–0.79: strong activity; Pz < 0.69: very strong activity; CSH = cell surface hydrophobicity.
Effect of antifungal agents on phospholipases, proteases, hemolytic activity and hydrophobicity of Candida albicans ATCC MYA-2876 biofilm cells surface.
| . | Effect of LL-III/43 and azoles on biofilm properties . | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||||
| . | LL-III/43 . | FLC . | CLT . | VOR . | LL-III/43 . | FLC . | CLT . | VOR . |
| 0 | 0.61 | 0.74 | 0.67 | 0.72 | 0.45 | 0.41 | 0.46 | 0.40 |
| 3.1 | 0.60 | 0.74 | 0.72 | 0.77 | 0.47 | 0.40 | 0.47 | 0.43 |
| 6.3 | 0.64 | 0.77 | 0.72 | 0.74 | 0.48 | 0.42 | 0.49 | 0.47 |
| 13 | 0.64 | 0.79 | 0.73 | 0.78 | 0.50 | 0.42 | 0.46 | 0.46 |
| 25 | 0.68 | 0.76 | 0.76 | 0.79 | 0.46 | 0.41 | 0.48 | 0.46 |
| 50 | 0.73 | 0.77 | 0.73 | 0.73 | 0.46 | 0.40 | 0.42 | 0.47 |
| Concentration (μM) | Hemolytic activity (Pz) | CSH index (%) | ||||||
| LL-III/43 | FLC | CLT | VOR | LL-III/43 | FLC | CLT | VOR | |
| 0 | 0.26 | 0.22 | 0.23 | 0.27 | 76 | 88 | 82 | 72 |
| 3.1 | 0.23 | 0.24 | 0.24 | 0.25 | 83 | 90 | 69 | 81 |
| 6.3 | 0.30 | 0.23 | 0.23 | 0.26 | 78 | 89 | 59 | 84 |
| 13 | 0.37 | 0.24 | 0.25 | 0.27 | 80 | 91 | 67 | 85 |
| 25 | 0.42 | 0.23 | 0.26 | 0.26 | 64 | 86 | 60 | 88 |
| 50 | 0.55 | 0.22 | 0.72 | 0.27 | 38 | 90 | 52 | 85 |
| . | Effect of LL-III/43 and azoles on biofilm properties . | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||||
| . | LL-III/43 . | FLC . | CLT . | VOR . | LL-III/43 . | FLC . | CLT . | VOR . |
| 0 | 0.61 | 0.74 | 0.67 | 0.72 | 0.45 | 0.41 | 0.46 | 0.40 |
| 3.1 | 0.60 | 0.74 | 0.72 | 0.77 | 0.47 | 0.40 | 0.47 | 0.43 |
| 6.3 | 0.64 | 0.77 | 0.72 | 0.74 | 0.48 | 0.42 | 0.49 | 0.47 |
| 13 | 0.64 | 0.79 | 0.73 | 0.78 | 0.50 | 0.42 | 0.46 | 0.46 |
| 25 | 0.68 | 0.76 | 0.76 | 0.79 | 0.46 | 0.41 | 0.48 | 0.46 |
| 50 | 0.73 | 0.77 | 0.73 | 0.73 | 0.46 | 0.40 | 0.42 | 0.47 |
| Concentration (μM) | Hemolytic activity (Pz) | CSH index (%) | ||||||
| LL-III/43 | FLC | CLT | VOR | LL-III/43 | FLC | CLT | VOR | |
| 0 | 0.26 | 0.22 | 0.23 | 0.27 | 76 | 88 | 82 | 72 |
| 3.1 | 0.23 | 0.24 | 0.24 | 0.25 | 83 | 90 | 69 | 81 |
| 6.3 | 0.30 | 0.23 | 0.23 | 0.26 | 78 | 89 | 59 | 84 |
| 13 | 0.37 | 0.24 | 0.25 | 0.27 | 80 | 91 | 67 | 85 |
| 25 | 0.42 | 0.23 | 0.26 | 0.26 | 64 | 86 | 60 | 88 |
| 50 | 0.55 | 0.22 | 0.72 | 0.27 | 38 | 90 | 52 | 85 |
FLC—fluconazole; CLT—clotrimazole; VOR—voriconazole; Pz = 1.0: no activity; Pz = 0.90–0.99: weak activity; Pz = 0.80–0.89: mild activity; Pz 0.70–0.79: strong activity; Pz < 0.69: very strong activity; CSH = cell surface hydrophobicity.
Effect of CLT and LL-III/43 in their combinations on phospholipases, proteases, hemolytic activity and hydrophobicity of Candida albicans ATCC MYA-2876 biofilm cells surface.
| . | Effect of LL-III/43 and CLT in their combinations on biofilm properties . | |||||
|---|---|---|---|---|---|---|
| CLT (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||
| . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . |
| 0 | 0.67 | 0.64 | 0.68 | 0.46 | 0.50 | 0.46 |
| 3.1 | 0.72 | 0.59 | 0.64 | 0.46 | 0.57 | 0.55 |
| 6.3 | 0.72 | 0.60 | 0.65 | 0.49 | 0.58 | 0.61 |
| 13 | 0.73 | 0.59 | 0.63 | 0.45 | 0.66 | 0.67 |
| 25 | 0.76 | 0.85 | 0.89 | 0.47 | 0.60 | 0.67 |
| 50 | 0.73 | 0.91 | 0.94 | 0.41 | 0.72 | 0.75 |
| Hemolytic activity (Pz) | CSH index (%) | |||||
| CLT (μM) | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 |
| 0 | 0.23 | 0.55 | 0.55 | 82 | 80 | 64 |
| 3.1 | 0.24 | 0.40 | 0.52 | 69 | 73 | 73 |
| 6.3 | 0.23 | 0.51 | 0.82 | 59 | 82 | 73 |
| 13 | 0.25 | 0.67 | 0.83 | 67 | 80 | 69 |
| 25 | 0.26 | 0.72 | 0.90 | 60 | 83 | 60 |
| 50 | 0.72 | 1.00 | 1.00 | 52 | 74 | 57 |
| . | Effect of LL-III/43 and CLT in their combinations on biofilm properties . | |||||
|---|---|---|---|---|---|---|
| CLT (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||
| . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . |
| 0 | 0.67 | 0.64 | 0.68 | 0.46 | 0.50 | 0.46 |
| 3.1 | 0.72 | 0.59 | 0.64 | 0.46 | 0.57 | 0.55 |
| 6.3 | 0.72 | 0.60 | 0.65 | 0.49 | 0.58 | 0.61 |
| 13 | 0.73 | 0.59 | 0.63 | 0.45 | 0.66 | 0.67 |
| 25 | 0.76 | 0.85 | 0.89 | 0.47 | 0.60 | 0.67 |
| 50 | 0.73 | 0.91 | 0.94 | 0.41 | 0.72 | 0.75 |
| Hemolytic activity (Pz) | CSH index (%) | |||||
| CLT (μM) | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 |
| 0 | 0.23 | 0.55 | 0.55 | 82 | 80 | 64 |
| 3.1 | 0.24 | 0.40 | 0.52 | 69 | 73 | 73 |
| 6.3 | 0.23 | 0.51 | 0.82 | 59 | 82 | 73 |
| 13 | 0.25 | 0.67 | 0.83 | 67 | 80 | 69 |
| 25 | 0.26 | 0.72 | 0.90 | 60 | 83 | 60 |
| 50 | 0.72 | 1.00 | 1.00 | 52 | 74 | 57 |
CLT—clotrimazole; Pz = 1.0: no activity; Pz = 0.90–0.99: weak activity; Pz = 0.80–0.89: mild activity; Pz 0.70–0.79: strong activity; Pz < 0.69: very strong activity; CSH = cell surface hydrophobicity.
Effect of CLT and LL-III/43 in their combinations on phospholipases, proteases, hemolytic activity and hydrophobicity of Candida albicans ATCC MYA-2876 biofilm cells surface.
| . | Effect of LL-III/43 and CLT in their combinations on biofilm properties . | |||||
|---|---|---|---|---|---|---|
| CLT (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||
| . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . |
| 0 | 0.67 | 0.64 | 0.68 | 0.46 | 0.50 | 0.46 |
| 3.1 | 0.72 | 0.59 | 0.64 | 0.46 | 0.57 | 0.55 |
| 6.3 | 0.72 | 0.60 | 0.65 | 0.49 | 0.58 | 0.61 |
| 13 | 0.73 | 0.59 | 0.63 | 0.45 | 0.66 | 0.67 |
| 25 | 0.76 | 0.85 | 0.89 | 0.47 | 0.60 | 0.67 |
| 50 | 0.73 | 0.91 | 0.94 | 0.41 | 0.72 | 0.75 |
| Hemolytic activity (Pz) | CSH index (%) | |||||
| CLT (μM) | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 |
| 0 | 0.23 | 0.55 | 0.55 | 82 | 80 | 64 |
| 3.1 | 0.24 | 0.40 | 0.52 | 69 | 73 | 73 |
| 6.3 | 0.23 | 0.51 | 0.82 | 59 | 82 | 73 |
| 13 | 0.25 | 0.67 | 0.83 | 67 | 80 | 69 |
| 25 | 0.26 | 0.72 | 0.90 | 60 | 83 | 60 |
| 50 | 0.72 | 1.00 | 1.00 | 52 | 74 | 57 |
| . | Effect of LL-III/43 and CLT in their combinations on biofilm properties . | |||||
|---|---|---|---|---|---|---|
| CLT (μM) . | Phospholipases activity (Pz) . | Proteases activity (Pz) . | ||||
| . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . | 0 μM LL III/43 . | 13 μM LL III/43 . | 25 μM LL III/43 . |
| 0 | 0.67 | 0.64 | 0.68 | 0.46 | 0.50 | 0.46 |
| 3.1 | 0.72 | 0.59 | 0.64 | 0.46 | 0.57 | 0.55 |
| 6.3 | 0.72 | 0.60 | 0.65 | 0.49 | 0.58 | 0.61 |
| 13 | 0.73 | 0.59 | 0.63 | 0.45 | 0.66 | 0.67 |
| 25 | 0.76 | 0.85 | 0.89 | 0.47 | 0.60 | 0.67 |
| 50 | 0.73 | 0.91 | 0.94 | 0.41 | 0.72 | 0.75 |
| Hemolytic activity (Pz) | CSH index (%) | |||||
| CLT (μM) | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 | 0 μM LL III/43 | 13 μM LL III/43 | 25 μM LL III/43 |
| 0 | 0.23 | 0.55 | 0.55 | 82 | 80 | 64 |
| 3.1 | 0.24 | 0.40 | 0.52 | 69 | 73 | 73 |
| 6.3 | 0.23 | 0.51 | 0.82 | 59 | 82 | 73 |
| 13 | 0.25 | 0.67 | 0.83 | 67 | 80 | 69 |
| 25 | 0.26 | 0.72 | 0.90 | 60 | 83 | 60 |
| 50 | 0.72 | 1.00 | 1.00 | 52 | 74 | 57 |
CLT—clotrimazole; Pz = 1.0: no activity; Pz = 0.90–0.99: weak activity; Pz = 0.80–0.89: mild activity; Pz 0.70–0.79: strong activity; Pz < 0.69: very strong activity; CSH = cell surface hydrophobicity.
Stability of LL-III/43 and LL-III/Nal during their interaction with the C. albicans biofilm cells
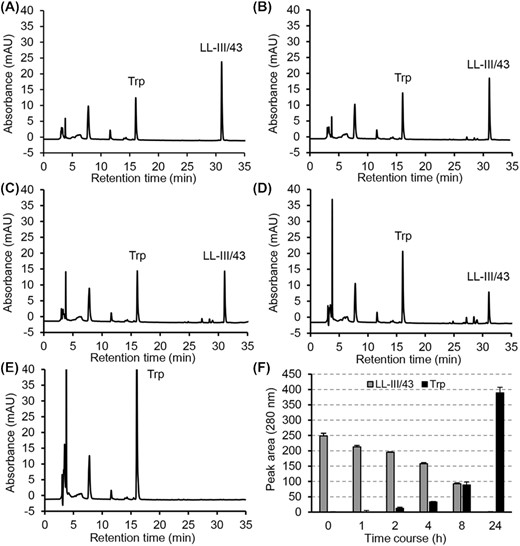
In order to understand how LL-III/43 inhibits biofilm formation; it was important to know how stable the peptide is during its interactions with the C. albicans cells. The stability of LL-III/43 in the presence of C. albicans ATCC MYA-2876 biofilm cells was examined over time by RP-HPLC. As shown in Fig. 3, the time course of the LL-III/43 indicates that the peptide is degraded by proteases secreted by C. albicans. This was manifested by continuous decrease in peptide peak area accompanied by a concomitant increase in the peak area of Trp (expressed as an increment relative to that Trp presented as a component of RPMI-1640 medium). After 4 h, the decrease of LL-III/43 peak was also accompanied by the appearance of two degradation peptide products containing Trp in their sequences. Their peak areas corresponded to approximately 4% (each) of initial LL-III/43 peak area. After 8 h, the peak areas of these two degradation products increased to 7%. After 24 h, the diminution of LL-III/43 resulted in the disappearance of its peak as well as the peaks of the peptide fragments. The notable increase in the Trp peak area indicated that the LL-III/43 had to have been cleaved by proteases secreted by the C. albicans cells into short peptide fragments which were then quickly converted to single amino acids.
Time course for degradation of LL-III/43 by Candida albicans ATCC MYA-2876 proteases followed by RP-HPLC at 280 nm. (A) 0 h, (B) 2 h, (C) 4 h, (D) 8 h, (E) 24 h. Panel F shows quantifications of LL-III/43 and tryptophan (Trp) in terms of their peak areas during the time course.
To support this assumption, we followed the degradation of LL-III/Nal containing non-coded amino acid Nal instead of Trp in its sequence (see Supplementary Fig. 2). Surprisingly, the degradation of this peptide proceeded analogously as in the case of LL-III/43. The peak of LL-III/Nal almost disappeared within 24 h and was accompanied by the quantitatively equal formation of the corresponding peak of Nal (see Supplementary Fig. 2E and F) whose identity was verified by mass spectrometry (see Supplementary Fig. 3).
HPLC analysis of the extract of remaining biofilm cells made at the end of this experiment did not show a presence of LL-III/43. This proved that the peptide had not been absorbed onto the biofilm surface but rather had been degraded by its proteases.
Effect of LL-III/43 and azoles and LL-III/43 in combination with CLT on phospholipases, proteases and hemolytic activity
Neither azoles nor LL-III/43 showed effective reduction of C. albicans ATCC MYA-2876 phospholipases activity (Table 2). This activity remained strong even at the highest tested concentration (50 µM) of azoles and of LL-III/43. Very similar results were achieved when we tested the effect of these agents on the proteases activity of C. albicans ATCC MYA-2876, which was very strong in all cases (Table 2). Only hemolytic activity was weakly inhibited by 50 µM CLT inasmuch as Pz reached a value of 0.72 (Table 2) compared to very strong activity of the control sample with Pz value of 0.23.
Because the use of antifungal agents alone had no obvious effect on phospholipases, proteases and hemolytic activity, we studied their effect on these virulence factors using a combination of CLT and LL-III/43. That combination in some cases showed significant inhibition in the production of these virulence factors (Table 3). Although the inhibitory effect on proteases activity was not great with the combination of 50 µM CLT and 25 µM LL-III/43 (strong activity, Pz value 0.75), it was notable compared to the effect of CLT alone (very strong activity, Pz value 0.42).
A more significant inhibitory effect of the CLT and LL-III/43 combination was observed in the case of phospholipases activity (Table 3). Whereas this activity remained strong across the tested concentration range of 3.1–50 µM using either CLT or LL-III/43 alone, the combination of 50 µM CLT and 13 µM LL-III/43 reduced phospholipases activity to the weak-activity level (Pz value 0.91). The most significant attenuation of enzymatic virulence factors was exhibited when using the combination of CLT (50 µM) and LL-III/43 (13 µM), which resulted in complete inhibition of hemolytic activity (Pz value = 1.0; Table 3) produced by C. albicans ATCC MYA-2876.
Localization of Fl-LL-III/43 in C. albicans adhered cells using SDCM
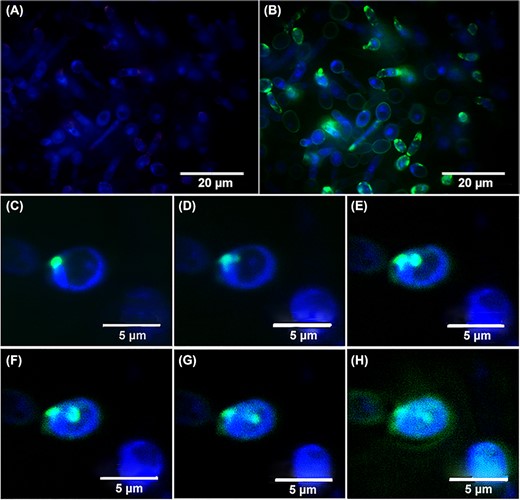
Although the mechanism of antifungal peptides action on C. albicans cells has been studied in several previous works, this topic remains a subject of further research. In order to contribute to elucidating this mechanism, we examined the interaction of fluorescently labeled peptide Fl-LL-III/43 with adhered C. albicans ATCC MYA-2876 cells using SDCM (Fig. 4).
Spinning disk confocal micrographs depicting localization of Fl-LL-III/43 (green signal) by double-staining of adhered cells of Candida albicans ATCC MYA-2876 with DAPI (blue signal). A—adhered cells stained by DAPI (0 min); B—adhered cells double-stained by DAPI and Fl-LL-III/43 (5.5 min); C—initial interaction of Fl-LL-III/43 with the cell surface (0.5 min); D—Fl-LL-III/43 forms a pore in cytoplasmic membrane (2.15 min); E–G—Fl-LL-III/43 penetrates into the cytoplasm through the created pore (3.5–4.5 min); H—Fl-LL-III/43 disperses in the cell and begins to leak from cytosol to cell's surroundings as the cell surface becomes disrupted (5.5 min).
After 5.5 min of this interaction, the surface of most cells in the mixture was covered with the peptide, and we also observed some cells with the peptide infiltrating into their intracellular space (Fig. 4B). Detailed visualization of a single adhered cell of C. albicans ATCC MYA-2876 within short time intervals showed that Fl-LL-III/43 accumulated in the cell membrane until 2.25 min of its action (Fig. 4C and D). Thereafter, the peptide penetrated through the created pore and the localization within the cell began to divide. As a part of the peptide remained incorporated into the cell membrane, a second part started to infiltrate into the intracellular space of the cell. Within a time interval of 3.75–4.5 min, we observed two clearly recognized locations of Fl-LL-III/43 inside the cell (Fig. 4E, F and G). After 5.5 min of the interaction, the entire surface of the single cell was covered with the peptide, and the portion of the peptide inside intracellular space began to disperse (Fig. 4H). Although the cell remained seemingly intact, its surface might have been disrupted (Fig. 4H). Further treatment of the cell with the peptide led to the formation of pores in cell membranes and finally to cell lysis.
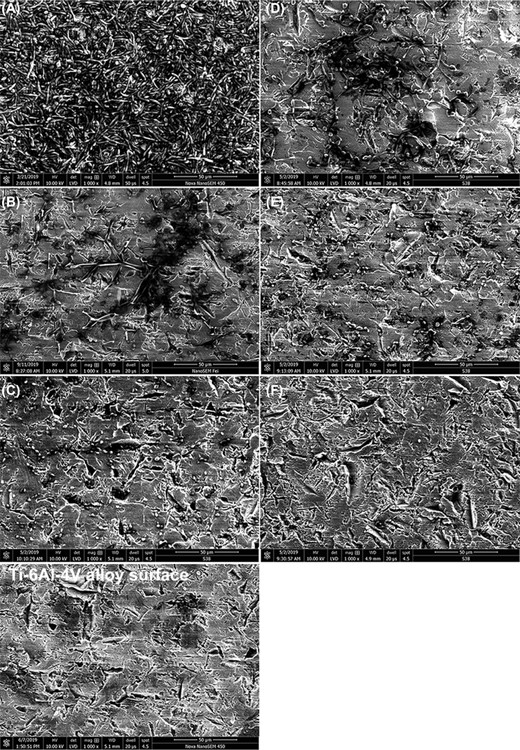
Effect of LL-III/43 on biofilm formation on Ti-6Al-4 V alloy visualized using SEM
The formation of C. albicans ATCC MYA-2876 biofilm on Ti-6Al-4 V alloy (material used for orthopedic surgeries) coupons and the evaluation of its overall architecture in YPD medium (a typical growth medium for conventional yeast cultivations without stimulation of hyphae formation) or RPMI-1640 medium (stimulates both adhesion and hyphae formation) was assessed using SEM. C. albicans ATCC MYA-2876 was able to form a thick biofilm containing hyphae (rather than yeast cells), which completely covered the surface of the coupons when grown in RPMI-1640 medium without antifungal agents (Fig. 5A). As expected, the ability of this strain to form biofilm on coupons in YPD medium was significantly weaker than in the cases of RPMI-1640 cultivations and resulted only in the formation of microcolonies consisting of the two cell types (Fig. 5D). The cultivation of C. albicans ATCC MYA-2876 cells in RPMI-1640 medium in the presence of 25 µM LL-III/43 allowed only partial formation of biofilm (Fig. 5B). The biofilm formed consisted of very long, branched hyphae. In the case of cultivation in YPD medium at the same peptide concentration, only the round cells were apparent, and these adhered solitarily, indicating that both biofilm formation and the occurrence of hyphae were suppressed (Fig. 5E). At the 50 µM concentration of LL-III/43, the formation of biofilm in RPMI-1640 medium was almost completely inhibited. There were only round separate cells adhered and no hyphae were observed (Fig. 5C). The 50 µM concentration of LL-III/43 completely suppressed the biofilm formation of C. albicans ATCC MYA-2876 in the case of YPD medium (Fig. 5F).
Effect of LL-III/43 on cell morphology and biofilm formation of Candida albicans ATCC MYA-2876 cultured on Ti-6Al-4 V alloy coupons presented as scanning electron micrographs. A—cells cultured in absence of LL-III/43 in RPMI-1640; B—cells cultured in presence of 25 µM LL-III/43 in RPMI-1640; C—cells cultured in presence of 50 µM LL-III/43 in RPMI-1640; D—cells cultured in absence of LL-III/43 in YPD; E—cells cultured in presence of 25 µM LL-III/43 in YPD; F—cells cultured in presence of 50 µM LL-III/43 in YPD. Scale bar = 50 µm, magnification: 1000×, detector: LVD, dwell time: 20 µs, spot size: 4.5.
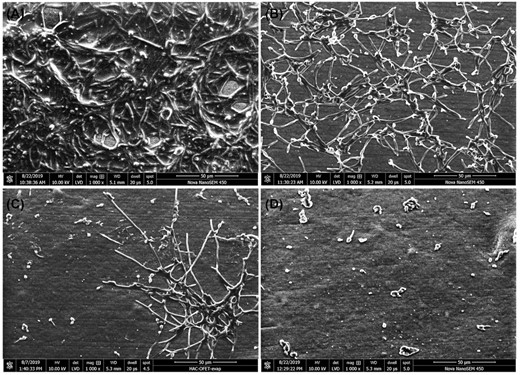
Effect of LL-III/43 alone and in combination with CLT on biofilm formation on silicone catheters visualized using SEM
The effect of LL-III/43 in the combination with CLT on the inhibition of C. albicans biofilm formation was further evaluated by visualization of biofilm formed on the pieces of silicone catheter using SEM. C. albicans ATCC MYA-2876 cultivated in RPMI-1640 medium (without antifungal agents) formed a complex biofilm consisting of both hyphae and yeast-like cells completely covering the surface of this material (Fig. 6A). The cells cultured in the presence of 3.1 µM CLT formed biofilm with lowered density, consisting mainly of hyphae. The biofilm was formed equally on the whole area of the catheter surface, but the cells, although attached to each other, were separated by large spaces in between. The surface was still covered in adhered hyphae, but the biofilm structure was apparently thinner (Fig. 6B). When the cells were cultured with 25 µM LL-III/43, the biofilm did not form within its typical structure. LL-III/43 inhibited adhesion of planktonic cells on the catheter surface, but some of the cells were able to adhere despite the peptide's interference and some clusters of hyphae developed (Fig. 6C). The difference between the antibiofilm effects of CLT and LL-III/43 is therefore more than obvious. The combination of CLT (3.1 µM) and LL-III/43 (25 µM) completely suppressed the presence of hyphae phenotype and the biofilm formation. The small number of adhered cells had only yeast-like morphology (Fig. 6D). Finally, this combination of antifungal agents led to almost complete inhibition of initial adhesion by C. albicans and thus of biofilm formation (see also Fig. 2C and D). It suppressed the hyphae phenotype of the cells and significantly weakened the virulence of its cells in general.
Effect of LL-III/43 and clotrimazole (CLT) combination on cell morphology and biofilm formation of Candida albicans ATCC MYA-2876 cultured on medical grade silicone catheters. A—cells cultured in absence of antifungal agents, B—cells cultured in presence of 3.1 µM CLT, C—cells cultured in presence of 25 µM LL-III/43, D—cells cultured in presence of 3.1 µM CLT and 25 µM LL-III/43. Scale bar = 50 µm, magnification: 1000×, detector: LVD, dwell time: 20 µs, spot size: 5.
Characterization of peptides excreted by C. albicans during its biofilm formation
A recent study has shown that C. albicans produces yet another virulence factor, a cytolytic peptide toxin known as candidalysin. This peptide of 31 amino acid residues secreted during the transition from yeast to filamentous hyphae is critical for epithelial immune activation and mucosal infection by C. albicans (Moyes et al. 2016). Using our methodology, we tried to isolate candidalysin during the process of biofilm formation by C. albicans ATCC MYA-2876 when this yeast switches its morphology from yeast-like cells to hyphae. We also wanted to find out whether the production of candidalysin could be suppressed by the presence of LL-III/43 during the transition of C. albicans cells to its biofilm form. As described in Supporting Information, we were able to detect and isolate two biologically active peptides (see Supplementary Fig. 4). These were identified as follows: The sequencing of the isolated peptide from peak A (at 21.5 min) by Edman degradation using 27 cycles gave the following N-terminal sequence: DVAPAAPAAPADQAPTVPAPQEFNTAI. The monoisotopic molecular mass (2730.35) of this peptide measured by ESI-MS (see Supplementary Fig. 5) was in good agreement with the calculated value of 2730.38 corresponding to the sequence determined by Edman degradation and elongated on its C-terminus by threonine residue. The sequence of this isolated peptide is identical to the Ece1-II32–61, which is derived from its parent protein Ece1p, a large parental preprotein of C. albicans consisting of 271 amino acid residues (Moyes et al. 2016). In addition, we characterized the peptide eluted in peak B (at 26.8 min). Its monoisotopic molecular mass measured by ESI-MS (Electrospray Ionization Mass Spectrometry) was 6902.6 (see Supplementary Fig. 6). Trypsin digestion of this peptide followed by LC-MS-MS (Liquid Chromatography—Tandem Mass Spectrometry) analysis revealed 80% sequence coverage of the 65 amino acid residue peptide corresponding to the C. albicans ATTC MYA-2876 white colony protein WHS11 (Srikantha and Soll 1993). Regrettably, we were not able to detect candidalysin by this procedure, probably due to its insolubility in the solvents used during the process of the isolation. HPLC analysis of the 3 kDa retentate obtained in the parallel experiment performed under the same experimental conditions, but in the presence of LL-III/43, revealed an absence of peptides (data not shown) that should correspond to peaks A and B in the HPLC profile (see Supplementary Fig. 4).
DISCUSSION
Candida albicans is well-known for its strong pathogenicity due to its wide range of virulence factors, including biofilm formation, morphology switching and secretion of extracellular hydrolases (especially phospholipases, proteases and hemolysins) and toxins. These virulence factors are the important tools C. albicans uses to develop infections in patients and acquire resistance to antimycotics. There is increasing demand, therefore, for the development of new antifungal agents capable of enhancing the antibiofilm activity of common antifungal agents.
The antimicrobial peptide lasioglossin (LL-III) originally isolated from the venom of wild bee Lasioglossum laticeps in our laboratory (Čeřovský et al. 2009) possesses broad activities against gram-positive and gram-negative bacteria (Mishra et al. 2013; Basu, Mishra and Leong 2015; Zaccaria et al. 2018) as well as antifungal activities against various strains of Candida spp. (Slaninová et al. 2011; Vráblíková et al. 2017). Since its discovery, LL-III (immobilized on biomedical materials) has been the subject of several studies describing also its inhibitory effect on bacterial biofilm formation (Mishra et al. 2013; Basu, Mishra and Leong 2015; Zaccaria et al. 2018). By contrast, there is a nearly complete absence of research related to antifungal and antibiofilm activity against fungal species. Therefore, we continued in the study of LL-III's properties and found that the replacement of L-Lys in position 9 with its D-Lys isomer, leading to the LL-III/43 analog, increased its antifungal activity (with MIC90 value 6.3 μM for C. albicans ATCC MYA-2876), including its action against yeast biofilm, while reducing its cytotoxicity (since its MIC90 is not toxic for human cells) (Kočendová et al. 2019). In view of the increasing emergence of biofilm-related resistance of Candida spp. to antimycotics (Nett 2014), we conducted in this work a detailed study of LL-III/43’s action against virulence factors of C. albicans. The well-known fact of C. albicans resistance to azoles (FLC, CLT and VOR) was also confirmed in this study, inasmuch as they had no significant effect on the inhibition of C. albicans biofilm formation.
On the contrary, LL-III/43 inhibited initial adhesion and subsequent biofilm formation of this yeast in polystyrene microtiter plates and efficiently prevented the overall biofilm formation on Ti-6Al-4 V alloy. It also affected the hyphae formation that promotes the adhesion of Candida spp. to both biotic and abiotic surfaces and induces the infection process in the host. Suppression of hyphae formation by LL-III was previously described for C. albicans in planktonic form (Vráblíková et al. 2017). Following on from this, we prepared the scanning electron micrographs confirmed that the application of LL-III/43 resulted in reduced ability of C. albicans biofilm cells to form hyphae, thus decreasing its virulence potential. Additionally, we examined the mixture of compounds which C. albicans secrets during the biofilm formation in hyphae-inducing RPMI-1640 medium. We isolated from this mixture peptide Ece1-II32–61, a derivative of Ece1p related to candidalysin, which is itself associated with the switching of yeast-like round cells to hyphae (Moyes et al. 2016). Ece1-II32–61 was not detected in C. albicans biofilm affected by LL-III/43, and this corresponds to this peptide's inhibition of hyphae formation as observed using SEM. Although antifungal agents able to suppress hyphae morphology of cells in favor of yeast-like round cells might act as potent anti-virulence drug candidates (Cui et al. 2015), neither azoles nor LL-III/43 when used alone were able to significantly decrease the phospholipases, proteases, or hemolytic activity of C. albicans.
The fast-killing action of LL-III against Candida spp. planktonic growth at low concentrations described by our colleagues (Kodedová and Sychrová 2016) corresponds to the comparable activity of LL-III/43 in inhibiting initial adhesion and biofilm formation of C. albicans observed in our work. In addition to demonstrating this ability for lasioglossins to kill cells before their adhesion to the surface, we also proved the effect of LL-III/43 in decreasing hydrophobicity of biofilm cells surface. As recently described, the interactions between predominantly anionic phospholipids of plasma membrane and cationic lasioglossins indicates a mainly electrostatic binding of peptides to the cell plasma membranes (Kodedová et al. 2019). We assume that LL-III/43 incorporates into the cytoplasmic membrane, but some part of its amount is bound to the cell surface due to electrostatic interaction. In the case of biofilm biomass (including extracellular polymeric substances), however, the hydrophobic side of the peptide may interact with the hydrophobic components of the biofilm matrix and thus the hydrophilic side of the peptide is directed to the surrounding environment. The hydrophobicity of C. albicans cells’ surface is considered to be a key factor in their ability to adhere to various surfaces and thus switch to the biofilm phenotype (Mah and O'Toole 2001). The decrease of the cells surface hydrophobicity by LL-III/43 led to inhibition of initial adhesion and reduced the virulence of C. albicans.
Localization of the fluorescently labelled peptide Fl-LL-III/43 in C. albicans adhered cells using SDCM also confirmed the presumption as to enveloping of the cells by LL-III/43, because some of the cells were covered in a homogenous layer of the peptide. Some other cells had a surface covered with an irregular layer of peptide with apparently denser locations accumulated probably at the site of the lipid rafts enriched in negatively charged phospholipids (Löffler et al. 2000). In addition, the cells also contained the peptide in cytoplasm, suggesting the peptide's penetration through the pores into the intracellular space. That could lead to DNA binding as designed in an experimental setting by Bandyopadhyay et al. 2013 in the case of the related lasioglossin LL-II. The formation of pores in the cell membrane caused deformation in the shape of the cells in comparison to cells that were not so extensively covered with the peptide and still retained their round shape. Further damage to the cells could lead to cell lysis. These findings extend the results obtained by Slaninová et al. 2011 who tested fluorescently labelled parent peptide LL-III (Fl-LL-III). Use of the more sensitive microscopic technique in our study, however, showed greater detail regarding the peptide's localization in C. albicans cells.
In order better to understand the interaction between LL-III/43 and C. albicans biofilm, we evaluated the integrity of the peptide when cultured with the biofilm cells. Interestingly, the peptide was degraded by the yeast into smaller fragments, which, together with the peptide, were further degraded until they disappeared completely. We assume that the peptide lyses the cells, the content of the cells spills out and includes a spectrum of various enzymes from lysosomes or cytosol. These enzymes, probably including secreted aspartyl proteases possessing broad substrate specificity, can degrade the peptide into fragments, ultimately resulting in a complete conversion of LL-III/43 to single amino acids, as indicated by an increasing amount of Trp during the time of cultivation. Although the peptide was completely degraded during this interaction, the adhesion and subsequent biofilm formation were inhibited due to its rapid action. The result of an analogous experiment using LL-III/Nal clearly demonstrated that the enzymes degraded the peptide to single amino acids and were able to cleave peptide bonds also at both the carboxylic and amino groups of non-coded amino acid Nal. The view that the degradation of LL-III/43 to single amino acids was thus supported by this experiment, because the content of Nal at the end of cultivation was equal to that in the initial concentration of the LL-III/Nal.
In summary, the peptide LL-III/43 clearly possesses antiadhesive and antibiofilm activity towards C. albicans. Its potential for use in treating biofilm-associated yeast infections is therefore obvious. Furthermore, we wanted to find out whether this peptide is capable of enhancing the antibiofilm effect of conventional antifungal agents. The combination of AMP with antibiotics has been used for the inhibition of planktonic growth or biofilm formation of bacteria in many studies (Giacometti et al. 1999; Cirioni et al. 2008; Dosler and Gerceker 2012; Dosler and Karaaslan 2014; Issam et al. 2015). To the best of our knowledge, there are just a few published studies combining the effect of antifungal peptides and antimycotics against yeast. Inhibition of planktonic growth of Candida spp. in combination predominantly with amphotericin B, caspofungin, or FLC has been described, for example, for magainin 2 (Van't Hof et al. 2000; Harris and Coote 2010), lactoferrin (Wakabayashi et al. 1996; Kuipers et al. 1999), mouse β-defensin 3 (Jiang et al. 2012) and histatin 5 (Van't Hof et al. 2000). To date, just three studies have described antibiofilm activity from combinations of antifungal peptides with antimycotics. Amphotericin B and caspofungin were combined with tyrocidines (cyclopeptides from Bacillus aneurinolyticus), which combination exhibited pronounced synergistic biofilm-eradicating activity towards C. albicans (Troskie et al. 2014). Further, the antibiofilm activity of the same antimycotics against C. albicans was also enhanced by plant defensin from coral bells (Heuchera sanguinea) HsAFP1 (Vriens et al. 2015) or recombinant radish defensin (r)RsAFP2 (Vriens et al. 2016).
We showed that LL-III/43 has ability to enhance the effect of azoles (especially CLT) in inhibiting initial adhesion and biofilm formation of C. albicans. The addition of LL-III/43 to CLT caused effective antiadhesive and antibiofilm activity of CLT at a very low concentration of 3.1 µM even though CLT was ineffective when used alone at even its highest tested concentration of 50 µM. The efficiency of the combination of LL-III/43 and CLT on the inhibition of C. albicans biofilm formation was finally confirmed by visualization using SEM of its biofilm formation on silicone urinary catheters in the presence of these antibiofilm agents. Moreover, the addition of LL-III/43 to CLT also led to significant suppression of phospholipases and proteases production and complete attenuation of hemolytic activity of C. albicans. This proves that LL-III/43 helps to overcome the resistance of C. albicans to CLT and points to a potent synergistic action from such combination which could also overcome the problem with frequently emerging recurrent vulvovaginal candidiasis (Kovács et al. 2019).
The common mechanism of action attributable to the LL-III/43 and CLT combination is meanwhile unknown to us. Although the antifungal azoles are known to inhibit the synthesis of ergosterol (Cleveland et al. 2012), the lasioglossin LL-III interacts only negligibly with ergosterol, as was found in Candida glabrata cells according to a recently published study (Kodedová et al. 2019). Those authors, however, observed increasing resistance to the peptide action after the ergosterol content was diminished in this yeast. Based on these facts, we can assume the pretreatment of C. albicans cells by fast-acting LL-III/43 results in the formation of pores in the cell membranes and secondary action in the intracellular space (e.g. DNA binding) with subsequent higher inhibitory effect of CLT on weakened C. albicans cells. Therefore, the antifungal peptide LL-III/43 is a suitable candidate for the enhancement of antimycotic drugs’ action in the treatment of C. albicans biofilm-associated infections.
CONCLUSION
This study shows that the analogue (LL-III/43) of the natural antimicrobial peptide LL-III could be used for the protection of medical materials (such as Ti-6Al-4 V alloy used in orthopedic surgeries and silicone urinary catheters) against C. albicans colonization. It suppresses hyphae formation, envelopes the cell surface and thus changes its hydrophobicity, creates pores, penetrates the intracellular space and subsequently causes the lysis of C. albicans cells. In addition, this peptide helps to overcome the resistance of C. albicans to CLT and the effective concentration of this azole in combination with LL-III/43 needed to achieve potent antibiofilm activity was greatly lowered. The combination of these antibiofilm agents also causes attenuation of other virulence factors of C. albicans, and particularly the hemolytic activity, which is not observed when these agents are used alone. We assume that the enhancement of antifungals by the action of LL-III/43 may lead to overcoming the important medical problem represented by biofilm resistance to common antimycotics, and it has promising potential as a candidate for future pharmaceutical research.
ACKNOWLEDGEMENTS
We thank our technical assistant L. Borovičková for her help with peptide synthesis and Květoslava Kertisová, Dr. Martin Hubálek and Dr. Radko Souček, for mass spectrometry measurement. We also thank G. A. Kirking at English Editorial Services, s.r.o. for assistance with the English.
FUNDING
This work was supported by the Czech Health Research Council [grant number 16-27726A] and the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic[grant number RVO 61388963].
Conflict of interest. None declared.