Article contents
Synthesis and crystal structure of (Ag,Pd)22Se6
Published online by Cambridge University Press: 13 March 2013
Abstract
The (Ag,Pd)22Se6 phase was synthesized from individual elements by silica glass tube technique and structurally characterized from powder X-ray diffraction data. The (Ag,Pd)22Se6 phase crystallizes in Fm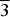 $\overline3$m symmetry, unit-cell parameters: a = 12.3169(2) Å, V = 1862.55(5) Å3, Z = 4, and Dc = 10.01 g/cm3. The crystal structure of the (Ag,Pd)22Se6 phase represents a stuffed 3a.3a.3a superstructure of the Pd structure (fcc), where only 4 from 108 available octahedral holes are occupied. Its crystal structure is related to the Cr23C6 structure type.
$\overline3$m symmetry, unit-cell parameters: a = 12.3169(2) Å, V = 1862.55(5) Å3, Z = 4, and Dc = 10.01 g/cm3. The crystal structure of the (Ag,Pd)22Se6 phase represents a stuffed 3a.3a.3a superstructure of the Pd structure (fcc), where only 4 from 108 available octahedral holes are occupied. Its crystal structure is related to the Cr23C6 structure type.
Keywords
- Type
- Technical Articles
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2013
References
- 1
- Cited by




