Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives ‡
Abstract
:1. Introduction
2. Plant Sources and Discovery of Betulinic Acid
3. Pharmacological Effects of Betulinic Acid
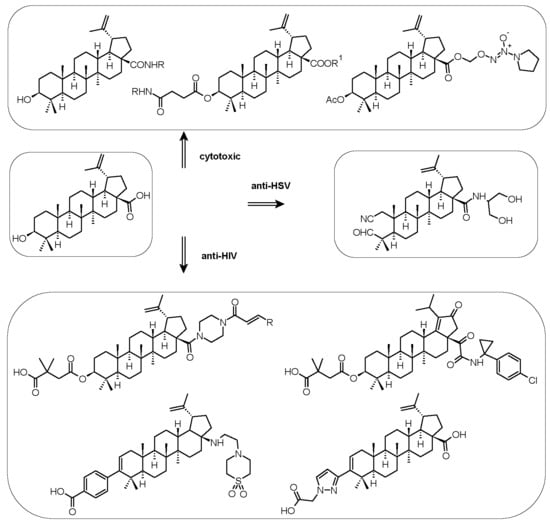
3.1. Cytotoxicity and Antitumor Activity
3.2. Antiviral Activity
3.3. Anti-Inflammatory Activity
3.4. Anti-Diabetic Activity
3.5. Anti-Hyperlipidemic Activity
3.6. Other Activity
4. Supramolecular Characteristics of Betulinic Acid
5. Derivatives of Betulinic Acid, Their Pharmacological Effects, and Supramolecular Characteristics
5.1. Cytotoxicity and Supramolecular Characteristics
5.2. Antiviral Activity
5.2.1. Anti-HIV Activity
5.2.2. Antiherpetic Activity
5.2.3. Antihepatitic Activity
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic acid: Recent advances in chemical modifications, effective delivery, and molecular mechanisms of a promising anticancer therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.G.; Ahmad, J.B.H.; Samzadeh-Kermani, A. Biological activity of betulinic acid: A review. Phamacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef]
- Rios, J.L.; Manez, S. New pharmacological opportunities for betulinic acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, F. Ueber Herba Gratiolae. Arch. Pharm. 1902, 240, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Šarek, J.; Kvasnica, M.; Vlk, M.; Urban, M.; Džubák, P.; Hajdúch, M. The potential of triterpenoids in the treatment of melanoma. In Research on Melanoma—A Glimpse into Current Directions and Future Trends; Murph, M., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 7; pp. 125–158. [Google Scholar]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Csuk, R.; Schmuck, K.; Schäfer, R. A practical synthesis of betulinic acid. Tetrahedron Lett. 2006, 47, 8769–8770. [Google Scholar] [CrossRef]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef]
- Sajfrtová, M.; Ličková, I.; Wimmerová, M.; Sovová, H.; Wimmer, Z. β-Sitosterol: Supercritical carbon dioxide extraction from sea buckthorn (Hippophae rhamnoides L.) seeds. Int. J. Mol. Sci. 2010, 11, 1842–1850. [Google Scholar] [CrossRef]
- Lepojevic, I.; Lepojevic, Z.; Pavlic, B.; Ristic, M.; Zekovic, Z. Solid-liquid and high-pressure (liquid and supercritical carbondioxide) extraction of Echinacea purpurea L. J. Supercrit. Fluids 2017, 119, 159–168. [Google Scholar] [CrossRef]
- Trumbull, E.R.; Bianchi, E.; Eckert, D.J.; Wiedhopf, R.M.; Cole, J.R. Tumor inhibitory agents from Vauquelinia-Corymbosa (Rosaceae). J. Pharm. Sci. 1976, 65, 1407–1408. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human-melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Jaggi, M.; Singh, M.K.; Mukherjee, R.; Burman, A.C. Pharmacological evaluation of C-3 modified betulinic acid derivatives with potent anticancer activity. Investig. New Drugs 2008, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Xu, H.G.; Wang, L.; Li, Y.J.; Sun, P.H.; Wu, X.M.; Wang, G.J.; Chen, W.M.; Ye, W.C. Betulinic acid and its derivatives as potential antitumor agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic acid as a potent and complex antitumor phytochemical: A minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Selzer, E.; Pimentel, E.; Wacheck, W.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J. Investig. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Selzer, E.; Thallinger, C.; Hoeller, C.; Oberkleiner, P.; Wacheck, W.; Pehamberger, H.; Jansen, B. Betulinic acid-induced Mcl-1 expression in human melanoma-mode of action and functional significance. Mol. Med. 2002, 8, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.W.; Adamson, C.S.; Heymann, J.B.; Freed, E.O.; Steven, A.C. HIV-1 maturation inhibitor bevirimat stabilizes the immature Gag lattice. J. Virol. 2011, 85, 1420–1428. [Google Scholar] [CrossRef]
- Suh, N.; Wang, Y.; Honda, T.; Gribble, G.W.; Dmitrovsky, E.; Hickey, W.F.; Maue, R.A.; Place, A.E.; Porter, D.M.; Spinella, M.J.; et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and antiinflammatory activity. Cancer Res. 1999, 59, 336–341. [Google Scholar] [PubMed]
- Willmann, M.; Wacheck, W.; Buckley, J.; Nagy, K.; Thalhammer, J.; Paschke, R.; Triche, T.; Jansen, B.; Selzer, E. Characterization of NVX-207, a novel betulinic acidderived anti-cancer compound. Eur. J. Clin. Investig. 2009, 384–394. [Google Scholar] [CrossRef]
- Dash, S.K.; Chattopadhyay, S.; Dash, S.S.; Tripathy, S.; Das, B.; Mahapatra, S.K.; Bag, B.G.; Karmakar, P.; Roy, S. Self-assembled nano fibers of betulinic acid: A selective inducer for ROS/TNF-alpha pathway mediated leukemic cell death. Bioorg. Chem. 2015, 63, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.; Chen, C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005, 11, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Sarin, P.; Gelmann, E.; Robert-Guroff, M.; Richardson, E.; Kalyanaraman, V.; Mann, D.; Sidhu, G.; Stahl, R.; Zolla-Pazner, S.; et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 1983, 220, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, Q.; Morris-Natschke, S.L.; Chen, C.-H.; Lee, K.-H. Incorporation of privileged structures into bevirimat can improve activity against wild-type and bevirimat-resistant HIV-1. J. Med. Chem. 2016, 59, 9262–9268. [Google Scholar] [CrossRef]
- Zhan, P.; Pannecouque, C.; De Clercq, E.; Liu, X. Anti-HIV drug discovery and development: Current innovations and future trends. J. Med. Chem. 2015, 59, 2849–2878. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.-S.; Lee, K.-H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef]
- Heidary, N.M.; Laszczyk-Lauer, M.N.; Reichling, J.; Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 2014, 21, 1273–1280. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Gou, Y.; Zhang, H.; Vlessidis, A.G.; Zhou, H.; Evmiridis, N.P.; Liu, Z. Betulinic acid-mediated inhibitory effect on hepatitis B virus by suppression of manganese superoxide dismutase expression. FEBS J. 2009, 276, 2599–2614. [Google Scholar] [CrossRef]
- Huguet, A.; Recio, M.C.; Máñez, S.; Giner, R.; Ríos, J.L. Effect of triterpenoids on the inflammation induced by protein kinase C activators, neuronally acting irritants and other agents. Eur. J. Pharmacol. 2000, 410, 69–81. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.; Oliveira, P.J.; Duarte, M.F. Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: Promise or illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabol. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Kim, D.Y.; Kim, S.J.; Jo, H.K.; Kim, G.W.; Chung, S.H. Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling pathway. Biochem. Pharmacol. 2013, 85, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Dash, S.S. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 2011, 3, 4564–4566. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Majumdar, R. Self-assembly of renewable nano-sized triterpenoids. Chem. Rec. 2017, 17, 841–873. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kamra, M.; Bhan, A.; Mandal, S.S.; Bhattacharya, S. New Fe(III) and Co(II) salen complexes with pendant distamycins: Selective targeting of cancer cells by DNA damage and mitochondrial pathways. Dalton Trans. 2016, 45, 9345–9353. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.-H.; Wang, M.; Xu, G.-B.; Liao, S.-G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 913–923. [Google Scholar] [CrossRef]
- Konysheva, A.V.; Nebogatikov, V.O.; Tolmacheva, I.A.; Dmitriev, M.V.; Grishko, V.V. Synthesis of cytotoxically active derivatives based on alkylated 2,3-seco-triterpenoids. Eur. J. Med. Chem. 2017, 140, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Grishko, V.V.; Tolmacheva, I.A.; Nebogatikov, V.O.; Galaiko, N.V.; Nazarov, A.V.; Dmitriev, M.V.; Ivshina, I.B. Preparation of novel ring-A fused azole derivatives of betulin and evaluation of their cytotoxicity. Eur. J. Med. Chem. 2017, 125, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Eignerová, B.; Tichý, M.; Krasulová, J.; Kvasnica, M.; Rárová, L.; Christová, R.; Urban, M.; Bednarczyk-Cwynar, B.; Hajdúch, M. Synthesis and antiproliferative properties of new hydrophilic esters of triterpenic acids. Eur. J. Med. Chem. 2017, 140, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Vida, N.; Rárová, L.; Kolář, M.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Kaletová, E.; Šaman, D.; Sievänan, E.; Kolehmainen, E.T.; Šlouf, M.; Wimmer, Z. Spectral and microscopic study of self-assembly of novel cationic spermine amides of betulinic acid. Steroids 2017, 117, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Bildziukevich, U.; Šaman, D.; Havlíček, L.; Rárová, L.; Navrátilová, L.; Wimmer, Z. Amphiphilic derivatives of (3β,17β)-3-hydroxyandrost-5-ene-17-carboxylic acid. Steroids 2017, 128, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Šaman, D.; Kolehmainen, E.T. Studies on supramolecular gel formation using DOSY NMR. Magn. Reson. Chem. 2015, 53, 256–260. [Google Scholar]
- Noponen, V.; Nonappa; Lahtinen, M.; Valkonen, A.; Salo, H.; Kolehmainen, E.; Sievänen, E. Bile acid–amino acid ester conjugates: Gelation, structural properties, and thermoreversible solid to solid phase transition. Soft Matter 2010, 6, 3789–3796. [Google Scholar] [CrossRef]
- Svobodová, H.; Nonappa; Wimmer, Z.; Kolehmainen, E. Design, synthesis and stimuli responsive gelation of novel stigmasterol–amino acid conjugates. J. Colloid Interface Sci. 2011, 361, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Coates, I.A.; Boucheteau, T.R.; Miravet, J.F.; Escuder, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Low-molecular-weight gelators: Elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. J. Am. Chem. Soc. 2008, 130, 9113–9121. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Rárová, L.; Šaman, D.; Wimmer, Z. Picolyl amides of betulinic acid as antitumor agents causing tumor cell apoptosis. Eur. J. Med. Chem. 2018, 145, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Rárová, L.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Amides derived from heteroaromatic amines and selected steryl hemiesters. Steroids 2013, 78, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, S.; Li, B.; Pan, J.; Jiang, L.; Zhou, G.; Gu, H.; Zhao, C.; Lu, H.; Ma, F. Combination of betulinic acid with diazen-1-ium-1,2-diolate nitric oxide moiety donating a novel anticancer candidate. OncoTargets Ther. 2018, 11, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II study of safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuccinyl) betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Margot, N.A.; Gibbs, C.S.; Miller, M.D. Phenotypic susceptibility to bevirimat in isolates from HIV-1-infected patients without prior exposure to bevirimat. Antimicrob. Agents Chemother. 2010, 54, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Bori, I.D.; Chen, C.-H.; Huang, L.; Lee, K.-H. Anti-AIDS agents 90. Novel C-28 modified bevirimat analogues as potent HIV maturation inhibitors. J. Med. Chem. 2012, 55, 8128–8136. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Park, S.W. An evolving role of piperazine moieties in drug design and discovery. Mini-Rev. Med. Chem. 2013, 13, 1579–1601. [Google Scholar] [CrossRef]
- Tagat, J.R.; McCombie, S.W.; Nazareno, D.; Labroli, M.A.; Xiao, Y.; Steensma, R.W.; Strizki, J.M.; Baroudy, B.M.; Cox, K.; Lachowicz, J.; et al. Piperazine-based CCR5 antagonists as HIV-1 inhibitors. IV. Discovery of 1-[(4,6-dimethyl-5-pyrimidinyl)carbonyl]-4-[4-{2-methoxy-1(R)-4-(trifluoromethyl)-phenyl}ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a potent, highly selective, and orally bioavailable CCR5 antagonist. J. Med. Chem. 2004, 47, 2405–2408. [Google Scholar]
- Thompson, T.N. Optimization of metabolic stability as a goal of modern drug design. Med. Res. Rev. 2001, 21, 412–449. [Google Scholar] [CrossRef]
- Liu, Z.; Swidorski, J.J.; Nowicka-Sans, B.; Terry, B.; Protack, T.; Lin, Z.; Samanta, H.; Zhang, S.; Li, Z.; Parker, D.D.; et al. C-3 benzoic acid derivatives of C-3 deoxybetulinic acid and deoxybetulin as HIV-1 maturation inhibitors. Bioorg. Med. Chem. 2016, 24, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jones, S.A.; Jeffrey, J.L.; Miranda, S.R.; Galardi, C.M.; Irlbeck, D.M.; Brown, K.W.; McDanal, C.B.; Johns, B.A. Discovery of a novel and potent class of anti-HIV-1 maturation inhibitors with improved virology profile against gag polymorphisms. Bioorg. Med. Chem. Lett. 2017, 27, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Swidorski, J.J.; Liu, Z.; Sit, S.-Y.; Chen, J.; Chen, Y.; Sin, N.; Venables, B.L.; Parker, D.D.; Nowicka-Sans, B.; Terry, B.J.; et al. Inhibitors of HIV-1 maturation: Development of structure–activity relationship for C-28 amides based on C-3 benzoic acid-modified triterpenoids. Bioorg. Med. Chem. Lett. 2016, 26, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Regueiro-Ren, A.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.-Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Zhu, J.; Nowicka-Sans, B.; et al. Discovery of BMS-955176, a second generation HIV-1 maturation inhibitor with broad spectrum antiviral activity. ACS Med. Chem. Lett. 2016, 7, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Soumeillant, M.; Savage, S.A.; Strotman, N.A.; Haley, M.; Benkovics, T.; Nye, J.; Xu, Z.; Tan, Y.; Ayers, S.; et al. Synthesis of HIV-Maturation Inhibitor BMS-955176 from betulin by an enabling oxidation strategy. J. Org. Chem. 2017, 82, 4958–4963. [Google Scholar] [CrossRef]
- Visalli, R.J.; Ziobrowski, H.; Badri, K.R.; He, J.J.; Zhang, X.; Arumugam, S.R.; Zhao, H. Ionic derivatives of betulinic acid exhibit antiviral activity against herpes simplex virus type-2 (HSV-2), but not HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2015, 25, 3168–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolmacheva, I.A.; Igosheva, E.V.; Savinova, O.V.; Boreko, E.I.; Grishko, V.V. Synthesis and antiviral activity of C-3(C-28)-substituted 2,3-seco-triterpenoids. Chem. Nat. Comp. 2014, 49, 1050–1058. [Google Scholar] [CrossRef]
- Konysheva, A.V.; Tolmacheva, I.A.; Savinova, O.V.; Boreko, E.I.; Grishko, V.V. Regioselective transformation of the cyano group of triterpene α,β-alkenenitriles. Chem. Nat. Comp. 2017, 53, 687–690. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wang, C.-M.; Cheng, H.-L.; Chen, H.-J.; Hsu, Y.-M.; Lin, Y.-C.; Chou, C.-H. Biological activity of oleanane triterpene derivatives obtained by chemical derivatization. Molecules 2013, 18, 13003–13019. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Z.-S.; Shen, Y.; Xu, J.; Miao, H.-H.; Xiong, Y.; Xu, F.; Li, B.-L.; Luo, J.; Song, B.-L. Inhibition of the sterol regulatory element-binding protein pathway suppresses hepatocellular carcinoma by repressing inflammation in mice. Hepatology 2017, 65, 1936–1947. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Chu, Z.; Ruan, Q.; Chen, X.; Kong, D.; Huang, X.; Li, H.; Tang, H.; Wu, H.; et al. Combination of betulinic acid and chidamide synergistically inhibits Epstein-Barr virus replication through over-generation of reactive oxygen species. Oncotarget 2017, 8, 61646–61661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Only samples of the compounds prepared by the authors of this review article are available from the authors. |
















| Compound | Cytotoxicity (IC50 [µM]) | |||
|---|---|---|---|---|
| CEM a | MCF7 b | HeLa c | BJ d | |
| 15a | 0.7 ± 0.0 | 2.4 ± 0.1 | 2.3 ± 0.0 | 2.6 ± 0.1 |
| 15b | 0.8 ± 0.0 | 7.8 ± 1.1 | 5.7 ± 1.5 | 6.2 ± 2.7 |
| 15c | 7.7 ± 1.6 | 3.3 ± 0.4 | 4.4 ± 1.5 | 3.9 ± 0.9 |
| 20a | 26.8 ± 7.2 | > 50 | > 50 | > 50 |
| 20b | 7.3 ± 0.3 | 35.5 ± 1.5 | 21.9 ± 7.0 | > 50 |
| 20c | 5.2 ± 2.3 | > 50 | 23.8 ± 3.7 | 42.9 ± 3.8 |
| Compound | Cytotoxicity (IC50 [µM]) | TI a | ||||
|---|---|---|---|---|---|---|
| CEM b | MCF7 c | HeLa d | G-361 e | BJ f | ||
| 22a | 6.9 ± 0.4 | 2.2 ± 0.2 | 2.3 ± 0.5 | 2.4 ± 0.0 | 46.2 ± 2.8 | 19.3 |
| 22b | 6.5 ± 1.5 | 1.4 ± 0.1 | 2.4 ± 0.4 | 0.5 ± 0.1 | 50.0 ± 0.0 | 100.0 |
| 22c | 22.6 ± 5.9 | ˃50 | 40.9 ± 4.7 | 32.1 ± 2.1 | ˃50 | ND g |
| 24a | 18.6 ± 1.0 | 27.0 ± 5.5 | 14.7 ± 0.2 | 16.4 ± 1.6 | 15.8 ± 1.4 | 1.0 |
| 24b | 25.3 ± 6.9 | 38.7 ± 1.0 | 23.6 ± 2.3 | 18.1 ± 0.1 | 17.5 ± 2.7 | 1.0 |
| 24c | 18.6 ± 3.7 | 21.1 ± 4.3 | 14.8 ± 0.2 | 11.6 ± 1.6 | 11.2 ± 1.4 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bildziukevich, U.; Özdemir, Z.; Wimmer, Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives ‡. Molecules 2019, 24, 3546. https://doi.org/10.3390/molecules24193546
Bildziukevich U, Özdemir Z, Wimmer Z. Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives ‡. Molecules. 2019; 24(19):3546. https://doi.org/10.3390/molecules24193546
Chicago/Turabian StyleBildziukevich, Uladzimir, Zülal Özdemir, and Zdeněk Wimmer. 2019. "Recent Achievements in Medicinal and Supramolecular Chemistry of Betulinic Acid and Its Derivatives ‡" Molecules 24, no. 19: 3546. https://doi.org/10.3390/molecules24193546