3.2. Chemistry
Ethyl (20S)-6β-hydroxy-3α,5-cyclo-5α-pregnane-20-carboxylate (2)
Potassium acetate (5.4 g; 55 mmol) was added to a solution of tosylate 1 (3.0 g; 5.54 mmol) in acetone (140 mL) and water (40 mL). The reaction was stirred under reflux for 7 h. Then, excess of acetone was removed under reduced pressure and the residual suspension was dissolved in diethyl ether and extracted twice with water. Organic solvents were dried with sodium sulfate and removed under reduced pressure. The crude product was purified on silica gel (mobile phase—30% ethyl acetate in cyclohexane) to afford hydroxyl ester 2 (2.1 g; 97%) as a white solid.
Mp 59–60 °C. 1H NMR (CDCl3) δ 0.29 (dd, 3H, J = 8.1 and 4.5 Hz); 0.53 (t, 1H, J = 4.5 Hz, H-4β); 0.74 (s, 3H, CH3); 1.06 (s, 3H, CH3); 1.19 (d, 3H, J = 6.7 Hz, CH3); 1.25 (t, 3H, J = 7.0 Hz, CH2CH3); 1.93 (dt, 1H, J = 12.5 and 4.0 Hz); 2.41 (dq, 1H, J = 10.6 and 8.0 Hz, H-20); 3.26 (t, 1H, J = 2.9 Hz, H-6α); 4.07–4.16 (m, 2H, CH2CH3). 13C NMR δ 11.57, 12.37, 14.25, 17.10, 20.20, 22.60, 24.22, 24.26, 24.98, 27.19, 29.85, 33.17, 37.03, 38.85, 39.92, 42.61, 42.78, 42.86, 47.57, 52.97, 56.00, 59.96, 73.68, 177.00. HRMS: (API+) calculated for C24H39O3 ([M + H]+) 375.2899, found 375.2899.
Ethyl (20S)-6-oxo-3α,5-cyclo-5α-pregnane-20-carboxylate (3)
Jones reagent (10 mL) was added to a solution of hydroxy ester 2 (2 g; 5.13 mmol) in acetone (150 mL) at 0 °C. The reaction was stirred at 0 °C for an additional 20 min and then quenched with isopropanol (20 mL). Excess of acetone was removed under reduced pressure. The suspension was diluted with diethyl ether and extracted twice with water. Organic solvents were dried with sodium sulfate and removed under reduced pressure. The crude product was purified on silica gel (mobile phase—10% ethyl acetate in cyclohexane) to afford oxoester 3 (1.7 g; 85%) as a white solid.
Mp 86–87 °C. 1H NMR (CDCl3) δ 0.73 (t, 1H, J = 4.9 Hz, H-4β); 0.74 (s, 3H, CH3); 1.01 (s, 3H, CH3); 1.20 (d, 3H, J = 6.7 Hz, CH3); 1.25 (t, 3H, J = 7.0 Hz, CH2CH3); 1.89–1.95 (m, 2H); 1.99 (t, 1H, J = 12.6 and 3.5 Hz); 2.38–2.45 (m, 2H, H-7β, H-20); 4.07–4.16 (m, 2H, CH2CH3). 13C NMR δ 11.65, 12.20, 14.24, 17.08, 19.65, 22.76, 24.10, 25.85, 27.04, 33.44, 34.72, 35.34, 39.45, 42.53, 42.75, 44.67, 45.98, 46.28, 46.70, 52.72, 56.48, 60.04, 176.75, 209.47. HRMS: (API+) calculated for C24H37O3 ([M + H]+) 373.2743, found 373.2744.
Ethyl (20S)-6-oxo-5α-pregn-2-ene-20-carboxylate (4)
Lithium bromide (165 mg; 1.9 mmol) and pyridinium p-toluensulfonate (165 mg; 0.66 mmol) were added to a solution of oxoester 3 (1.65 g; 4.2 mmol) in dimethylacetamide (60 mL). The reaction mixture was stirred under reflux for 5 h. Dimethylacetamide was then removed under reduced pressure. The crude mixture was diluted in diethyl ether and extracted twice with water. Organic solvents were dried with sodium sulfate and removed under reduced pressure. The crude product was purified on silica gel (mobile phase—10% ethyl acetate in cyclohexane) to afford unsaturated ester 4 (1.45 g; 88%) as a white solid.
Mp 118–120 °C. 1H NMR (CDCl3) δ 0.70 (s, 3H, CH3); 0.72 (s, 3H, CH3); 1.20 (d, 3H, J = 6.7 Hz, CH3); 1.25 (t, 3H, J = 7.0 Hz, CH2CH3); 1.71–1.79 (m, 2H); 1.97–2.05 (m, 4H); 2.26 (m, 1H); 2.34–2.36 (m, 2H); 2.41 (dq, 1H, J = 10.4 and 7.0 Hz, H-20); 4.07–4.16 (m, 2H, CH2CH3); 5.57, 5.69 (both m, 1H, H-2 and H-3). 13C NMR δ 12.11, 13.48, 14.24, 17.07, 21.03, 21.68, 23.99, 26.92, 37.61, 39.23, 39.29, 39.99, 42.51, 42.84, 46.89, 52.73, 53.28, 53.78, 56.25, 60.04, 124.45, 124.93, 176.74, 211.81. HRMS: (API+) calculated for C24H37O3 ([M + H]+) 373.2743, found 373.2745.
(20S)-6-oxo-5α-pregn-2-ene-20-carboxylic acid (5)
Potassium hydroxide (1.0 g; 18 mmol) was added to a solution of ester
4 (1.4 g; 3.63 mmol) in ethanol (100 mL) and water (5 mL). The reaction mixture was stirred under reflux for 8 h. The excess ethanol was then removed under reduced pressure. The suspension was diluted with ethyl acetate and extracted with 5% aqueous hydrochloric acid and twice with water. Organic solvents were dried with sodium sulfate and evaporated under reduced pressure. The crude product was purified on silica gel (mobile phase—50–80% ethyl acetate in cyclohexane) to afford acid
5 (1.18 g; 91%) as a white solid. All data correspond to the data published [
34].
General procedure for amide formation from acids 5, 12 and 18
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (1.1 equivalents) was added to a solution of either acid 5 (100 mg; 0.28 mmol), acid 12 (200 mg; 0.43 mmol) or acid 18 (200 mg; 0.36 mmol), secondary amine (1.5 equivalents) and 1-hydroxybenzotriazole hydrate (1.1 equivalents) in dimethylformamide (10 mL). The reaction mixture was stirred at 40 °C for 12 h. Then, it was diluted with ethyl acetate and extracted twice with water. The combined organic fractions were dried with sodium sulfate and evaporated under reduced pressure. The crude product was purified on silica gel (mobile phase at each experiment).
1H-benzo[d][1,2,3]triazol-1-yl (20S)-6-oxo-5α-pregn-2-ene-20-carboxylate (6)
Compound 6 can be obtained as a white solid intermediate (quant. yield) in amide formation according to the general procedure after 5 h at room temperature.
Mp > 160 °C (decomp.). 1H NMR (CDCl3) δ 0.75 (s, 3H, CH3); 0.84 (s, 3H, CH3); 1.55 (d, 3H, J = 6.9 Hz, CH3); 1.78–1.91 (m, 2H); 2.00–2.13 (m, 4H); 2.28 (m, 1H); 2.34–2.42 (m, 2H); 2.96 (m, 1H, H-20); 5.59, 5.71 (both m, 1H, H-2 and H-3); 7.38 (dt, 1H, J = 8.5 and 0.9 Hz, HAr); 7.44 (ddd, 1H, J = 8.5, 7.1 and 0.9 Hz, HAr); 7.56 (ddd, 1H, J = 8.5, 7.1 and 0.9 Hz, HAr); 8.08 (dt, 1H, J = 8.5 and 0.9 Hz, HAr). 13C NMR δ 12.23, 13.52, 17.17, 21.05, 21.68, 24.12, 27.37, 37.58, 39.21, 39.29, 39.96, 40.38, 43.31, 46.79, 52.37, 53.20, 53.80, 56.15, 108.10, 120.58, 124.37, 124.78, 124.97, 128.50, 128.71, 143.48, 172.22, 211.48. HRMS: (API+) calculated for C28H36N3O3 ([M + H]+) 462.2757, found 462.2758.
N,N-dimethyl-(20S)-6-oxo-5α-pregn-2-ene-20-carboxamide (7a)
The general procedure for amide formation with acid 5 and dimethylamine and chromatography on silica gel (isopropanol/cyclohexane—1/9) yielded 79 mg (76%) of the title compound 7a as a colorless oil:
1H NMR (CDCl3) δ 0.73 (s, 3H, CH3); 0.74 (s, 3H, CH3); 1.15 (d, 3H, J = 6.7 Hz, CH3); 1.75 (dtd, 1H, J = 12.4, 10.8 and 4.3 Hz); 1.81–1.89 (m, 2H); 1.97–2.05 (m, 4H); 2.27 (m, 1H); 2.33–2.39 (m, 2H); 2.96 (m, 1H, H-20); 2.76 (dq, 1H, J = 9.8 and 6.7 Hz, H-20); 3.01 (bs, 6H, 2×NCH3); 5.58, 5.69 (both m, 1H, H-2 and H-3). 13C NMR δ 12.35, 13.48, 17.04, 21.05, 21.67, 23.97, 27.35, 37.66 (2×C), 37.68, 39.25, 39.29 (2×C), 40.05, 42.62, 46.92, 52.88, 53.24, 53.76, 56.12, 124.47, 124.90, 176.56, 211.97. HRMS: (API+) calculated for C24H38NO2 ([M + H]+) 372.2903, found 372.2903.
N,N-diethyl-(20S)-6-oxo-5α-pregn-2-ene-20-carboxamide (7b)
The general procedure for amide formation with acid 5 and diethylamine and chromatography on silica gel (isopropanol/cyclohexane—1/19) yielded 87 mg (78%) of the title compound 7b as a colorless oil:
1H NMR (CDCl3) δ 0.72 (s, 3H, CH3); 0.73 (s, 3H, CH3); 1.10 (t, 3H, J = 7.1 Hz, CH3); 1.16 (d, 3H, J = 6.7 Hz, CH3); 1.19 (t, 3H, J = 7.1 Hz, CH3); 1.74 (m, 1H); 1.80–1.90 (m, 2H); 1.97–2.05 (m, 4H); 2.26 (m, 1H); 2.34 (dd, 1H, J = 13.3 and 4.1 Hz); 2.37 (dd, 1H, J = 11.1 and 4.9 Hz); 2.64 (dq, 1H, J = 9.7 and 6.6 Hz, H-20); 3.22–3.33 (m, 2H, 2×NCH); 3.37–3.51 (m, 2H, 2×NCH); 5.58, 5.69 (both m, 1H, H-2 and H-3). 13C NMR δ 12.43, 13.01, 13.50, 14.94, 17.69, 21.08, 21.68, 23.96, 27.10, 37.53, 37.72, 39.28, 39.30, 40.07, 40.48, 42.11, 42.61, 46.92, 53.04, 53.26, 53.77, 56.12, 124.49, 124.91, 175.71, 212.02. HRMS: (API+) calculated for C26H42NO2 ([M + H]+) 400.3216, found 400.3215.
1-((20S)-6-oxo-5α-pregn-2-ene-20-carbonyl)pyrrolidine (7c)
The general procedure for amide formation with acid 5 and pyrrolidine and chromatography on silica gel (isopropanol/cyclohexane—1/19) yielded 91 mg (82%) of the title compound 7c as a colorless oil:
1H NMR (CDCl3) δ 0.71 (s, 6H, 2×CH3); 1.16 (d, 3H, J = 6.7 Hz, CH3); 1.74 (m, 1H); 1.81–1.89 (m, 4H); 1.92–2.05 (m, 6H); 2.25 (m, 1H); 2.34 (dd, 1H, J = 13.1 and 4.3 Hz); 2.38 (dd, 1H, J = 11.0 and 4.9 Hz); 2.55 (dq, 1H, J = 9.8 and 6.7 Hz, H-20); 3.43–3.55 (m, 4H, 4×NCH); 5.57, 5.69 (both m, 1H, H-2 and H-3). 13C NMR δ 12.38, 13.48, 16.92, 21.05, 21.67, 23.97, 24.35, 26.09, 27.25, 37.71, 39.20, 39.28, 40.06, 40.29, 42.58, 45.56, 46.79, 46.92, 52.71, 53.24, 53.75, 56.10, 124.48, 124.89, 175.05, 212.01. HRMS: (API+) calculated for C26H40NO2 ([M + H]+) 398.3059, found 398.3062.
1-((20S)-6-oxo-5α-pregn-2-ene-20-carbonyl)piperidine (7d)
The general procedure for amide formation with acid 5 and piperidine and chromatography on silica gel (isopropanol/cyclohexane—1/19) yielded 99 mg (86%) of the title compound 7d as a colorless oil:
1H NMR (CDCl3) δ 0.720 (s, 3H, CH3); 0.724 (s, 3H, CH3); 1.15 (d, 3H, J = 6.7 Hz, CH3); 1.75 (dtd, 1H, J = 12.5, 10.7 and 4.3 Hz); 1.82–1.91 (m, 2H); 1.98–2.05 (m, 4H); 2.26 (m, 1H); 2.35 (dd, 1H, J = 13.1 and 4.3 Hz); 2.37 (dd, 1H, J = 11.0 and 4.9 Hz); 2.76 (dq, 1H, J = 9.7 and 6.7 Hz, H-20); 3.46–3.68 (m, 4H, 4×NCH); 5.58, 5.69 (both m, 1H, H-2 and H-3). 13C NMR δ 12.33, 13.50, 17.43, 21.06, 21.68, 24.01, 24.71, 25.88 (vbs), 26.89 (vbs, 2×C), 27.62, 37.70, 39.27, 39.30, 40.07, 42.67, 42.92 (vbs), 46.81 (vbs), 46.93, 52.74, 53.26, 53.77, 56.17, 124.49, 124.91, 174.79, 212.02. HRMS: (API+) calculated for C27H42NO2 ([M + H]+) 412.3216, found 412.3216.
4-((20S)-6-oxo-5α-pregn-2-ene-20-carbonyl)morpholine (7e)
The general procedure for amide formation with acid 5 and morpholine and chromatography on silica gel (isopropanol/cyclohexane—1/19) yielded 97 mg (84%) of the title compound 7e as a colorless oil:
1H NMR (CDCl3) δ 0.718 (s, 3H, CH3); 0.720 (s, 3H, CH3); 1.16 (d, 3H, J = 6.7 Hz, CH3); 1.75 (dtd, 1H, J = 12.4, 10.6 and 4.2 Hz); 1.82–1.91 (m, 2H); 1.98–2.05 (m, 4H); 2.26 (m, 1H); 2.35 (dd, 1H, J = 13.1 and 4.3 Hz); 2.38 (dd, 1H, J = 11.0 and 4.6 Hz); 2.70 (dq, 1H, J = 10.0 and 6.8 Hz, H-20); 3.54–3.69 (m, 8H, 8×Hmorpholine); 5.57, 5.69 (both m, 1H, H-2 and H-3). 13C NMR δ 12.44, 13.61, 17.47, 21.16, 21.79, 24.11, 27.74, 37.41 (bs), 37.77, 39.37, 39.41, 40.16, 42.15 (bs), 42.81, 46.40 (bs), 47.01, 52.80, 53.34, 53.89, 56.24, 66.97 (bs), 67.18 (bs), 124.57, 125.03, 175.26, 212.01. HRMS: (API+) calculated for C26H40NO3 ([M + H]+) 414.3008, found 414.3012.
General procedure for Upjohn dihydroxylation
A solution (50 mg/mL) of osmium tetroxide in t-butanol (0.1 mL; 0.02 mmol) was added to a solution of unsaturated amide (~0.15 mmol) and N-methylmorpholine-N-oxide (88 mg; 0.75 mmol) in acetone (4 mL), tetrahydrofuran (4 mL) and water (0.5 mL). The reaction mixture was stirred for 12 h at room temperature. A saturated aqueous solution of sodium sulfite (2 mL) was then added, and the mixture was stirred for an additional 30 min. The reaction was diluted with ethyl acetate and extracted twice with water. The organic phase was dried with sodium sulfate and solvents were evaporated under reduced pressure. The crude product was purified on silica gel (mobile phase at each experiment).
N,N-dimethyl-(20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-carboxamide (8a)
The general procedure for dihydroxylation with amide 7a and chromatography on silica gel (isopropanol/ethyl acetate—1/19) yielded 52 mg (85%) of the title compound 8a as a white solid:
Mp > 230 °C (decomp.). 1H NMR (CDCl3) δ 0.68 (s, 3H, CH3); 0.73 (s, 3H, CH3); 1.10 (d, 3H, J = 6.7 Hz, CH3); 1.86 (dt, 1H, J = 15.2 and 3.4 Hz); 1.94–2.02 (m, 2H); 2.24 (dd, 1H, J = 13.1 and 4.6 Hz); 2.67 (dd, 1H, J = 12.5 and 3.1 Hz); 2.73 (dq, 1H, J = 9.7 and 6.8 Hz, H-20); 2.91 (s, 3H, NCH3); 3.05 (s, 3H, NCH3); 3.67 (m, 1H, Σ J = 19.6 Hz, H-2β); 3.98 (m, 1H, H-3β). 13C NMR δ 12.36, 13.43, 16.87, 21.06, 23.89, 26.21, 27.20, 35.66, 37.61 (2×C), 37.62, 39.13, 39.93, 42.56, 42.71, 46.59, 50.70, 52.78, 53.54, 55.98, 68.01, 68.06, 176.98, 212.76. HRMS: (API+) calculated for C24H40NO4 ([M + H]+) 406.2957, found 406.2957. Anal. Calcd. for C24H39NO4: C, 71.07; H, 9.69; N, 3.45. Found: C, 71.02; H, 9.73; N, 3.42%.
N,N-diethyl-(20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-carboxamide (8b)
The general procedure for dihydroxylation with amide 7b and chromatography on silica gel (isopropanol/ethyl acetate—1/19) yielded 57 mg (88%) of the title compound 8b as a white solid:
Mp > 200 °C (decomp.). 1H NMR (CDCl3) δ 0.72 (s, 3H, CH3); 0.78 (s, 3H, CH3); 1.10 (bt, 3H, J = 6.7 Hz, CH3); 1.16 (d, 3H, J = 6.7 Hz, CH3); 1.20 (bt, 3H, J = 6.7 Hz, CH3); 1.93 (dt, 1H, J = 15.0 and 3.4 Hz); 1.98–2.05 (m, 2H); 2.30 (dd, 1H, J = 13.1 and 4.6 Hz); 2.64 (dq, 1H, J = 9.9 and 6.8 Hz, H-20); 2.73 (dd, 1H, J = 12.7 and 2.9 Hz); 3.24–3.32 (m, 2H, NCH2); 3.39–3.47 (m, 2H, NCH2); 3.75 (m, 1H, Σ J = 19.6 Hz, H-2β); 4.08 (m, 1H, H-3β). 13C NMR δ 12.52, 12.93, 13.57, 14.86, 17.59, 21.19, 23.97, 26.31, 27.05, 37.59, 37.67, 39.34, 40.31, 40.82 (bs), 42.33 (bs), 42.67, 42.80, 46.76, 50.85, 52.96, 53.85, 56.21, 68.43, 68.50, 176.14, 212.06. HRMS: (API+) calculated for C26H44NO4 ([M + H]+) 434.3270, found 434.3270. Anal. Calcd. for C26H43NO4: C, 72.02; H, 10.00; N, 3.23. Found: C, 71.95; H, 10.06; N, 3.20%.
1-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-carbonyl)pyrrolidine (8c)
The general procedure for dihydroxylation with amide 7c and chromatography on silica gel (isopropanol/ethyl acetate—1/19) yielded 55 mg (85%) of the title compound 8c as a white solid:
Mp > 180 °C (decomp.). 1H NMR (CDCl3) δ 0.71 (s, 3H, CH3); 0.77 (s, 3H, CH3); 1.16 (d, 3H, J = 6.7 Hz, CH3); 1.92 (dt, 1H, J = 15.1 and 3.3 Hz); 1.93–2.03 (m, 4H); 2.29 (dd, 1H, J = 13.1 and 4.6 Hz); 2.55 (dq, 1H, J = 9.8 and 6.5 Hz, H-20); 2.73 (dd, 1H, J = 12.5 and 2.8 Hz); 3.43–3.55 (m, 4H, 2×NCH2); 3.75 (m, 1H, Σ J = 19.6 Hz, H-2β); 4.07 (m, 1H, H-3β). 13C NMR δ 12.46, 13.54, 16.81, 21.17, 23.98, 24.29, 26.02, 26.31, 27.20, 37.67, 39.30, 40.27, 40.33, 42.66, 42.78, 45.84, 46.74, 47.02, 50.84, 52.73, 53.84, 56.22, 68.41, 68.48, 175.45, 212.12. HRMS: (API+) calculated for C26H42NO4 ([M + H]+) 432.3114, found 432.3116. Anal. Calcd. for C26H41NO4: C, 72.35; H, 9.57; N, 3.25. Found: C, 72.29; H, 9.61; N, 3.22%.
1-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-carbonyl)piperidine (8d)
The general procedure for dihydroxylation with amide 7d and chromatography on silica gel (isopropanol/ethyl acetate—1/19) yielded 58 mg (87%) of the title compound 8d as a white solid:
Mp > 230 °C (decomp.). 1H NMR (CDCl3) δ 0.71 (s, 3H, CH3); 0.77 (s, 3H, CH3); 1.14 (d, 3H, J = 6.7 Hz, CH3); 1.93 (dt, 1H, J = 15.1 and 3.5 Hz); 1.98–2.04 (m 2H); 2.29 (dd, 1H, J = 13.1 and 4.6 Hz); 2.73 (dd, 1H, J = 12.7 and 2.9 Hz); 2.76 (dq, 1H, J = 9.8 and 6.7 Hz, H-20); 3.48–3.60 (m, 4H, 2×NCH2); 3.74 (m, 1H, Σ J = 19.6 Hz, H-2β); 4.07 (m, 1H, H-3β). 13C NMR δ 12.41, 13.56, 17.37, 21.17, 24.01, 24.61, 26.29, ~26.76 (vbm 3×C), 37.64, 39.35, 40.30, 42.67, 42.83, 43.02 (vbs), 46.76, 46.88 (vbs), 50.84, 52.77, 53.26, 53.85, 56.28, 68.41, 68.44, 175.03, 212.08. HRMS: (API+) calculated for C27H44NO4 ([M + H]+) 446.3270, found 446.3271. Anal. Calcd. for C27H43NO4: C, 72.77; H, 9.73; N, 3.14. Found: C, 72.75; H, 9.78; N, 3.10%.
4-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-carbonyl)morpholine (8e)
The general procedure for dihydroxylation with amide 7e and chromatography on silica gel (isopropanol/ethyl acetate—1/9) yielded 56 mg (84%) of the title compound 8e as a white solid:
Mp > 270 °C (decomp.). 1H NMR (CDCl3+MeOD) δ 0.68 (s, 3H, CH3); 0.73 (s, 3H, CH3); 1.13 (d, 3H, J = 6.7 Hz, CH3); 1.87 (dt, 1H, J = 15.3 and 3.5 Hz); 1.95–2.03 (m 2H); 2.26 (dd, 1H, J = 13.1 and 4.6 Hz); 2.65–2.71 (m, 2H); 3.54–3.68 (m, 8H, 8×Hmorpholine); 3.69 (m, 1H, H-2β); 3.99 (m, 1H, H-3β). 13C NMR δ 12.34, 13.46, 17.22, 21.06, 23.92, 26.22, 27.51, 37.23 (bs), 37.59, 39.13, 39.97, 42.05 (bs), 42.54, 42.79, 46.26 (bs), 46.58, 50.69, 52.58, 53.54, 56.00, 66.77 (bs), 66.97 (bs), 68.00, 68.07, 175.38, 212.52. HRMS: (API+) calculated for C26H42NO5 ([M + H]+) 448.3063, found 448.3063. Anal. Calcd. for C26H41NO5: C, 69.77; H, 9.23; N, 3.13. Found: C, 69.72; H, 9.25; N, 3.09%.
Methyl (20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carboxylate (11)
p-Toluensulfonic acid (50 mg; 0.29 mmol) was added to a solution of ester 9 (1.0 g; 2.55 mmol) in dry acetone (100 mL). The reaction mixture was stirred at room temperature for 5 h. The excess acetone was removed under reduced pressure and the suspension was diluted in ethyl acetate and extracted with a saturated solution of sodium bicarbonate and twice with water. The organic phase was dried over sodium sulfate and solvents were evaporated under reduced pressure. Partially protected ester 10 was dissolved in 2,2-dimethyl-1,3-dioxolan (20 mL) and p-toluensulfonic acid (10 mg; 0.06 mmol) was added. The reaction mixture was stirred at 90 °C for 10 h. The mixture was diluted with ethyl acetate and extracted with a saturated solution of sodium bicarbonate and twice with water. The organic phase was dried over sodium sulfate and solvents were evaporated under reduced pressure. The crude product was purified on silica gel (mobile phase—30% ethyl acetate in cyclohexane) to afford protected methyl ester 11 (888 mg; 74% after 2 steps) as a colorless oil.
1H NMR (DMSO-d6) δ 0.62 (s, 3H, CH3); 0.76 (s, 3H, CH3); 1.10 (d, 3H, J = 6.7 Hz, CH3); 1.21 (s, 3H, CH3); 1.35 (s, 3H, CH3); 1.62–1.71 (m, 3H); 1.79 (dd, 1H, J = 12.5 and 7.0 Hz); 1.86 (m, 1H); 1.96 (m, 1H); 2.32 (dq, 1H, J = 10.3 and 6.9 Hz, H-20); 3.55 (s, 3H, CH3); 3.67 (m, 1H, CH2O); 3.80–3.91 (m, 3H, CH2O); 4.01 (m, 1H, H-2β); 4.18 (m, 1H, H-3β). 13C NMR δ 11.83, 13.18, 16.91, 20.34, 21.46, 23.87, 26.65, 26.74, 28.65, 32.53, 37.61, 38.82, 40.64, 41.83, 42.09, 42.44, 45.12, 51.21, 52.16, 52.65, 54.81, 63.72, 65.18, 72.04, 72.27, 106.80, 108.86, 176.27. HRMS: (API+) calculated for C28H45O6 ([M + H]+) 477.3216, found 477.3220.
(20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carboxylic acid (12)
Potassium hydroxide (500 mg; 8.9 mmol) was added to a solution of methyl ester 11 (850 mg; 1.79 mmol) in isopropanol (80 mL) and water (10 mL). The reaction mixture was stirred under reflux for 4 h. After cooling to room temperature, the excess of isopropanol was removed under reduced pressure and the residue was diluted in ethyl acetate and extracted with 5% aqueous solution of citric acid and twice with water. The organic phase was dried over sodium sulfate and solvents were evaporated under reduced pressure to obtain acid 12 (795 mg; 96%) in adequate purity for other reactions.
1H NMR (DMSO-d6) δ 0.62 (s, 3H, CH3); 0.77 (s, 3H, CH3); 1.09 (d, 3H, J = 6.9 Hz, CH3); 1.22 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.79 (dd, 1H, J = 12.7 and 6.9 Hz); 1.87 (m, 1H); 1.97 (m, 1H); 2.20 (dq, 1H, J = 10.4 and 6.8 Hz, H-20); 3.67 (m, 1H, CH2O); 3.80–3.91 (m, 3H, CH2O); 4.01 (m, 1H, H-2β); 4.19 (m, 1H, H-3β); 11.94 (bs, 1H, COOH). 13C NMR δ 11.84, 13.15, 17.02, 20.32, 21.42, 23.90, 26.60, 26.95, 28.60, 32.48, 37.57, 38.92, 40.60, 41.95, 42.10, 42.40, 45.08, 52.15, 52.42, 54.87, 63.67, 65.13, 72.00, 72.22, 106.74, 108.82, 177.40. HRMS: (API+) calculated for C27H43O6 ([M + H]+) 463.3060, found 463.3059.
N,N-dimethyl-(20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carboxamide (13a)
The general procedure for amide formation with acid 12 and dimethylamine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 180 mg (85%) of the title compound 13a as a colorless oil:
1H NMR (DMSO-d6) δ 0.67 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.98 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.79 (dd, 1H, J = 12.7 and 6.9 Hz); 1.88 (m, 1H); 1.97 (m, 1H); 2.72 (m, 1H, H-20); 2.78 (s, 3H, CH3); 3.00 (s, 3H, CH3); 3.67 (m, 1H, CH2O); 3.80–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 12.12, 13.16, 17.02, 20.34, 21.42, 23.87, 26.36, 26.62, 27.00, 28.61, 32.47, 35.02, 37.04, 37.57, 39.08, 40.64, 41.76, 42.40, 45.07, 52.18, 52.91, 54.90, 63.65, 65.15, 72.02, 72.23, 106.75, 108.86, 175.35. HRMS: (API+) calculated for C29H48NO5 ([M + H]+) 490.3532, found 490.3534.
N,N-diethyl-(20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carboxamide (13b)
The general procedure for amide formation with acid 12 and diethylamine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 186 mg (83%) of the title compound 13b as a colorless oil:
1H NMR (DMSO-d6) δ 0.67 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.97 (t, 3H, J = 7.0 Hz, CH3); 1.00 (d, 3H, J = 6.7 Hz, CH3); 1.10 (t, 3H, J = 7.0 Hz, CH3); 1.22 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.80 (dd, 1H, J = 12.7 and 6.9 Hz); 1.89 (m, 1H); 1.97 (m, 1H); 2.58 (m, 1H, H-20); 3.13 (m, 1H, NCH); 3.24–3.40 (m, 3H, 3×NCH); 3.67 (m, 1H, CH2O); 3.80–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 12.14, 12.97, 13.17, 14.94, 17.65, 20.35, 21.42, 23.78, 26.35, 26.61, 26.76, 28.60, 32.47, 36.70, 37.56, 39.09, 40.65, 41.52, 41.75, 42.39, 45.06, 52.18, 53.02, 54.87, 63.65, 65.15, 72.01, 72.22, 106.74, 108.86, 174.50. HRMS: (API+) calculated for C31H52NO5 ([M + H]+) 518.3845, found 518.3849.
1-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carbonyl)pyrrolidine (13c)
The general procedure for amide formation with acid 12 and pyrrolidine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 196 mg (88%) of the title compound 13c as a colorless oil:
1H NMR (DMSO-d6) δ 0.66 (s, 3H, CH3); 0.77 (s, 3H, CH3); 1.00 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.73–1.81 (m, 3H); 1.82–1.89 (m, 3H); 1.97 (m, 1H); 2.51 (m, 1H, H-20); 3.21–3.24 (m, 2H, 2×NCH); 3.40–3.46 (m, 2H, 2×NCH); 3.67 (m, 1H, CH2O); 3.80–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 12.18, 13.20, 16.93, 20.38, 21.46, 23.89, 23.96 (2×C), 25.73, 26.65, 26.92, 28.65, 32.53, 37.61, 39.09, 40.68, 41.76, 42.43, 45.11, 45.25, 46.22, 52.22, 52.81, 54.91, 63.69, 65.19, 72.05, 72.27, 106.80, 108.89, 173.81. HRMS: (API+) calculated for C31H50NO5 ([M + H]+) 516.3689, found 516.3690.
1-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carbonyl)piperidine (13d)
The general procedure for amide formation with acid 12 and piperidine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 192 mg (84%) of the title compound 13d as a colorless oil:
1H NMR (DMSO-d6) δ 0.66 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.99 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.64–1.74 (m, 3H); 1.79 (dd, 1H, J = 12.5 and 6.7 Hz); 1.89 (m, 1H); 1.97 (m, 1H); 2.72 (m, 1H, H-20); 3.39–3.50 (m, 4H, 4×NCH); 3.67 (m, 1H, CH2O); 3.80–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 12.09, 13.17, 17.40, 20.34, 21.44, 23.89, 24.24, 25.55, 26.56, 26.63, 27.31, 28.62, 32.47, 37.58, 39.10, 39.27, 40.67, 41.81, 42.07, 42.40, 45.09, 46.11, 52.19, 52.75, 54.94, 63.67, 65.17, 72.03, 72.24, 106.76, 108.87, 173.64. HRMS: (API+) calculated for C32H51NO5 ([M + H]+) 530.3845, found 530.3846.
4-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-carbonyl)morpholine (13e)
The general procedure for amide formation with acid 12 and morpholine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 193 mg (84%) of the title compound 13b as a colorless oil:
1H NMR (DMSO-d6, 45 °C) δ 0.68 (s, 3H, CH3); 0.78 (s, 3H, CH3); 1.02 (d, 3H, J = 6.7 Hz, CH3); 1.23 (s, 3H, CH3); 1.37 (s, 3H, CH3); 1.61–1.75 (m, 5H); 1.80 (dd, 1H, J = 12.5 and 7.0 Hz); 1.89 (m, 1H); 1.98 (m, 1H); 2.73 (m, 1H, H-20); 3.44–3.56 (m, 8H, 8×Hmorpholine); 3.68 (m, 1H, CH2O); 3.81–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 12.10, 13.18, 17.29, 20.35, 21.45, 23.92, 26.64, 27.21, 28.64 (2×C), 32.49, 37.60, 39.10, 40.68, 41.59, 41.88, 42.42, 45.11, 45.76, 52.20, 52.71, 54.92, 63.69, 65.19, 66.32, 66.48, 72.04, 72.26, 106.79, 108.88, 174.22. HRMS: (API+) calculated for C31H50NO6 ([M + H]+) 532.3638, found 532.3639.
General procedure for reduction of amides with lithium aluminum hydride
An amount of 1M solution of lithium aluminum hydride in diethyl ether (1.0 mL; 1.0 mmol) was added dropwise to a solution of amide (150 mg) in dry dioxane (10 mL). The reaction mixture was stirred under reflux for 3 h. After cooling, a saturated aqueous solution of sodium potassium tartrate (1 mL) was added, and the mixture was stirred for an additional 10 min. Then, it was diluted with ethyl acetate and extracted twice with water. The organic phase was dried with sodium sulfate and solvents were evaporated under reduced pressure. The crude product was purified on silica gel (mobile phase at each experiment).
N,N-dimethyl-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-methyl)amine (14a)
The general procedure for amide reduction with amide 13a (150 mg; 0.31 mmol) and chromatography on silica gel (2–4% methanol in ammonia chloroform) yielded 106 mg (73%) of the title compound 14a as a colorless oil:
1H NMR (DMSO-d6, 55 °C) δ 0.66 (s, 3H, CH3); 0.79 (s, 3H, CH3); 0.96 (d, 3H, J = 6.5 Hz, CH3); 1.23 (s, 3H, CH3); 1.37 (s, 3H, CH3); 1.64–1.75 (m, 3H); 1.76 (m, 1H); 1.81 (dd, 1H, J = 12.5 and 7.0 Hz); 1.94–2.01 (m, 2H); 2.19 (bs, 6H, 2×CH3), 3.69 (m, 1H, CH2O); 3.81–3.91 (m, 3H, CH2O); 4.03 (m, 1H, H-2β); 4.20 (m, 1H, H-3β). 13C NMR δ 11.86, 13.16, 17.59, 20.34, 21.42, 24.00, 26.61, 27.65, 28.62, 32.47, 34.02 (vb), 37.56, 39.07, 40.65, 42.34, 42.42, 45.08, 45.33 (vb, 2×C), 52.20, 54.54 (b), 54.98, 59.78, 63.67, 65.14, 72.01, 72.23, 106.74, 108.86. HRMS: (API+) calculated for C29H50NO4 ([M + H]+) 476.3740, found 476.3742.
N,N-diethyl-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-methyl)amine (14b)
The general procedure for amide reduction with amide 13b (150 mg; 0.29 mmol) and chromatography on silica gel (2–4% methanol in ammonia chloroform) yielded 104 mg (71%) of the title compound 14b as a colorless oil:
1H NMR (DMSO-d6, 55 °C) δ 0.64 (s, 3H, CH3); 0.75 (s, 3H, CH3); 0.89–1.00 (m, 9H, 3×CH3), 1.61–1.72 (m, 3H); 1.78 (dd, 1H, J = 12.7 and 6.9 Hz); 1.80 (m, 1H); 1.91–1.98 (m, 2H); 2.50–3.10 (vbs, 6H, 6×NCH), 3.66 (m, 1H, CH2O); 3.78–3.88 (m, 3H, CH2O); 3.99 (m, 1H, H-2β); 4.17 (m, 1H, H-3β). 13C NMR δ 11.76, 13.15, 14.55 (vb, 2×C), 17.65 (b), 20.32, 21.42, 23.90 (b), 26.61, 27.59 (b), 28.61, 32.47, 37.55, 39.08, 40.63, 42.34, 42.40, 45.06, 51.97 (vb, 2×C), 52.11, 55.01, 63.22 (vb, C), 63.66, 65.15, 71.99, 72.22, 106.74, 108.84, 2×C n. f. HRMS: (API+) calculated for C31H54NO4 ([M + H]+) 504.4053, found 504.4050.
1-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-methyl)pyrrolidine (14c)
The general procedure for amide reduction with amide 13c (150 mg; 0.29 mmol) and chromatography on silica gel (2–4% methanol in ammonia chloroform) yielded 110 mg (75%) of the title compound 14c as a colorless oil:
1H NMR (DMSO-d6) δ 0.65 (s, 3H, CH3); 0.76 (s, 3H, CH3); 1.04 (d, 3H, J = 6.7 Hz, CH3); 1.21 (s, 3H, CH3); 1.35 (s, 3H, CH3); 1.62–1.72 (m, 3H); 1.73–1.81 (m, 2H); 1.91–1.98 (m, 2H); 2.66–3.14 (vbs, 6H, 6×NCH), 3.67 (m, 1H, CH2O); 3.80–3.84 (m, 2H, CH2O); 3.89 (m, 1H, CH2O); 4.01 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 11.81, 13.19, 17.29, 20.38, 21.45, 22.66 (2×C), 23.90, 26.65, 27.53, 28.66, 32.52, 37.60, 39.08, 40.65, 42.37, 42.46, 45.12, 52.14, 53.66 (vb, 2×C), 55.02, 60.49 (vb), 63.73, 65.19, 72.04, 72.27, 106.80, 108.88, 2×C n. f. HRMS: (API+) calculated for C31H52NO4 ([M + H]+) 502.3896, found 502.3895.
1-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-methyl)piperidine (14d)
The general procedure for amide reduction with amide 13d (150 mg; 0.28 mmol) and chromatography on silica gel (2–4% methanol in ammonia chloroform) yielded 107 mg (73%) of the title compound 14d as a colorless oil:
1H NMR (DMSO-d6) δ 0.67 (s, 3H, CH3); 0.79 (s, 3H, CH3); 1.04 (d, 3H, J = 6.7 Hz, CH3); 1.23 (s, 3H, CH3); 1.37 (s, 3H, CH3); 1.64–1.83 (m, 5H); 1.93–2.01 (m, 2H); 2.65–3.00 (vbm, 4H, 4×NCH), 3.14–3.55 (vbs, 2H, 2×NCH), 3.70 (m, 1H, CH2O); 3.81–3.92 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.20 (m, 1H, H-3β). 13C NMR δ 11.76, 13.14, 17.67, 20.30, 21.41, 23.24, 23.90 (vb, 2×C), 26.60, 27.52, 28.61, 32.46, 37.55, 39.08, 40.60, 42.35, 42.39, 45.05, 52.08, 54.62 (vb, 2×C), 54.96, 60.23 (vb), 63.66, 65.12, 71.97, 72.20, 106.73, 108.82, 3×C n. f. HRMS: (API+) calculated for C32H54NO4 ([M + H]+) 516.4053, found 516.4055.
4-((20S)-2α,3α-(1-methylethylidene)dioxy-6,6-ethylenedioxy-5α-pregnane-20-methyl)morpholine (14e)
The general procedure for amide reduction with amide 13e (150 mg; 0.28 mmol) and chromatography on silica gel (2–4% methanol in ammonia chloroform) yielded 115 mg (79%) of the title compound 14e as a colorless oil:
1H NMR (DMSO-d6, 50 °C) δ 0.65 (s, 3H, CH3); 0.78 (s, 3H, CH3); 0.97 (d, 3H, J = 6.7 Hz, CH3); 1.23 (s, 3H, CH3); 1.37 (s, 3H, CH3); 1.63–1.72 (m, 3H); 1.78–1.82 (m, 2H); 1.89–2.00 (m, 3H); 2.13–2.19 (m, 3H, 3×NCH); 2.38–2.42 (m, 2H, 2×NCH); 3.51–3.59 (m, 4H, 2×CH2Omorpholine); 3.69 (m, 1H, CH2O); 3.81–3.91 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.20 (m, 1H, H-3β). 13C NMR δ 11.67, 12.92, 17.72, 20.16, 21.29, 23.82, 26.17, 27.40, 28.38, 32.32, 32.99, 37.38, 38.97, 40.51, 42.21, 42.32, 44.97, 52.19, 53.80 (2×C), 54.59, 54.86, 63.43, 64.47, 64.95, 66.14 (2×C), 71.85, 72.08, 106.53, 108.72. HRMS: (API+) calculated for C31H52NO5 ([M + H]+) 518.3845, found 518.3849.
General procedure for deprotection of short side chain amine ketals
A 5% aqueous solution of hydrochloric acid (1 mL) was added to a solution of ketals 14a–e (80 mg) in tetrahydrofuran (5 mL) and the mixture was stirred at 45 °C for 2 h. After cooling, the saturated solution of sodium bicarbonate (2 mL) was added, and the reaction was stirred at room temperature for an additional 15 min. The mixture was diluted with ethyl acetate and extracted twice with water. The organic phase was dried with sodium sulfate and solvents were evaporated under reduced pressure. The crude product was washed with diethylether to obtain a pure product (no chromatography needed).
(20S)-20-(N,N-dimethylaminomethyl)-2α,3α-dihydroxy-5α-pregnane-6-one (15a)
The general procedure for deprotection with ketal 14a (80 mg; 0.17 mmol) yielded 57 mg (88%) of the title compound 15a as an off-white solid:
Mp 219–221 °C; >240 (decomp.). 1H NMR (DMSO-d6) δ 0.64 (s, 3H, CH3); 0.65 (s, 3H, CH3); 0.96 (d, 3H, J = 6.5 Hz, CH3); 1.67 (m, 1H); 1.79 (m, 1H); 1.97–2.13 (m, 3H); 2.19 (vbs, 6H, 2×NCH3); 2.60 (dd, 1H, J = 12.2 and 3.4 Hz); 3.46 (m, 1H, H-2β); 3.75 (m, 1H, H-3β); 4.20 (d, 1H, J = 2.4 Hz, OH); 4.34 (d, 1H, J = 6.1 Hz, OH). 13C NMR δ 11.90, 13.36, 17.55, 20.78, 23.67, 26.84, 27.57, 33.92 (vb), 36.98, 38.97, 41.84, 42.81, 45.22 (vb, 2×C), 46.00, 50.34, 52.91, 54.36 (vb), 55.61, 65.00 (vb), 67.11, 67.49, 211.50, 1×C n. f. HRMS: (API+) calculated for C24H42NO3 ([M + H]+) 392.3165, found 392.3164. Anal. Calcd. for C24H41NO3: C, 73.61; H, 10.55; N, 3.58. Found: C, 73.57; H, 10.59; N, 3.57%.
(20S)-20-(N,N-diethylaminomethyl)-2α,3α-dihydroxy-5α-pregnane-6-one (15b)
The general procedure for deprotection with ketal 14b (80 mg; 0.16 mmol) yielded 56 mg (85%) of the title compound 15b as an off-white solid:
Mp > 190 °C (decomp.). 1H NMR (DMSO-d6) δ 0.64 (s, 3H, CH3); 0.65 (s, 3H, CH3); 0.94–1.08 (m, 9H, 3×CH3); 1.67 (m, 1H); 1.83 (m, 1H); 1.97–2.13 (m, 3H); 2.37 (vbs, 2H, 2×NCH); 2.60 (dd, 1H, J = 12.1 and 3.2 Hz); 2.61 (vbs, 2H, 2×NCH); 3.46 (m, 1H, H-2β); 3.76 (m, 1H, H-3β); 4.21 (bs, 1H, OH); 4.35 (bs, 1H, OH). 13C NMR δ 11.88, 13.36, 17.80, 19.08 (vb, 2×C), 20.77, 23.69, 26.84, 27.62, 34.43 (vb), 37.00, 38.95, 41.84, 42.80, 46.01, 46.93 (vb, 2×C), 50.33, 52.91, 54.34 (vb), 55.65, 58.55 (vb), 67.11, 67.49, 211.52, 1×C n. f. HRMS: (API+) calculated for C26H46NO3 ([M + H]+) 420.3478, found 420.3476. Anal. Calcd. for C26H45NO3: C, 74.42; H, 10.81; N, 3.34. Found: C, 74.39; H, 10.86; N, 3.31%.
(20S)-20-(pyrrolidin-1-ylmethyl)-2α,3α-dihydroxy-5α-pregnane-6-one (15c)
The general procedure for deprotection with ketal 14c (80 mg; 0.16 mmol) yielded 58 mg (88%) of the title compound 15c as an off-white solid:
Mp > 240 °C (decomp.). 1H NMR (DMSO-d6) δ 0.64 (s, 3H, CH3); 0.65 (s, 3H, CH3); 1.01 (d, 3H, J = 6.5 Hz, CH3); 1.63–1.84 (m, 6H); 1.98–2.13 (m, 3H); 2.25–2.75 (vbm, 4H, 4×NCH); 2.60 (dd, 1H, J = 12.1 and 3.5 Hz); 3.46 (m, 1H, H-2β); 3.73 (m, 1H, H-3β); 4.21 (d, 1H, J = 2.4 Hz, OH); 4.34 (d, 1H, J = 6.1 Hz, OH). 13C NMR δ 11.85, 13.36, 17.69, 20.77, 22.94 (vb, 2×C), 23.63, 26.83, 27.59, 35.06 (vb), 36.94, 38.93, 41.82, 42.75, 45.98, 50.33, 52.88, 54.11 (vb, 3×C), 55.61, 61.35 (vb), 67.11, 67.48, 211.48, 1×C n. f. HRMS: (API+) calculated for C26H44NO3 ([M + H]+) 418.3321, found 418.3322. Anal. Calcd. for C26H43NO3: C, 74.77; H, 10.38; N, 3.35. Found: C, 74.74; H, 10.42; N, 3.30%.
(20S)-20-(piperidin-1-ylmethyl)-2α,3α-dihydroxy-5α-pregnane-6-one (15d)
The general procedure for deprotection with ketal 14d (80 mg; 0.16 mmol) yielded 57 mg (85%) of the title compound 15d as an off-white solid:
Mp > 210 °C (decomp.). 1H NMR (DMSO-d6) δ 0.64 (s, 6H, 2×CH3); 0.96 (d, 3H, J = 6.5 Hz, CH3); 1.66 (m, 1H); 1.79 (m, 1H); 1.92–2.13 (m, 5H, incl. 2×NCH); 2.10–2.33 (m, 2H, 2×NCH); 2.43–2.62 (m, 2H, 2×NCH); 2.60 (dd, 1H, J = 12.1 and 3.5 Hz); 3.47 (m, 1H, H-2β); 3.75 (m, 1H, H-3β); 4.20 (d, 1H, J = 2.1 Hz, OH); 4.34 (d, 1H, J = 5.8 Hz, OH). 13C NMR δ 11.89, 13.36, 17.90, 20.77, 23.69, 23.93 (vb), 25.32 (b, 2×C), 26.84, 27.58, 33.40 (vb), 36.99, 38.98, 41.84, 42.82, 46.01, 50.34, 52.91, 54.52 (vb, 2×C), 55.62, 64.52 (vb), 67.11, 67.49, 211.51, 1×C n. f. HRMS: (API+) calculated for C27H46NO3 ([M + H]+) 432.3478, found 432.3474. Anal. Calcd. for C26H43NO3: C, 74.77; H, 10.38; N, 3.35. Found: C, 74.74; H, 10.42; N, 3.30%.
(20S)-20-(morpholin-4-ylmethyl)-2α,3α-dihydroxy-5α-pregnane-6-one (15e)
The general procedure for deprotection with ketal 14e (80 mg; 0.15 mmol) and chromatography on silica gel yielded 57 mg (86%) of the title compound 15e as an off-white solid:
Mp > 210 °C (decomp.). 1H NMR (DMSO-d6) δ 0.64 (s, 6H, 2×CH3); 0.96 (d, 3H, J = 6.5 Hz, CH3); 1.66 (m, 1H); 1.80 (m, 1H); 1.92 (dd, 1H, J = 11.9 and 10.5 Hz); 1.98–2.19 (m, 7H, incl. 4×NCH); 2.39 (vbs, 2H, 2×NCH); 2.59 (dd, 1H, J = 12.2 and 3.4 Hz); 3.46 (m, 1H, H-2β); 3.50–3.58 (m, 4H, 2×CH2Omorpholine); 3.75 (m, 1H, H-3β); 4.20 (d, 1H, J = 2.4 Hz, OH); 4.33 (d, 1H, J = 6.1 Hz, OH). 13C NMR δ 11.90, 13.35, 17.82, 20.76, 23.70, 26.83, 27.62, 33.11, 36.98, 38.95, 41.82, 42.82, 46.01, 50.33, 52.92, 53.92 (b, 2×C), 54.56, 55.61, 64.55, 66.30 (2×C), 67.10, 67.47, 211.48. HRMS: (API+) calculated for C26H44NO4 ([M + H]+) 434.3270, found 434.3267. Anal. Calcd. for C26H43NO4: C, 72.02; H, 10.00; N, 3.23. Found: C, 71.98; H, 10.04; N, 3.22%.
General procedure for preparation of aminium chlorides
A 5% aqueous solution of hydrochloric acid (0.5 mL) was added to a solution of amine (20 mg; ~0.05 mmol) in methanol (2 mL). The mixture was shaken, evaporated to dryness and washed with diethyl ether. The prepared salts obtained in quantitative yield had sufficient purity.
N,N-dimethyl-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-methyl)aminium chloride (16a)
Mp > 290 °C (decomp.). 1H NMR (D2O) δ 0.67 (s, 3H, CH3); 0.70 (s, 3H, CH3); 1.00 (d, 3H, J = 6.7 Hz, CH3); 1.75–1.83 (m, 2H); 1.88 (m, 1H); 1.99 (m, 1H); 2.17–2.20 (m, 2H); 2.68 (dd, 1H, J = 11.1 and 4.9 Hz); 2.77 (s, 3H, NCH3); 2.83 (s, 3H, NCH3); 2.87 (dd, 1H, J = 12.9 and 11.3 Hz, H-22a); 3.04 (dd, 1H, J = 12.9 and 3.2 Hz, H-22b); 3.73 (m, 1H, ΣJ = 20.2 Hz, H-2β); 3.98 (m, 1H, H-3β). 13C NMR δ 11.22, 12.72, 15.69, 20.79, 23.43, 26.01, 27.23, 32.23, 38.03, 38.79, 38.92, 40.88, 43.04, 43.09, 45.18, 46.11, 50.87, 52.98, 53.00, 55.55, 63.62, 67.72, 68.11, 219.52. HRMS: (API+) calculated for C24H42NO3 ([M − Cl]+) 392.3165, found 392.3164. Anal. Calcd. for C24H42ClNO3: C, 67.34; H, 9.89; N, 3.27. Found: C, 67.28; H, 9.96; N, 3.23%.
N,N-diethyl-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-methyl)aminium chloride (16b)
Mp > 270 °C (decomp.). 1H NMR (CD3OD) δ 0.77 (s, 3H, CH3); 0.80 (s, 3H, CH3); 1.15 (d, 3H, J = 6.5 Hz, CH3); 1.87–2.00 (m, 2H); 2.09–2.15 (m, 2H); 2.21 (dd, 1H, J = 13.1 and 4.9 Hz); 2.73 (dd, 1H, J = 12.4 and 3.2 Hz); 2.89 (dd, 1H, J = 13.3 and 11.2 Hz, H-22a); 3.08 (dd, 1H, J = 13.3 and 2.4 Hz, H-22b); 3.15–3.22 (m, 2H, 2×NCH); 3.25–3.34 (m, 2H, 2×NCH); 3.66 (m, 1H, ΣJ = 19.9 Hz, H-2β); 3.95 (m, 1H, H-3β). 13C NMR δ 8.41 (vb), 9.55 (vb), 12.51, 14.01, 17.69, 22.44, 25.07, 27.99, 29.03, 34.30, 39.16, 40.72, 41.10, 43.68, 44.71, 47.50, 47.77 (vb), 50.73 (vb), 52.22, 55.03, 55.14, 57.61, 59.51, 69.23, 69.61, 214.89. HRMS: (API+) calculated for C26H46NO3 ([M − Cl]+) 420.3478, found 420.3480. Anal. Calcd. for C26H46ClNO3: C, 68.47; H, 10.17; N, 3.07. Found: C, 68.40; H, 10.23; N, 3.02%.
1-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-methyl)pyrrolidin-1-ium chloride (16c)
Mp > 260 °C (decomp.). 1H NMR (DMSO-d6) δ 0.61 (s, 3H, CH3); 0.63 (s, 3H, CH3); 1.06 (d, 3H, J = 6.5 Hz, CH3); 1.83–1.97 (m, 5H); 2.00 (dd, 1H, J = 13.1 and 4.9 Hz); 2.08 (bt, 1H, J = 12.3 Hz); 2.57 (dd, 1H, J = 11.9 and 3.4 Hz); 2.86–3.00 (m, 4H, 4×NCH); 3.43 (m, 1H, H-2β); 3.46–3.53 (m, 2H, 2×NCH); 3.72 (m, 1H, H-3β); 4.18 (d, 1H, J = 2.4 Hz, OH); 4.32 (d, 1H, J = 6.1 Hz, OH); 9.62 (bs. 1H, NH). 13C NMR δ 11.79, 13.36, 17.26, 20.78, 22.41, 22.69, 23.49, 26.84, 27.39, 33.51, 36.93, 38.85, 41.81, 42.78, 45.91, 50.34, 52.11, 52.80, 53.23, 55.50, 55.61, 60.15, 67.11, 67.49, 211.46, 1×C n. f. HRMS: (API+) calculated for C26H44NO3 ([M − Cl]+) 418.3321, found 418.3321. Anal. Calcd. for C26H44ClNO3: C, 68.77; H, 9.77; N, 3.08. Found: C, 68.71; H, 9.83; N, 3.03%.
1-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-methyl)piperidin-1-ium chloride (16d)
Mp > 280 °C (decomp.). 1H NMR (DMSO-d6) δ 0.65 (s, 3H, CH3); 0.67 (s, 3H, CH3); 1.08 (d, 3H, J = 6.5 Hz, CH3); 1.83–1.93 (m, 2H); 1.99 (m, 1H); 2.04 (dd, 1H, J = 13.1 and 4.9 Hz); 2.11 (bt, 1H, J = 12.4 Hz); 2.60 (dd, 1H, J = 12.1 and 3.5 Hz); 2.73–2.80 (m, 2H, 2×NCH); 2.89–2.97 (m, 2H, 2×NCH); 3.29–3.34 (m, 2H, 2×NCH); 3.46 (m, 1H, H-2β); 3.76 (m, 1H, H-3β); 9.25 (bs. 1H, NH). 13C NMR δ 11.78, 13.36, 17.87, 20.76, 21.39, 22.01, 22.04, 23.48, 26.84, 27.41, 31.50, 36.93, 38.85, 41.81, 42.81, 45.91, 50.34, 50.76, 52.77, 53.33, 54.64, 55.62, 61.99, 67.11, 67.49, 211.47, 1×C n. f. HRMS: (API+) calculated for C27H46NO3 ([M − Cl]+) 432.3478, found 432.3475. Anal. Calcd. for C27H46ClNO3: C, 69.28; H, 9.90; N, 2.99. Found: C, 69.20; H, 9.98; N, 2.95%.
4-((20S)-2α,3α-dihydroxy-6-oxo-5α-pregnane-20-methyl)morpholin-4-ium chloride (16e)
Mp > 240 °C (decomp.). 1H NMR (DMSO-d6) δ 0.62 (s, 3H, CH3); 0.64 (s, 3H, CH3); 1.03 (d, 3H, J = 6.5 Hz, CH3); 1.65 (m, 1H); 1.76 (m, 1H); 1.84 (m, 1H); 1.96 (m, 1H); 2.03 (dd, 1H, J = 13.1 and 4.9 Hz); 2.08 (bt, 1H, J = 12.5 Hz); 2.58 (dd, 1H, J = 12.4 and 3.5 Hz); 2.83 (dd, 1H, J = 12.9 and 11.0 Hz, NCH); 2.96 (td, 1H, J = 12.2 and 3.8 Hz, NCH) 3.02 (dd, 1H, J = 12.9 and 2.3 Hz, NCH); 3.13 (td, 1H, J = 12.2 and 3.8 Hz, NCH); 3.30 (bd, 1H, J = 11.6 Hz, NCH); 3.43–3.48 (m, 2H, H-2β, NCH); 3.70 (m, 1H, OCHmorph); 3.75–3.81 (m, 2H, H-3β and OCHmorph); 3.88–3.93 (m, 2H, 2×OCHmorph). 13C NMR δ 12.15, 13.75, 17.63, 21.19, 23.90, 27.12, 27.69, 31.43, 37.48, 40.23, 42.34, 43.28, 46.34, 50.38, 50.79, 53.20, 53.49, 53.73, 55.96, 62.65, 63.34, 63.46, 67.44, 67.79, 212.71, 1×C n. f. HRMS: (API+) calculated for C26H44NO4 ([M − Cl]+) 434.3270, found 434.3272. Anal. Calcd. for C26H44ClNO4: C, 66.43; H, 9.43; N, 2.98. Found: C, 66.38; H, 9.48; N, 2.95%.
(20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-oic acid (18)
Potassium hydroxide (570 mg; 10.18 mmol) was added to a solution of ethyl ester 17 (1.2 g; 2.03 mmol) in methanol (80 mL) and water (10 mL). The reaction mixture was stirred under reflux for 1 h. After cooling to room temperature, the excess of methanol was removed under reduced pressure, and the residue was diluted in ethyl acetate and extracted with 5% aqueous solution of citric acid and twice with water. The organic phase was dried over sodium sulfate and solvents were evaporated under reduced pressure to obtain acid 18 (795 mg; 96%) as a colorless oil in adequate purity for other reactions.
1H NMR (DMSO-d6) δ 0.61 (s, 3H, CH3); 0.76 (s, 3H, CH3); 0.89 (d, 3H, J = 6.5 Hz, CH3); 1.21 (s, 3H, CH3); 1.26 (s, 3H, CH3); 1.33 (s, 3H, CH3); 1.35 (s, 3H, CH3); 1.76–1.86 (m, 2H); 1.91 (m, 1H); 1.96 (m, 1H); 3.67 (m, 1H, CH2O); 3.78–3.90 (m, 3H, CH2O); 4.01 (m, 1H, H-2β); 4.11–4.14 (m, 2H, H-22, H-23); 4.18 (m, 1H, H-3β). HRMS: (API-) calculated for C32H49O8 ([M-H]-) 561.3427, found 561.3426.
N,N-dimethyl-(20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-amide (19a)
The general procedure for amide formation with acid 18 and dimethylamine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 191 mg (91%) of the title compound 19a as a colorless oil:
1H NMR (DMSO-d6) δ 0.60 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.91 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.23 (s, 3H, CH3); 1.33 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.77–1.86 (m, 2H); 1.91 (td, 1H, J = 11.9 and 3.2 Hz); 1.97 (m, 1H); 2.83 (s, 3H, NCH3); 3.04 (s, 3H, NCH3); 3.67 (m, 1H, CH2O); 3.81–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β); 4.33 (d, 1H, J = 7.6 Hz, H-23); 4.53 (dd, 1H, J = 7.6 and 1.1 Hz, H-22). 13C NMR δ 11.54, 12.61, 13.12, 20.36, 21.42, 23.73, 25.98, 26.60, 26.70, 27.14, 28.62, 32.54, 35.07, 35.55, 36.48, 37.55, 40.65, 41.93, 42.40, 45.08, 52.09, 53.23, 55.03, 63.64, 65.14, 72.00, 72.22, 73.65, 78.97, 106.73, 108.61, 108.85, 168.43, 1×C n. f. HRMS: (API+) calculated for C34H56NO7 ([M + H]+) 590.4057, found 590.4061.
N,N-diethyl-(20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-amide (19b)
The general procedure for amide formation with acid 18 and diethylamine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 193 mg (88%) of the title compound 19b as a colorless oil:
1H NMR (DMSO-d6) δ 0.60 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.91 (d, 3H, J = 6.7 Hz, CH3); 1.01 (t, 3H, J = 7.0 Hz, CH3); 1.11 (t, 3H, J = 7.0 Hz, CH3); 1.218 (s, 3H, CH3); 1.221 (s, 3H, CH3); 1.33 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.77–1.86 (m, 2H); 1.91 (td, 1H, J = 11.9 and 3.1 Hz); 1.97 (m, 1H); 3.18–3.41 (m, 4H, 2×NCH2); 3.67 (m, 1H, CH2O); 3.81–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β); 4.27 (d, 1H, J = 7.2 Hz, H-23); 4.53 (dd, 1H, J = 7.2 and 1.2 Hz, H-22). 13C NMR δ 11.59, 12.65, 12.71, 13.17, 14.39, 20.40, 21.46, 23.77, 25.84, 26.65, 26.72, 27.25, 28.66, 32.58, 35.92, 37.60, 39.04, 39.57, 40.68, 41.07, 41.98, 42.45, 45.12, 52.12, 53.23, 55.07, 63.69, 65.18, 72.04, 72.27, 74.04, 79.09, 106.78, 108.70, 108.88, 168.14. HRMS: (API+) calculated for C36H60NO7 ([M + H]+) 618.4370, found 618.4375.
1-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-oyl) pyrrolidine (19c)
The general procedure for amide formation with acid 18 and pyrrolidine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 195 mg (89%) of the title compound 19c as a colorless oil:
1H NMR (DMSO-d6) δ 0.60 (s, 3H, CH3); 0.76 (s, 3H, CH3); 0.91 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.24 (s, 3H, CH3); 1.32 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.72 (m, 3H); 1.73–1.88 (m, 6H); 1.91 (td, 1H, J = 11.8 and 3.1 Hz); 1.97 (m, 1H); 3.25–3.33 (m, 2H, NCH2); 3.54 (t, 2H, J = 6.7 Hz, NCH2); 3.67 (m, 1H, CH2O); 3.81–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β); 4.24 (d, 1H, J = 7.4 Hz, H-23); 4.49 (dd, 1H, J = 7.4 and 1.1 Hz, H-22). 13C NMR δ 11.56, 12.62, 13.14, 20.38, 21.43, 23.46, 23.73, 25.64, 25.92, 26.61, 26.70, 27.19, 28.63, 32.55, 35.76, 37.56, 40.65, 41.94, 42.41, 45.09, 45.83, 45.94, 52.10, 53.18, 55.04, 63.65, 65.15, 72.01, 72.23, 75.15, 79.03, 106.74, 108.75, 108.86, 167.32, 1×C n. f. HRMS: (API+) calculated for C36H58NO7 ([M + H]+) 616.4213, found 616.4218.
1-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-oyl) piperidine (19d)
The general procedure for amide formation with acid 18 and piperidine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 188 mg (84%) of the title compound 19d as a colorless oil:
1H NMR (DMSO-d6) δ 0.60 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.90 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 6H, 2×CH3); 1.33 (s, 3H, CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.77–1.86 (m, 2H); 1.91 (m, 1H); 1.97 (m, 1H); 3.41–3.60 (m, 4H, 2×NCH2); 3.67 (m, 1H, CH2O); 3.81–3.90 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β); 4.32 (d, 1H, J = 7.3 Hz, H-23); 4.58 (dd, 1H, J = 7.3 and 0.9 Hz, H-22). 13C NMR δ 11.56, 12.65, 13.13, 20.38, 21.42, 23.74, 24.05, 25.35, 25.91, 26.12, 26.61, 26.69, 27.18, 28.62, 32.54, 35.85, 37.56, 40.65, 41.95, 42.41, 42.48, 45.09, 45.86, 52.11, 53.23, 55.04, 63.64, 65.14, 72.01, 72.23, 73.84, 78.89, 106.74, 108.60, 108.86, 167.02, 1×C n. f. HRMS: (API+) calculated for C37H60NO7 ([M + H]+) 630.4370, found 630.4370.
4-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-oyl) morpholine (19e)
The general procedure for amide formation with acid 18 and morpholine and chromatography on silica gel (40–60% ethyl acetate in cyclohexane) yielded 191 mg (85%) of the title compound 19e as a colorless oil:
1H NMR (DMSO-d6) δ 0.62 (s, 3H, CH3); 0.78 (s, 3H, CH3); 0.92 (d, 3H, J = 6.7 Hz, CH3); 1.23 (s, 3H, CH3); 1.24 (s, 3H, CH3); 1.34 (s, 3H, CH3); 1.37 (s, 3H, CH3); 1.63–1.75 (m, 3H); 1.78–1.87 (m, 2H); 1.92 (td, 1H, J = 11.7 and 2.9 Hz); 1.99 (m, 1H); 3.43–3.63 (m, 8H, 8×Hmorph); 3.68 (m, 1H, CH2O); 3.81–3.91 (m, 3H, CH2O); 4.02 (m, 1H, H-2β); 4.20 (m, 1H, H-3β); 4.36 (d, 1H, J = 7.3 Hz, H-23); 4.58 (dd, 1H, J = 7.3 and 1.2 Hz, H-22). 13C NMR δ 11.60, 12.65, 13.15, 20.39, 21.46, 23.77, 25.92, 26.64, 26.67, 27.20, 28.65, 32.58, 35.87, 37.59, 40.68, 41.99, 42.05, 42.44, 45.12, 45.66, 52.13, 53.24, 55.07, 63.68, 65.18, 66.09, 66.32, 72.04, 72.26, 73.69, 78.85, 106.78, 108.81, 108.88, 167.63, 1×C n. f. HRMS: (API+) calculated for C36H58NO8 ([M − Cl]+) 632.4162, found 632.4168.
General procedure for deprotection of long side chain amide ketals
A 5% aqueous solution of hydrochloric acid (1 mL) was added to a solution of ketals 19a–e (20 mg) in tetrahydrofuran (5 mL) and the mixture was stirred at 45 °C for 2 h. After cooling, the mixture was diluted with ethyl acetate and extracted twice with water. The organic phase was dried with sodium sulfate and solvents were evaporated under reduced pressure. The crude product was purified on silica gel (mobile phase at each experiment).
N,N-dimethyl-(20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-amide (20a)
The general procedure for amide deprotection with ketal 19a (50 mg; 0.08 mmol) and chromatography on silica gel (5–10% isopropanol in chloroform) yielded 14 mg (86%) of the title compound 20a as an off-white solid:
Mp > 210 °C (decomp.). 1H NMR (CD3OD+CDCl3) δ 0.65 (s, 3H, CH3); 0.73 (s, 3H, CH3); 0.99 (d, 3H, J = 6.7 Hz, CH3); 1.75–1.82 (m, 2H); 1.95 (m, 1H); 1.99–2.05 (m, 2H); 2.23 (dd, 1H, J = 13.1 and 4.6 Hz); 2.68 (dd, 1H, J = 12.4 and 2.9 Hz); 2.95 (s, 3H, NCH3); 3.08 (s, 3H, NCH3); 3.64 (m, 1H, H-2β); 3.77 (dd, 1H, J = 5.7 and 1.2 Hz, H-22); 3.94 (m, 1H, H-3β); 4.32 (d, 1H, J = 5.7 Hz, H-23). 13C NMR δ 12.33, 13.45, 14.00, 21.90, 24.57, 27.14, 28.42, 36.41, 37.56, 38.67, 39.89, 40.14, 40.47, 43.38, 43.53, 47.30, 51.65, 53.34, 54.42, 57.31, 68.10, 68.90, 71.50, 73.91, 173.67, 214.81. HRMS: (API+) calculated for C26H44NO6 ([M + H]+) 466.3169, found 466.3172. Anal. Calcd. for C26H43NO6: C, 67.07; H, 9.31; N, 3.01. Found: C, 68.40; H, 10.23; N, 3.02%.
N,N-diethyl-(20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-amide (20b)
The general procedure for amide deprotection with ketal 19b (50 mg; 0.08 mmol) and chromatography on silica gel (5–10% isopropanol in chloroform) yielded 14 mg (88%) of the title compound 20b as an off-white solid:
Mp > 160 °C (decomp.). 1H NMR (CD3OD+CDCl3) δ 0.64 (s, 3H, CH3); 0.73 (s, 3H, CH3); 0.99 (d, 3H, J = 6.7 Hz, CH3); 1.10 (t, 3H, J = 7.2 Hz, CH3); 1.22 (t, 3H, J = 7.2 Hz, CH3); 1.94 (m, 1H), 1.99–2.05 (m, 2H); 2.23 (dd, 1H, J = 13.1 and 4.6 Hz); 2.68 (dd, 1H, J = 12.5 and 2.8 Hz); 3.27 (m, 1H, NCH); 3.37–3.42 (m, 2H, 2×NCH); 3.47 (m, 1H, NCH); 3.64 (m, 1H, H-2β); 3.76 (d, 1H, J = 6.2 Hz, H-22); 3.94 (m, 1H, H-3β); 4.24 (d, 1H, J = 6.2 Hz, H-23). 13C NMR δ 12.27, 13.01, 13.61, 13.95, 14.78, 21.81, 24.49, 27.03, 28.29, 38.58, 39.21, 40.03, 40.38, 41.22, 42.50, 43.30, 43.46, 47.23, 51.56, 53.27, 54.32, 57.22, 68.57, 68.80, 71.21, 74.44, 172.76, 214.71. HRMS: (API+) calculated for C28H48NO6 ([M + H]+) 494.3482, found 494.3481. Anal. Calcd. for C28H47NO6: C, 68.12; H, 9.60; N, 2.84. Found: C, 68.06; H, 9.66; N, 2.80%.
1-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-oyl)pyrrolidine (20c)
The general procedure for amide deprotection with ketal 19c (50 mg; 0.08 mmol) and chromatography on silica gel (5–10% isopropanol in chloroform) yielded 13 mg (83%) of the title compound 20c as an off-white solid:
Mp > 200 °C (decomp.). 1H NMR (CD3OD+CDCl3) δ 0.69 (s, 3H, CH3); 0.76 (s, 3H, CH3); 1.00 (d, 3H, J = 6.7 Hz, CH3); 1.84–2.07 (m, 6H); 2.10 (bt, 1H, J = 12.5 Hz); 2.22 (dd, 1H, J = 12.8 and 4.8 Hz); 2.78 (dd, 1H, J = 12.5 and 3.1 Hz); 3.38–3.48 (m, 2H, 2×NCH); 3.56 (m, 1H, NCH); 3.64-3.71 (m, 2H, H-2β, NCH); 3.83 (d, 1H, J = 7.1 Hz, H-22); 3.95 (m, 1H, H-3β); 4.22 (d, 1H, J = 7.1 Hz, H-23). 13C NMR δ 12.40, 13.93, 14.02, 22.48, 25.04 (2×C); 27.23, 27.97, 28.81, 39.31, 39.55, 40.95, 41.11, 43.76, 44.07, 47.45, 47.63, 47.98, 52.21, 54.12, 55.16, 58.01, 69.26, 69.90, 74.01, 74.82, 173.02, 215.17. HRMS: (API+) calculated for C28H46NO6 ([M + H]+) 492.3325, found 492.3325. Anal. Calcd. for C28H45NO6: C, 68.40; H, 9.23; N, 2.85. Found: C, 68.35; H, 9.30; N, 2.81%.
1-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-oyl)piperidine (20d)
The general procedure for amide deprotection with ketal 19d (50 mg; 0.08 mmol) and chromatography on silica gel (5–10% isopropanol in chloroform) yielded 14 mg (85%) of the title compound 20d as an off-white solid:
Mp > 200 °C (decomp.). 1H NMR (DMSO-d6) δ 0.58 (s, 3H, CH3); 0.63 (s, 3H, CH3); 0.87 (d, 3H, J = 6.5 Hz, CH3); 1.80–1.86 (m, 2H); 1.94 (m, 1H); 2.03 (dd, 1H, J = 13.0 and 4.7 Hz); 2.1 (m, 1H); 2.60 (dd, 1H, J = 12.1 and 3.2 Hz); 3.34–3.52 (m, 5H, H-2β, 2×NCH2); 3.60 (d, 1H, J = 6.8 Hz, H-22); 3.75 (bs, 1H, H-3β); 4.16–4.21 (m, 2H, H-23, OH); 4.34 (bd, 1H, J = 5.9 Hz, OH); 4.37 (bd, 1H, J = 4.6 Hz, OH); 4.94 (bd, 1H, J = 4.9 Hz, OH). 13C NMR δ 11.73, 13.00, 13.37, 20.82, 23.48, 24.08, 25.45, 26.20, 26.85, 27.21, 29.03, 37.08, 37.90, 41.85, 42.21, 42.40, 45.69, 46.01, 50.33, 52.23, 52.88, 55.98, 67.11, 67.49, 71.47, 72.48, 170.11, 211.61, 1×C n. f. HRMS: (API+) calculated for C29H48NO6 ([M + H]+) 506.3482, found 506.3479. Anal. Calcd. for C29H47NO6: C, 68.88; H, 9.37; N, 2.77. Found: C, 68.82; H, 9.42; N, 2.75%.
4-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-oyl)morpholine (20e)
The general procedure for amide deprotection with ketal 19e (50 mg; 0.08 mmol) and chromatography on silica gel (5–10% isopropanol in chloroform) yielded 14 mg (87%) of the title compound 20e as an off-white solid:
Mp > 210 °C (decomp.). 1H NMR (CD3OD+CDCl3) δ 0.66 (s, 3H, CH3); 0.74 (s, 3H, CH3); 0.98 (d, 3H, J = 6.7 Hz, CH3); 1.76–1.81 (m, 2H); 1.96 (m, 1H); 2.00–2.06 (m, 2H); 2.23 (dd, 1H, J = 13.0 and 4.6 Hz); 2.69 (dd, 1H, J = 12.4 and 2.9 Hz); 3.56–3.69 (m, 9H, H-2β, 8×Hmorph); 3.78 (dd, 1H, J = 6.2 and 0.9 Hz, H-22); 3.95 (m, 1H, H-3β); 4.30 (d, 1H, J = 6.2 Hz, H-23). 13C NMR δ 12.42, 13.42, 14.01, 21.95, 24.62, 27.20, 28.49, 30.39, 38.72, 39.45, 40.20, 40.51, 43.41, 43.46, 43.59, 48.95, 47.33, 51.70, 53.35, 54.47, 57.37, 67.48 (2×C), 68.69, 68.94, 72.08, 74.14, 172.27, 214.87. HRMS: (API+) calculated for C28H46NO7 ([M + H]+) 508.3274, found 508.3272. Anal. Calcd. for C28H45NO7: C, 66.25; H, 8.93; N, 2.76. Found: C, 66.20; H, 8.99; N, 2.73%.
4-((20S,22R,23S)-2α,3α-dihydroxy-22,23-(1-methylethylidene)dioxy-6-oxo-5α-cholan-24-oyl)morpholine (23)
The general procedure for amide deprotection (at room temperature) with ketal 19e (50 mg; 0.08 mmol) and chromatography on silica gel (isopropanol/ethyl acetate—1/19) yielded 31 mg (72%) of the title compound 23 as a colorless oil. The product was obtained during optimization of the reaction condition.
1H NMR (DMSO-d6, 45 °C) δ 0.63 (s, 3H, CH3); 0.65 (s, 3H, CH3); 0.93 (d, 3H, J = 6.7 Hz, CH3); 1.25 (s, 3H, CH3); 1.34 (s, 3H, CH3); 1.68 (qd, 1H, J = 10.9 and 5.7 Hz); 1.87 (m, 1H); 1.97 (m, 1H); 2.03–2.11 (m, 2H); 2.61 (dd, 1H, J = 12.4 and 3.5 Hz); 3.44–3.64 (m, 9H, H-2β, 8×Hmorph); 3.77 (m, 1H, H-3β); 4.06 (bd, 1H, J = 2.4 Hz, OH); 4.21 (bd, 1H, J = 6.1 Hz, OH); 4.37 (d, 1H, J = 7.2 Hz, H-23); 4.59 (dd, 1H, J = 7.2 and 1.4 Hz, H-22). 13C NMR δ 11.61, 12.63, 13.35, 20.82, 23.39, 25.92, 26.63, 26.83, 27.09, 31.31, 35.93, 37.09, 38.91, 41.83, 42.06, 42.45, 45.65, 46.02, 50.34, 52.88, 53.08, 55.72, 66.08, 66.29, 66.98, 67.51, 73.77, 78.85, 108.82, 167.65, 211.50. HRMS: (API+) calculated for C31H50NO7 ([M + H]+) 548.3587, found 548.3586.
N,N-dimethyl-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-yl)amine (21a)
The general procedure for amide reduction with amide 19a (150 mg; 0.25 mmol) and chromatography on silica gel (0–5% methanol in ethyl acetate) yielded 114 mg (78%) of the title compound 21a as a colorless oil:
1H NMR (DMSO-d6) δ 0.59 (s, 3H, CH3); 0.73 (s, 3H, CH3); 0.87 (d, 3H, J = 6.5 Hz, CH3); 1.18 (s, 3H, CH3); 1.22 (s, 3H, CH3); 1.23 (s, 3H, CH3); 1.32 (s, 3H, CH3); 1.58–1.70 (m, 3H); 1.74–1.82 (m, 2H); 1.86–1.94 (m, 2H); 2.13 (s, 6H, 2×NCH3); 2.24 (dd, 1H, J = 12.9 and 6.7 Hz, H-24a); 2.34 (dd, 1H, J = 12.9 and 3.4 Hz, H-24b); 3.61–3.66 (m, 2H, CH2O, H-22); 3.72–3.87 (m, 4H, 3×CH2O, H-23); 3.99 (m, 1H, H-2β); 4.15 (m, 1H, H-3β). 13C NMR δ 11.45, 12.53, 13.04, 20.29, 21.34, 23.62, 26.46, 26.93, 26.96, 27.25, 28.47, 32.42, 35.14, 37.47, 40.53, 41.89, 42.32, 44.96, 45.26 (b, 2×C), 52.18, 52.92, 55.12, 61.27, 63.55, 65.03, 71.99, 72.19, 74.76 (b), 80.64, 106.63, 107.74, 108.81, 1×C n. f. HRMS: (API+) calculated for C34H58NO6 ([M + H]+) 576.4264, found 576.4369.
N,N-diethyl-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-yl)amine (21b)
The general procedure for amide reduction with amide 19b (150 mg; 0.24 mmol) and chromatography on silica gel (0–5% methanol in ethyl acetate) yielded 110 mg (75%) of the title compound 21b as a colorless oil:
1H NMR (DMSO-d6) δ 0.62 (s, 3H, CH3); 0.76 (s, 3H, CH3); 0.90 (d, 3H, J = 6.7 Hz, CH3); 0.92–0.99 (m, 6H, 2×CH3); 1.22 (s, 3H, CH3); 1.27 (s, 6H, 2×CH3); 1.36 (s, 3H, CH3); 1.60–1.74 (m, 4H); 1.77–1.85 (m, 2H); 1.90–1.98 (m, 2H); 2.34–2.67 (vb m, 6H, 6×NCH); 3.67 (m, 1H, CH2O); 3.72 (d, 1H, J = 8.3 Hz, H-22); 3.80–3.86 (m, 3H, 2×CH2O, H-23); 3.89 (m, 1H, CH2O); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 11.53, 11.74 (vb, 2×C), 12.64, 13.15, 20.40, 21.44, 23.70, 26.62, 27.04, 27.14, 27.19, 28.64, 32.58, 35.22, 37.56, 39.11, 40.65, 41.95, 42.42, 45.11, 47.07, 52.14, 53.18, 55.09, 55.67 (vb), 63.67, 65.15, 72.01, 72.23, 74.84 (vb) 81.34 (vb), 106.75, 107.32 (vb), 108.87, 1×C n. f. HRMS: (API+) calculated for C36H62NO6 ([M + H]+) 604.4577, found 604.4579.
1-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-yl) pyrrolidine (21c)
The general procedure for amide reduction with amide 19c (150 mg; 0.24 mmol) and chromatography on silica gel (0–5% methanol in ethyl acetate) yielded 113 mg (77%) of the title compound 21c as a colorless oil:
1H NMR (DMSO-d6) δ 0.62 (s, 3H, CH3); 0.76 (s, 3H, CH3); 0.91 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.28 (s, 6H, 2×CH3); 1.35 (s, 3H, CH3); 1.62–1.74 (m, 7H); 1.76–1.84 (m, 2H); 1.89–1.99 (m, 2H); 2.55–2.71 (vb m, 6H, 6×NCH); 3.65–3.69 (m, 2H, CH2O, H-22); 3.80–3.90 (m, 4H, 3×CH2O, H-23); 4.01 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 11.51, 12.70, 13.14, 20.38, 21.43, 23.01 (2×C); 23.70, 26.61, 27.04, 27.14, 27.27, 28.63, 32.55, 35.17, 37.56, 40.65, 41.95, 42.42, 45.10, 52.11, 53.11, 54.13 (2×C), 55.07, 63.66, 65.15, 72.00, 72.23, 75.46, 80.77, 106.74, 107.82 (vb), 108.86, 2×C n. f. HRMS: (API+) calculated for C36H60NO6 ([M + H]+) 602.4421, found 602.4426.
1-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-yl) piperidine (21d)
The general procedure for amide reduction with amide 19d (150 mg; 0.24 mmol) and chromatography on silica gel (0–5% methanol in ethyl acetate) yielded 106 mg (72%) of the title compound 21d as a colorless oil:
1H NMR (DMSO-d6) δ 0.65 (s, 3H, CH3); 0.78 (s, 3H, CH3); 0.91 (d, 3H, J = 6.7 Hz, CH3); 1.23 (s, 3H, CH3); 1.268 (s, 3H, CH3); 1.274 (s, 3H, CH3); 1.37 (s, 3H, CH3); 1.61–1.75 (m, 4H); 1.79–1.87 (m, 2H); 1.91–2.01 (m, 2H); 2.33–2.50 (vb m, 6H, 6×NCH); 3.67–3.72 (m, 2H, CH2O, H-22); 3.77–3.91 (m, 4H, 3×CH2O, H-23); 4.02 (m, 1H, H-2β); 4.20 (m, 1H, H-3β). 13C NMR δ 11.55, 12.74, 13.15, 20.39, 21.43, 23.75, 25.50 (2×C), 26.62, 27.10, 27.18, 27.26, 28.63, 32.57, 35.20, 37.56, 40.65, 41.98, 42.43, 45.11, 52.13, 53.20, 54.73 (b, 2×C), 55.10, 61.34 (vb), 63.67, 65.14, 72.02, 72.24, 74.80 (vb), 81.34 (b), 106.75, 107.52, 108.87, 1×C n. f. HRMS: (API+) calculated for C37H62NO6 ([M + H]+) 616.4577, found 616.4578.
4-((20S,22R,23S)-2α,3α;22,23-bis((1-methylethylidene)dioxy)-6,6-ethylenedioxy-5α-cholan-24-yl) morpholine (21e)
The general procedure for amide reduction with amide 19e (150 mg; 0.24 mmol) and chromatography on silica gel (0–5% methanol in ethyl acetate) yielded 109 mg (74%) of the title compound 21e as a colorless oil:
1H NMR (DMSO-d6) δ 0.64 (s, 3H, CH3); 0.77 (s, 3H, CH3); 0.90 (d, 3H, J = 6.7 Hz, CH3); 1.22 (s, 3H, CH3); 1.26 (s, 6H, 2×CH3); 1.36 (s, 3H, CH3); 1.62–1.74 (m, 3H); 1.78–1.85 (m, 2H); 1.90–1.99 (m, 2H); 2.37–2.45 (m, 6H, 6×NCH); 3.52–3.55 (m, 4H, 4×CH2Omorph); 3.65–3.70 (m, 2H, CH2O, H-22); 3.80–3.91 (m, 4H, 3×CH2O, H-23); 4.02 (m, 1H, H-2β); 4.19 (m, 1H, H-3β). 13C NMR δ 11.55, 12.75, 13.15, 20.39, 21.44, 23.74, 26.62, 27.12, 27.18, 27.32, 28.64, 32.57, 35.22, 37.57, 40.65, 41.98, 42.42, 45.11, 52.13, 53.21, 54.04 (2×C), 55.10, 60.99, 63.67, 65.16, 66.23 (2×C), 72.02, 72.24, 75.05, 81.06, 106.75, 107.58, 108.87, 1×C n. f. HRMS: (API+) calculated for C36H60NO7 ([M + H]+) 618.4370, found 618.4376.
General procedure for deprotection of long side chain amine ketals
A 5% aqueous solution of hydrochloric acid (1 mL) was added to a solution of ketals 21a–e (20 mg; 0.03 mmol) in tetrahydrofuran (5 mL) and the mixture was stirred at 45 °C for 2 h. During the reaction, white crystals appeared. After cooling, solvents were evaporated under reduced pressure and the crude product was washed twice with ethyl acetate. The prepared salts obtained in quantitative yield had sufficient purity.
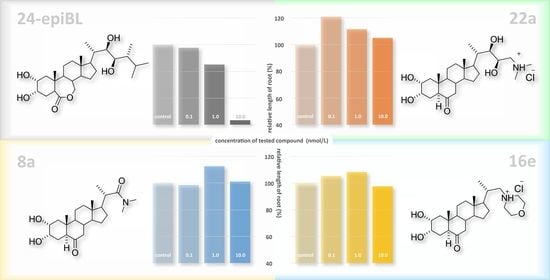
N,N-dimethyl-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-yl)aminium chloride (22a)
Mp > 270 °C (decomp.). 1H NMR (CD3OD+D2O) δ 0.62 (s, 3H, CH3); 0.67 (s, 3H, CH3); 0.87 (d, 3H, J = 6.4 Hz, CH3); 1.85 (m, 1H); 1.95 (m, 1H); 2.07–2.16 (m, 2H); 2.65 (dd, 1H, J = 11.9 and 2.8 Hz); 3.00 (bt, 1H, J = 12.5 Hz, H-24a); 3.10 (dd, 1H, J = 12.5 and 2.9 Hz. H-24b); 3.38 (bd, 1H, J = 7.0 Hz, H-22); 3.64 (m, 1H, H-2β); 3.84 (ddd, 1H, J = 10.7, 7.5, and 2.9 Hz, H-23); 3.92 (m, 1H, H-3β). 13C NMR δ 12.30, 12.89, 13.90, 22.17, 24.69, 27.45, 28.47, 39.00, 39.31, 40.46 (2×C), 42.01, 43.82, 44.00, 45.89, 47.39, 52.10, 53.22, 54.56, 57.44, 60.28, 68.34, 69.00, 69.33, 75.87, 218.18. HRMS: (API+) calculated for C26H46NO5 ([M − Cl]+) 452.3376, found 452.3377. Anal. Calcd. for C26H46ClNO5: C, 63.98; H, 9.50; N, 2.87. Found: C, 63.95; H, 9.55; N, 2.85%.
N,N-diethyl-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-yl)aminium chloride (22b)
Mp > 250 °C (decomp.). 1H NMR (CD3OD) δ 0.75 (s, 3H, CH3); 0.77 (s, 3H, CH3); 1.00 (d, 3H, J = 6.7 Hz, CH3); 1.33 (t, 3H, J = 7.3 Hz, CH3); 1.34 (t, 3H, J = 7.3 Hz, CH3); 1.99 (m, 1H); 2.05–2.14 (m, 2H); 2.22 (dd, 1H, J = 13.1 and 4.9 Hz); 2.73 (dd, 1H, J = 12.4 and 3.2 Hz); 3.05 (dd, 1H, J = 13.2 and 11.0 Hz, H-24a); 3.17 (dd, 1H, J = 13.2 and 3.2 Hz, H-24b); 3.21–3.37 (m, 4H, 4×NCH); 3.44 (dd, 1H, J = 6.7 and 1.4 Hz, H-22); 3.66 (ddd, 1H, J = 11.8, 4.8, and 3.2 Hz, H-2β); 3.87 (ddd, 1H, J = 11.1, 6.7, and 3.1 Hz, H-23); 3.95 (m, 1H, H-3β). 13C NMR δ 8.90, 9.39, 12.50, 13.20, 14.02, 22.51, 25.05, 27.99, 28.78, 39.33, 39.78, 41.04, 41.12, 43.76, 44.11, 47.61, 47.87, 50.30, 52.23, 53.72, 55.16, 55.35, 58.01, 68.59, 69.26, 69.63, 75.77, 215.17. HRMS: (API+) calculated for C28H50NO5 ([M − Cl]+) 480.3689, found 480.3689. Anal. Calcd. for C28H50ClNO5: C, 65.15; H, 9.76; N, 2.71. Found: C, 65.09; H, 9.81; N, 2.68%.
1-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-yl)pyrrolidinium chloride (22c)
Mp > 250 °C (decomp.). 1H NMR (CD3OD+D2O) δ 0.63 (s, 3H, CH3); 0.67 (s, 3H, CH3); 0.88 (d, 3H, J = 6.4 Hz, CH3); 1.86 (m, 1H); 1.93–1.99 (m, 3H); 2.05–2.16 (m, 3H); 2.66 (dd, 1H, J = 12.4 and 3.0 Hz); 2.97–3.15 (m, 4H, 4×NCH); 3.39 (bd, 1H, J = 7.0 Hz, H-22); 3.58–3.66 (m, 3H, 2×NCH, H-2β); 3.81 (m, H-23); 3.92 (m, 1H, H-3β). 13C NMR δ 12.32, 12.95, 13.90, 22.19, 23.78, 23.96, 24.72, 27.49, 28.51, 39.13, 39.31, 40.52, 43.85, 43.98, 47.39, 49.78, 52.12, 53.30, 54.08, 54.61, 56.83, 57.50, 58.04, 69.03, 69.37, 69.73, 75.79, 217.93. HRMS: (API+) calculated for C28H48NO5 ([M − Cl]+) 478.3532, found 478.3535. Anal. Calcd. for C28H48ClNO5: C, 65.41; H, 9.41; N, 2.72. Found: C, 65.36; H, 9.48; N, 2.69%.
1-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-yl)piperidinium chloride (22d)
Mp > 250 °C (decomp.). 1H NMR (CD3OD) δ 0.74 (s, 3H, CH3); 0.76 (s, 3H, CH3); 0.99 (d, 3H, J = 6.7 Hz, CH3); 1.93–2.02 (m, 2H); 2.05–2.14 (m, 2H); 2.20 (dd, 1H, J = 13.4 and 4.8 Hz); 2.73 (dd, 1H, J = 12.2 and 3.1 Hz); 2.92 (td, 1H, J = 12.0 and 2.9 Hz, NCH); 3.01 (dd, 1H, J = 12.9 and 11.5 Hz, H-24a); 3.05 (td, 1H, J = 12.0 and 4.4 Hz, NCH); 3.15 (dd, 1H, J = 12.9 and 2.4 Hz, H-24b); 3.41 (dd, 1H, J = 6.9 and 1.4 Hz, H-22); 3.49 (bd, 1H, J = 11.9 Hz, NCH); 3.62–3.68 (m, 2H, NCH, H-2β); 3.92 (m, 1H, H-23); 3.95 (m, 1H, H-3β). 13C NMR δ 12.48, 13.05, 14.01, 22.50, 22.89, 24.03, 24.08, 25.03, 27.99, 28.80, 39.32, 39.68, 41.03, 41.12, 43.76, 44.11, 47.60, 52.24, 52.74, 53.64, 55.16, 56.36, 57.96, 60.09, 67.88, 69.26, 69.62, 75.86, 215.24. HRMS: (API+) calculated for C28H48NO6 ([M − Cl]+) 494.3482, found 494.3485. Anal. Calcd. for C29H50ClNO5: C, 65.95; H, 9.54; N, 2.65. Found: C, 65.89; H, 9.60; N, 2.63%.
4-((20S,22R,23S)-2α,3α,22,23-tetrahydroxy-6-oxo-5α-cholan-24-yl)morpholinium chloride (22e)
Mp > 270 °C (decomp.). 1H NMR (CD3OD+D2O) δ 0.59 (s, 3H, CH3); 0.64 (s, 3H, CH3); 0.84 (d, 3H, J = 6.7 Hz, CH3); 1.71 (qd, 1H, J = 10.3 and 7.2 Hz); 1.79 (m, 1H); 1.92 (m, 1H); 2.07–2.13 (m, 2H); 2.63 (dd, 1H, J = 11.6 and 4.3 Hz); 3.03 (dd, 1H, J = 13.0 and 11.3 Hz, H-24a); 3.08 (m, 1H, NCH); 3.14 (dd, 1H, J = 13.0 and 3.1 Hz, H-24b); 3.18 (m, 1H, NCH); 3.38 (dd, 1H, J = 6.9 and 1.2 Hz, H-22); 3.39 (m, 1H, NCH); 3.52 (bd, 1H, J = 12.2 Hz, NCH); 3.64 (m, 1H, H-2β); 3.73–3.81 (m, 2H, CH-O); 3.89–3.94 (m, 2H, H-3β, H-23); 3.96–4.02 (m, 2H, CH-O). 13C NMR δ 12.25, 12.84, 13.85, 22.05, 24.56, 27.31, 28.37, 38.79, 39.28, 40.27, 40.30, 43.73, 44.09, 47.32, 51.57, 52.05, 53.11, 54.37, 54.53, 57.26, 59.87, 64.66 (2×C), 67.44, 68.90, 69.26, 75.89, 219.27. HRMS: (API+) calculated for C28H48NO6 ([M − Cl]+) 494.3482, found 494.3482. Anal. Calcd. for C28H48ClNO6: C, 63.44; H, 9.13; N, 2.64. Found: C, 63.40; H, 9.18; N, 2.61%.
4-((20S,22R,23S)-2α,3α-dihydroxy-22,23-(1-methylethylidene)dioxy-6-oxo-5α-cholan-24-yl) morpholinium chloride (24)
The product was obtained during optimization of the reaction condition (deprotection of long side chain amine ketals at room temperature) as white crystals.
Mp > 210 °C (decomp.). 1H NMR (D2O) δ 0.63 (s, 3H, CH3); 0.70 (s, 3H, CH3); 0.95 (d, 3H, J = 6.7 Hz, CH3); 1.37 (s, 3H, CH3); 1.38 (s, 3H, CH3); 1.77–1.90 (m, 2H); 1.98 (m, 1H); 2.20 (d, 2H, J = 8.3 Hz); 2.69 (dd, 1H, J = 11.2 and 5.0 Hz); 3.10–3.63 (vb, 4H, 4×NCH); 3.30 (dd, 1H, J = 13.6 and 1.9 Hz, H-24a); 3.35 (dd, 1H, J = 13.6 and 9.5 Hz, H-24b); 3.73 (m, 1H, H-2β); 3.78–4.13 (vb, 4H, 4×CH-O); 3.94 (d, 1H, J = 8.3 Hz, H-22); 3.98 (m, 1H, H-3β); 4.22 (m, 1H, H-23). 13C NMR δ 10.91, 12.09, 12.72, 20.82, 23.32, 25.93, 25.94, 26.02, 27.21, 35.11, 38.17, 38.85, 38.92, 42.75, 43.09, 46.14, 50.88, 52.77 (2×C), 53.01 (2×C), 55.78, 59.34, 63.64 (2×C), 67.74, 68.12, 71.64, 81.16, 110.75, 219.66. HRMS: (API+) calculated for C31H52NO6 ([M − Cl]+) 534.3795, found 534.3796.