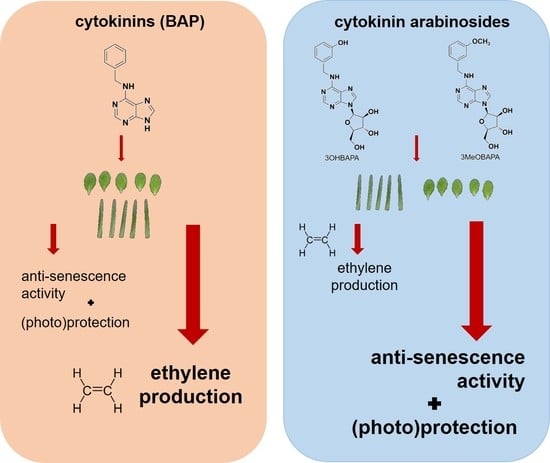
The Anti-Senescence Activity of Cytokinin Arabinosides in Wheat and Arabidopsis Is Negatively Correlated with Ethylene Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Senescence-Induced Changes in Detached Leaves of Wheat and Arabidopsis
2.2. Comparison of the Anti-Senescence Activity of BAP and CK Arabinosides in Wheat and Arabidopsis Leaves
2.3. Species-Specific Effects of BAPAs Treatment in the Protection Against Induced Oxidative Damage
2.4. On the Mechanism of Specific Anti-Senescence Activity of CK Arabinosides
3. Materials and Methods
3.1. Plant Material
3.2. High-Light Treatment
3.3. Determination of Chlorophyll, Carotenoid and Xanthophyll Content
3.4. Confocal Laser Scanning Microscopy
3.5. Chlorophyll Fluorescence Parameters (PSII Functioning)
3.6. Ethylene Production
3.7. Ultra-Weak Photon Emission
3.8. Ion Leakage
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | antheraxanthin |
| AHK | Arabidopsis histidine kinase |
| BAP | 6-benzylaminopurine |
| car | carotenoid |
| Chl | chlorophyll |
| CK | cytokinin |
| dad | day after detachment |
| DEPS | de-epoxidation state of xanthophylls |
| DIC | differential interference contrast |
| DMSO | dimethylsulfoxide |
| (dV/dt)0 | the initial slope of the O–J Chl fluorescence rise |
| FV/FM | the maximum quantum yield of photosystem II photochemistry |
| HL | high light |
| JA | jasmonic acid |
| LHCII | light-harvesting complexes of photosystem II |
| LOOH | lipid hydroperoxide |
| OJIP | chlorophyll fluorescence induction transient |
| PSII | photosystem II |
| QA | primary electron acceptor of photosystem II |
| RCII | reaction center of photosystem II |
| SAG | senescence-associated gene |
| UPE | ultra-weak photon emission |
| V | violaxanthin |
| VJ | the relative variable fluorescence at the J step of OJIP curve |
| Z | zeaxanthin |
| 3OHBAPA | 6-(3-hydroxybenzylamino)-9-β-d-arabinofuranosylpurine |
| 3MeOBAPA | 6-(3-methoxybenzylamino)-9-β-d-arabinofuranosylpurine |
| Φf,D | the quantum yield of constitutive non-regulatory dissipation processes |
| ΦNPQ | the quantum yield of regulatory light-induced non-photochemical quenching |
| ΦP | the effective quantum yield of photosystem II photochemistry (for the light-adapted state) |
References
- Špundová, M.; Popelková, H.; Ilík, P.; Skotnica, J.; Novotný, R.; Nauš, J. Ultra-structural and functional changes in the chloroplasts of detached barley leaves senescing under dark and light conditions. J. Plant Physiol. 2003, 160, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Janečková, H.; Husičková, A.; Ferretti, U.; Prčina, M.; Pilařová, E.; Plačková, L.; Pospíšil, P.; Doležal, K.; Špundová, M. The interplay between cytokinins and light during senescence in detached Arabidopsis leaves. Plant Cell Environ. 2018, 41, 1870–1885. [Google Scholar] [CrossRef] [PubMed]
- Janečková, H.; Husičková, A.; Lazár, D.; Ferretti, U.; Pospíšil, P.; Špundová, M. Exogenous application of cytokinin during dark senescence eliminates the acceleration of photosystem II impairment caused by chlorophyll b deficiency in barley. Plant Physiol. Biochem. 2019, 136, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holub, L.; Hanuš, J.; Hanke, D.E.; Strnad, M. Biological activity of cytokinins derived from ortho- and meta-hydroxybenzyladenine. Plant Growth Regul. 1998, 26, 109–115. [Google Scholar] [CrossRef]
- Oh, M.H.; Kim, J.H.; Zulfugarov, I.S.; Moon, Y.H.; Rhew, T.H.; Lee, C.H. Effects of benzyladenine and abscisic acid on the disassembly process of photosystems in an Arabidopsis delayed-senescence mutant, ore9. J. Plant Biol. 2005, 48, 170–177. [Google Scholar] [CrossRef]
- Vlčková, A.; Špundová, M.; Kotabová, E.; Novotný, R.; Doležal, K.; Nauš, J. Protective cytokinin action switches to damaging during senescence of detached wheat leaves in continuous light. Physiol. Plant. 2006, 126, 257–267. [Google Scholar] [CrossRef]
- Zavaleta-Mancera, H.A.; López-Delgado, H.; Loza-Tavera, H.; Mora-Herrera, M.; Trevilla-García, C.; Vargas-Suárez, M.; Ougham, H. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J. Plant Physiol. 2007, 164, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Zeng, H.; Cai, Q.; Zhou, X.; Yin, C. Exogenous jasmonic acid and cytokinin antagonistically regulate rice flag leaf senescence by mediating chlorophyll degradation, membrane deterioration, and senescence-associated genes expression. J. Plant Growth Regul. 2016, 35, 366–376. [Google Scholar] [CrossRef]
- Weaver, L.M.; Gan, S.; Quirino, B.; Amasino, R.M. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol. Biol. 1998, 37, 455–469. [Google Scholar] [CrossRef]
- Vylíčilová, H.; Husičková, A.; Spíchal, L.; Srovnal, J.; Doležal, K.; Plíhal, O.; Plíhalová, L. C2-substituted aromatic cytokinin sugar conjugates delay the onset of senescence by maintaining the activity of the photosynthetic apparatus. Phytochemistry 2016, 122, 22–33. [Google Scholar] [CrossRef]
- Schippers, J.H.M.; Schmidt, R.; Wagstaff, C.; Jing, H.C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef] [Green Version]
- Koprna, R.; De Diego, N.; Dundálková, L.; Spíchal, L. Use of cytokinins as agrochemicals. Bioorg. Med. Chem. 2016, 24, 484–492. [Google Scholar] [CrossRef]
- Galuszka, P.; Popelková, H.; Werner, T.; Frébortová, J.; Pospíšilová, H.; Mik, V.; Köllmer, I.; Schmülling, T.; Frébort, I. Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J. Plant Growth Regul. 2007, 26, 255–267. [Google Scholar] [CrossRef]
- Aremu, A.O.; Bairu, M.W.; Doležal, K.; Finnie, J.F.; Van Staden, J. Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 2012, 108, 1–16. [Google Scholar] [CrossRef]
- Woodward, E.J.; Marshall, C. Effects of plant-growth regulators and nutrient supply on tiller bud outgrowth in barley (Hordeum distichum L.). Ann. Bot. 1998, 61, 347–354. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; Strnad, M.; Van Ockelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Doležal, K.; Van Staden, J. Optimizing the micropropagation protocol for the endangered Aloe polyphylla: Can meta-topolin and its derivatives serve as replacement for benzyladenine and zeatin? Plant Cell Tissue Organ. Cult. 2007, 90, 15–23. [Google Scholar] [CrossRef]
- Rulcová, J.; Pospíšilová, J. Effect of benzylaminopurine on rehydration of bean plants after water stress. Biol. Plant. 2001, 44, 75–81. [Google Scholar] [CrossRef]
- Prokopová, J.; Špundová, M.; Sedlářová, M.; Husičková, A.; Novotný, R.; Doležal, K.; Nauš, J.; Lebeda, A. Photosynthetic responses of lettuce to downy mildew infection and cytokinin treatment. Plant Physiol. Biochem. 2010, 48, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Plíhalová, L.; Vylíčilová, H.; Doležal, K.; Zahajská, L.; Zatloukal, M.; Strnad, M. Synthesis of aromatic cytokinins for plant biotechnology. N. Biotechnol. 2016, 33, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Tarkowská, D.; Doležal, K.; Tarkowski, P.; Åstot, C.; Holub, J.; Fuksová, K.; Schmülling, T.; Sandberg, G.; Strnad, M. Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus x canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography frit-fast atom bombardment mass spectrometry. Physiol. Plant. 2003, 117, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Szüčová, L.; Spíchal, L.; Doležal, K.; Zatloukal, M.; Greplová, J.; Galuszka, P.; Kryštof, V.; Voller, J.; Popa, I.; Massino, F.J.; et al. Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives. Bioorg. Med. Chem. 2009, 17, 1938–1947. [Google Scholar] [CrossRef]
- Doležal, K.; Plíhalová, L.; Vylíčilová, H.; Zatloukal, M.; Plíhal, O.; Voller, J.; Strnad, M.; Bryksová, M.; Vostálová, J.; Rajnochová Svobodová, A.; et al. 6-aryl-9-glycosylpurines and use thereof. U.S. Patent 10,100,077, 16 October 2018. [Google Scholar]
- Bryksová, M.; Dabravolski, S.; Kučerová, Z.; Zavadil Kokáš, F.; Špundová, M.; Plíhalová, L.; Takáč, T.; Grúz, J.; Hudeček, M.; Hloušková, V.; et al. Aromatic cytokinin arabinosides promote PAMP-like responses and positively regulate leaf longevity. ACS Chem. Biol. 2020, 15, 1949–1963. [Google Scholar] [CrossRef]
- Nisler, J.; Zatloukal, M.; Sobotka, R.; Pilný, J.; Zdvihalová, B.; Novák, O.; Strnad, M.; Spíchal, L. New urea derivatives are effective anti-senescence compounds acting most likely via a cytokinin-independent mechanism. Front. Plant Sci. 2018, 9, 1225. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: New York, NY, USA, 2000; pp. 443–480. [Google Scholar]
- Leong, T.-Y.; Anderson, J.M. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. II. Regulation of electron transport capacities, electron carriers, coupling factor (CF1) activity and rates of photosynthesis. Photosynth. Res. 1984, 5, 117–128. [Google Scholar] [CrossRef]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signalling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Špundová, M.; Vlčková, A.; Doležal, K.; Habertová, A.; Nauš, J.; Strnad, M. Effect of meta-topolin and bohemine derived from benzylaminopurine on PSII function in artificially senescing wheat leaves. In Proceedings of the 12th International Congress on Photosynthesis; CSIRO Publishing: Collingwood, Victoria, Australia, 2001; S22-012. [Google Scholar]
- Cary, J.A.; Liu, W.; Howell, S.H. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995, 107, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Zdarska, M.; Dobisová, T.; Gelová, Z.; Pernisová, M.; Dabravolski, S.; Hejátko, J. Illuminating light, cytokinin, and ethylene signalling crosstalk. J. Exp. Bot. 2015, 66, 4913–4931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceusters, J.; Van de Poel, B. Ethylene exerts species-specific and age-dependent control of photosynthesis. Plant Physiol. 2018, 176, 2601–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wi, S.J.; Jang, S.J.; Park, K.Y. Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol. Cells 2010, 30, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photoch Photobiol. B 2014, 139, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Pinnola, A.; Bassi, R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 2018, 46, 467–482. [Google Scholar] [CrossRef]
- Wu, A.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.I.; Asensi-Fabado, M.A.; Munné-Bosch, S.; Antonio, C.; Tohge, T.; et al. JUNGBRUNNEN1, a reactive oxygen species-responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 2012, 24, 482–506. [Google Scholar] [CrossRef] [Green Version]
- Hutin, C.; Nussaume, L.; Moise, N.; Moya, I.; Kloppstech, K.; Havaux, M. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl. Acad. Sci. USA 2003, 100, 4921–4926. [Google Scholar] [CrossRef] [Green Version]
- Meyer, G.; Kloppstech, K. A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur. J. Biochem. 1984, 138, 201–207. [Google Scholar] [CrossRef]
- Binyamin, L.; Falah, M.; Portnoy, V.; Soudry, E.; Gepstein, S. The early light-induced protein is also produced during leaf senescence of Nicotiana tabacum. Planta 2001, 212, 591–597. [Google Scholar] [CrossRef]
- Humbeck, K.; Kloppstech, K.; Krupinska, K. Expression of early-light inducible proteins in flag leaves of field-grown barley. Plant Physiol. 1994, 105, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Riefler, M.; Novák, O.; Strnad, M.; Schmülling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2016, 18, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortleven, A.; Nitschke, S.; Klaumünzer, M.; Abdelgawad, H.; Asard, H.; Grimm, B.; Riefler, M.; Schmülling, T. A novel protective function for cytokinin in the light stress response is mediated by the Arabidopsis histidine kinase2 and Arabidopsis histidine kinase3 receptors. Plant Physiol. 2014, 164, 1470–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, K.; Li, Z.; Yang, Z.; Chen, J.; Wu, S.; Zhu, X.; Gao, S.; Gao, J.; Ren, G.; Kuai, B.; et al. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PLoS Genet. 2015, 11, e1005399. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Di, C.; Zhang, Q.; Zhang, K.; Wang, C.; You, Q.; Yan, H.; Dai, S.Y.; Yuan, J.S.; et al. JAZ7 negatively regulates dark-induced senescence in Arabidopsis. J. Exp. Bot. 2016, 67, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Björkman, O. Adenine nucleotides and the xanthophyll cycle in leaves—I. Effects of CO2- and temperature-limited photosynthesis on adenylate energy charge and violaxanthin de-epoxidation. Planta 1994, 192, 526–536. [Google Scholar] [CrossRef]
- Sedlářová, M.; Petřivalský, M.; Piterková, J.; Luhová, L.; Kočířová, J.; Lebeda, A. Influence of nitric oxide and reactive oxygen species on development of lettuce downy mildew in Lactuca spp. Eur. J. Plant Pathol. 2011, 129, 267–280. [Google Scholar] [CrossRef]
- Prasad, A.; Pospíšil, P. Towards the two-dimensional imaging of spontaneous ultra-weak photon emission from microbial, plant and animal cells. Sci. Rep. 2013, 3, 1211. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučerová, Z.; Rác, M.; Mikulík, J.; Plíhal, O.; Pospíšil, P.; Bryksová, M.; Sedlářová, M.; Doležal, K.; Špundová, M. The Anti-Senescence Activity of Cytokinin Arabinosides in Wheat and Arabidopsis Is Negatively Correlated with Ethylene Production. Int. J. Mol. Sci. 2020, 21, 8109. https://doi.org/10.3390/ijms21218109
Kučerová Z, Rác M, Mikulík J, Plíhal O, Pospíšil P, Bryksová M, Sedlářová M, Doležal K, Špundová M. The Anti-Senescence Activity of Cytokinin Arabinosides in Wheat and Arabidopsis Is Negatively Correlated with Ethylene Production. International Journal of Molecular Sciences. 2020; 21(21):8109. https://doi.org/10.3390/ijms21218109
Chicago/Turabian StyleKučerová, Zuzana, Marek Rác, Jaromír Mikulík, Ondřej Plíhal, Pavel Pospíšil, Magdaléna Bryksová, Michaela Sedlářová, Karel Doležal, and Martina Špundová. 2020. "The Anti-Senescence Activity of Cytokinin Arabinosides in Wheat and Arabidopsis Is Negatively Correlated with Ethylene Production" International Journal of Molecular Sciences 21, no. 21: 8109. https://doi.org/10.3390/ijms21218109