The Identification of Metabolites and Effects of Albendazole in Alfalfa (Medicago sativa)
Abstract
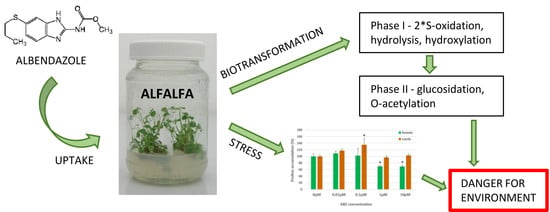
:1. Introduction
2. Results and Discussion
2.1. Effect of ABZ on Germination of Alfalfa Seeds
2.2. Effect of ABZ on Proline Accumulation in Alfalfa Regenerants
2.3. ABZ Biotransformation in Alfalfa Regenerants
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Their Cultivation
3.3. ABZ Phytotoxicity
3.4. ABZ Uptake and Biotransformation
3.5. UHPLC-MS/MS Conditions
3.6. Quantification of ABZ, ABZSO and ABZSO2
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABZ | albendazole |
| ABZSO | albendazole sulphoxide |
| ABZSO2 | albendazole sulphone |
| DME | drug-metabolizing enzyme |
| DMSO | dimethyl sulfoxide |
| DW | weight of dry tissue |
| ESI | electrospray ionization |
| M | metabolite |
| MBZ | mebendazole |
| MS | mass spectrometry |
| N/A | not available |
| UHPLC | ultrahigh-performance liquid chromatography |
References
- Bártíková, H.; Podlipna, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Serra, A.-A.; Gouesbet, G.; Couée, I. Xenobiotic sensing and signalling in higher plants. J. Exp. Bot. 2012, 63, 3999–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagil, M.; Białk-Bielińska, A.; Puckowski, A.; Wychodnik, K.; Maszkowska, J.; Mulkiewicz, E.; Kumirska, J.; Stepnowski, P.; Stolte, S. Toxicity of anthelmintic drugs (fenbendazole and flubendazole) to aquatic organisms. Environ. Sci. Pollut. Res. 2014, 22, 2566–2573. [Google Scholar] [CrossRef] [Green Version]
- Vokřál, I.; Michaela, Š.; Radka, P.; Jiří, L.; Lukáš, P.; Dominika, S.; Kateřina, L.; Barbora, S.; Lenka, S. Ivermectin environmental impact: Excretion profile in sheep and phytotoxic effect in Sinapis alba. Ecotoxicol. Environ. Saf. 2019, 169, 944–949. [Google Scholar] [CrossRef]
- Syslová, E.; Landa, P.; Navrátilová, M.; Stuchlíková, L.R.; Matoušková, P.; Skálová, L.; Szotáková, B.; Vaněk, T.; Podlipna, R. Ivermectin biotransformation and impact on transcriptome in Arabidopsis thaliana. Chemosphere 2019, 234, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Syslová, E.; Landa, P.; Stuchlíková, L.R.; Matoušková, P.; Skálová, L.; Szotáková, B.; Navrátilová, M.; Vaněk, T.; Podlipna, R. Metabolism of the anthelmintic drug fenbendazole in Arabidopsis thaliana and its effect on transcriptome and proteome. Chemosphere 2019, 218, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Prchal, L.; Podlipná, R.; Lamka, J.; Dědková, T.; Skálová, L.; Vokřál, I.; Lecová, L.; Vaněk, T.; Szotáková, B. Albendazole in environment: Faecal concentrations in lambs and impact on lower development stages of helminths and seed germination. Environ. Sci. Pollut. Res. 2016, 23, 13015–13022. [Google Scholar] [CrossRef] [PubMed]
- Stuchlíková, L.R.; Jakubec, P.; Langhansová, L.; Podlipna, R.; Navrátilová, M.; Szotáková, B.; Skálová, L. The uptake, effects and biotransformation of monepantel in meadow plants used as a livestock feed. Chemosphere 2019, 237, 124434. [Google Scholar] [CrossRef]
- Bártíková, H.; Skálová, L.; Stuchlíková, L.R.; Vokřál, I.; Vaněk, T.; Podlipna, R. Xenobiotic-metabolizing enzymes in plants and their role in uptake and biotransformation of veterinary drugs in the environment. Drug Metab. Rev. 2015, 47, 374–387. [Google Scholar]
- Podlipna, R.; Skálová, L.; Seidlová, H.; Szotáková, B.; Kubíček, V.; Stuchlíková, L.R.; Jirásko, R.; Vaněk, T.; Vokřál, I. Biotransformation of benzimidazole anthelmintics in reed (Phragmites australis) as a potential tool for their detoxification in environment. Bioresour. Technol. 2013, 144, 216–224. [Google Scholar] [CrossRef]
- Raisová, L.S.; Podlipna, R.; Szotáková, B.; Syslová, E.; Skálová, L. Evaluation of drug uptake and deactivation in plant: Fate of albendazole in ribwort plantain (Plantago laceolata) cells and regenerants. Ecotoxicol. Environ. Saf. 2017, 141, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Stuchlíková, L.R.; Jirásko, R.; Skálová, L.; Pavlík, F.; Szotáková, B.; Holcapek, M.; Vaněk, T.; Podlipna, R. Metabolic pathways of benzimidazole anthelmintics in harebell (Campanula rotundifolia). Chemosphere 2016, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Horvat, A.; Babic, S.; Pavlović, D.M.; Ašperger, D.; Pelko, S.; Kaštelan-Macan, M.; Petrovic, M.; Mance, A. Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. TrAC Trends Anal. Chem. 2012, 31, 61–84. [Google Scholar] [CrossRef]
- Jaeger, L.H.; Carvalho-Costa, F.A. Status of benzimidazole resistance in intestinal nematode populations of livestock in Brazil: A systematic review. BMC Veter. Res. 2017, 13, 358. [Google Scholar] [CrossRef] [Green Version]
- McKellar, Q. Ecotoxicology and residues of anthelmintic compounds. Veter. Parasitol. 1997, 72, 413–435. [Google Scholar] [CrossRef]
- Porto, R.; Rodrigues-Silva, C.; Schneider, J.; Rath, S. Benzimidazoles in wastewater: Analytical method development, monitoring and degradation by photolysis and ozonation. J. Environ. Manag. 2019, 232, 729–737. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Boil. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Navrátilová, M.; Stuchlíková, L.R.; Skálová, L.; Szotáková, B.; Langhansová, L.; Podlipná, R. Pharmaceuticals in environment: The effect of ivermectin on ribwort plantain (Plantago lanceolata L.). Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, F.; Zhang, X.-W.; Bhutani, H.; Ye, B. Characterizations and bioactivities of abendazole sulfoxide-loaded thermo-sensitive hydrogel. Parasitol. Res. 2016, 116, 921–928. [Google Scholar] [CrossRef]
- Sahin, A.; Gul, A.; Karaca, M.; Akkan, H.A.; Keles, I. The Efficacy of Ricobendazole and Ivermectin on Naturally Infected Sheep with Trichostrongylidae sp in the Region of Van. J. Anim. Vet. Adv. 2009, 8, 2756–2759. [Google Scholar]
- Arnold, K.E.; Boxall, A.B.A.; Brown, A.R.; Cuthbert, R.J.; Gaw, S.; Hutchinson, T.H.; Jobling, S.; Madden, J.C.; Metcalfe, C.D.; Naidoo, V.; et al. Assessing the exposure risk and impacts of pharmaceuticals in the environment on individuals and ecosystems. Boil. Lett. 2013, 9, 20130492. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assay with tabacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Máchová, J.; Svobodová, Z.; Vykusová, B. Ekotoxikologické hodnocení výluhů tuhých průmyslových odpadů; Výzkumný ústav rybářský a hydrobiologický: Vodňany, Czech Republic, 1994. [Google Scholar]
- Sánchez-Martín, J.; Heald, J.; Kingston-Smith, A.; Winters, A.; Rubiales, D.; Sanz, M.; Mur, L.; Prats, E. A metabolomic study in oats (Avena sativa) highlights a drought tolerance mechanism based upon salicylate signalling pathways and the modulation of carbon, antioxidant and photo-oxidative metabolism. Plant Cell Environ. 2015, 38, 1434–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vokřál, I.; Jirásko, R.; Stuchlíková, L.R.; Bártíková, H.; Szotáková, B.; Lamka, J.; Várady, M.; Skálová, L. Biotransformation of albendazole and activities of selected detoxification enzymes in Haemonchus contortus strains susceptible and resistant to anthelmintics. Veter. Parasitol. 2013, 196, 373–381. [Google Scholar] [CrossRef] [PubMed]



| tR [min] | Theoretical m/z Values of [M+H]+ Ions | Molecular Formula | Description of Metabolite Formation | Product Ions of [M+H]+, m/z | Metabolite Designation | |
|---|---|---|---|---|---|---|
| Phase I | Phase II | |||||
| 1.96 | 444.1435 | C18H25N3O8S | S-oxidation | N-glucosidation | 282, 240, 208, 191, 159 | M1 |
| 2.04 | 460.1384 | C18H25N3O9S | 2*S-oxidation, hydrolysis, hydroxylation | Glucosidation, O-acetylation | 240, 198 | M31 |
| 2.09 | 460.1384 | C18H25N3O9S | 2*S-oxidation, hydrolysis, hydroxylation | Glucosidation, O-acetylation | 240 | M2 |
| 2.18 | 444.1435 | C18H25N3O8S | S-oxidation | N-glucosidation | 282, 240, 208, 191, 159 | M30 |
| 2.24 | 444.1435 | C18H25N3O8S | S-oxidation | N-glucosidation | 282, 240, 208, 191, 159 | M29 |
| 2.25 | 460.1384 | C18H25N3O9S | 2*S-oxidation, hydrolysis, hydroxylation | Glucosidation, O-acetylation | 240, 198 | M4 |
| 2.30 | 460.1384 | C18H25N3O9S | 2*S-oxidation, hydrolysis, hydroxylation | Glucosidation, O-acetylation | 240 | M33 |
| 2.44 | 314.0805 | C12H15N3O5S | 2*S-oxidation, hydroxylation | − | 238, 159 | M6 |
| 2.46 | 444.1435 | C18H25N3O8S | S-oxidation | N-glucosidation | 282, 240, 208 | M5 |
| 2.61 | 460.1384 | C18H25N3O9S | 2*S-oxidation, hydrolysis, hydroxylation | Glucosidation, O-acetylation | 240, 198 | M32 |
| 2.86 | 460.1384 | C18H25N3O9S | 2*S-oxidation | N-glucosidation | 298, 266, 224, 191, 159 | M7 |
| 2.99 | 460.1384 | C18H25N3O9S | 2*S-oxidation, hydrolysis, hydroxylation | Glucosidation, O-acetylation | 240, 133 | M8 |
| 3.22 | 282.0907 | C12H15N3O3S | S-oxidation | − | 240, 208, 191,159 | M10 |
| 3.36 | 460.1384 | C18H25N3O9S | 2*S-oxidation | N-glucosidation | 298, 266, 224, 191, 159 | M9 |
| 3.56 | 370.1431 | C16H23N3O5S | Hydrolysis | N-glucosidation | 208, 166 | M11 |
| 4.50 | 370.1431 | C16H23N3O5S | Hydrolysis | N-glucosidation | 208, 166 | M25 |
| 4.74 | 428.1486 | C18H25N3O7S | +O, hydrolysis | Glucosidation, O-acetylation | 208 | M16 |
| 5.00 | 298.0856 | C12H15N3O4S | 2*S-oxidation | − | 266, 224, 159 | M14 |
| 5.54 | 428.1486 | C18H25N3O7S | − | N-glucosidation | 266, 234 | M20 |
| 6.06 | 428.1486 | C18H25N3O7S | +O, hydrolysis, | Glucosidation, O-acetylation | 208, 250 | M21 |
| 6.56 | 428.1486 | C18H25N3O7S | − | N-glucosidation | 266, 234 | M22 |
| 7.23 | 266.0958 | C12H15N3O2S | − | − | 234 | ABZ |
| M. sativa Roots | M. sativa Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ABZ (µM) | ABZ (µM) | ||||||||
| 0.01 | 0.1 | 1.0 | 10 | 0.01 | 0.1 | 1.0 | 10 | ||
| M1 | − | − | + | + | + | + | + | ||
| M30 | − | − | − | + | − | − | − | − | |
| M29 | − | − | − | + | − | − | − | + | |
| M31 | − | − | − | + | − | − | − | + | |
| M4 | − | − | − | + | − | − | − | + | |
| M32 | − | − | − | + | − | − | − | − | |
| M7 | − | − | − | + | − | − | − | + | |
| M9 | − | − | − | + | − | − | − | + | |
| M25 | − | − | − | + | − | − | − | − | |
| M21 | − | − | − | + | − | − | − | − | |
| M5 | − | − | − | + | − | − | − | − | |
| M2 | − | − | − | + | − | − | − | + | |
| M33 | − | − | − | + | − | − | − | − | |
| M8 | − | + | + | + | − | − | − | + | |
| M11 | − | − | − | + | − | + | + | + | |
| M16 | − | − | − | + | − | − | − | − | |
| M20 | − | − | − | + | − | − | − | − | |
| M22 | − | − | − | + | − | − | − | − | |
| M10 | + | + | + | + | + | + | + | + | |
| M14 | + | + | + | + | + | + | + | + | |
| M6 | − | − | − | + | − | − | − | + | |
| Exposition of Alfalfa to ABZ (µM) | |||||
|---|---|---|---|---|---|
| 0.01 | 0.1 | 1.0 | 10 | ||
| Roots | ABZ | 0.008 ± 0.002 | 0.019 ± 0.01 | 0.015 ± 0.005 | 0.162 ± 0.011 |
| ABZSO | 1.9 ± 1.2 | 4.1 ± 1.1 | 113.6 ± 23.7 | 1073 ± 209 | |
| ABZSO2 | 0.25 ± 0.12 | 0.54 ± 0.08 | 6.59 ± 1.37 | 42.7 ± 6.21 | |
| Leaves | ABZ | 0.005 ± 0.002 | 0.005 ± 0.003 | 0.012 ± 0.005 | 0.006 ± 0.001 |
| ABZSO | 0.444 ± 0.134 | 0.44 ± 0.19 | 3.25 ± 0.25 | 61.6 ± 2.19 | |
| ABZSO2 | 0.094 ± 0.016 | 0.09 ± 0.02 | 0.64 ± 0.24 | 6.21 ± 0.10 | |
| Standard Curve (y = ax + b) | r2 | LOD (ng/mL) | LOQ (ng/mL) | |
|---|---|---|---|---|
| ABZ | Y = (16.9401)X + (0.892919) | 0.9959 | 0.0008 | 0.0028 |
| ABZSO | Y = (0.731182)X + (0.0388037) | 0.9972 | 0.0104 | 0.0350 |
| ABZSO2 | Y = (0.463911)X + (−0.00401502) | 0.9934 | 0.0110 | 0.0367 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raisová Stuchlíková, L.; Navrátilová, M.; Langhansová, L.; Moťková, K.; Podlipná, R.; Szotáková, B.; Skálová, L. The Identification of Metabolites and Effects of Albendazole in Alfalfa (Medicago sativa). Int. J. Mol. Sci. 2020, 21, 5943. https://doi.org/10.3390/ijms21165943
Raisová Stuchlíková L, Navrátilová M, Langhansová L, Moťková K, Podlipná R, Szotáková B, Skálová L. The Identification of Metabolites and Effects of Albendazole in Alfalfa (Medicago sativa). International Journal of Molecular Sciences. 2020; 21(16):5943. https://doi.org/10.3390/ijms21165943
Chicago/Turabian StyleRaisová Stuchlíková, Lucie, Martina Navrátilová, Lenka Langhansová, Kateřina Moťková, Radka Podlipná, Barbora Szotáková, and Lenka Skálová. 2020. "The Identification of Metabolites and Effects of Albendazole in Alfalfa (Medicago sativa)" International Journal of Molecular Sciences 21, no. 16: 5943. https://doi.org/10.3390/ijms21165943