Multi-Component Antioxidative System and Robust Carbohydrate Status, the Essence of Plant Arsenic Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Cultivation of Plant Material
2.2. Arsenic Content Determination
2.3. Photosynthetic Characteristics Determinations
2.4. Oxidative Stress Level Determination
2.5. Antioxidant Enzymes Activities
2.6. Antioxidant Compounds Determinations
2.7. Carbohydrate Content Determination
2.8. Statistical Analysis
3. Results
3.1. Arsenic Content and Effect on Biomass Accumulation in Tobacco Roots and Leaves
3.2. Effect of Arsenic on Photosynthetic Characteristics
3.3. ROS Content and Lipid Peroxidation under Arsenic Exposure
3.4. Effects of Arsenic on Antioxidant Enzymes Activities
3.5. Effects of Arsenic on Antioxidant Molecules Levels
3.6. Effects of Arsenic on the Carbohydrate Status
4. Discussion
4.1. Plant Growth and Photosynthesis Under As Stress
4.2. Arsenic-Induced ROS Accumulation
4.3. Antioxidant Enzyme Activities as Affected by As Stress
4.4. Antioxidant Molecules Accumulation Resulting from As Stress
4.5. Carbohydrate Status Under As Stress
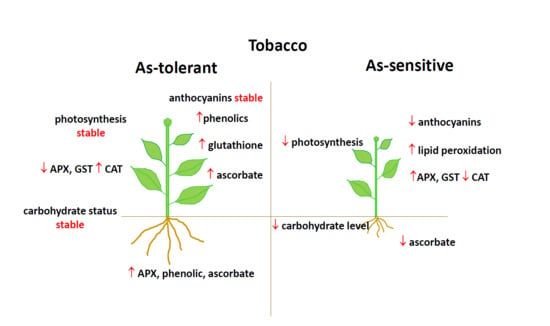
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Panda, S.K.; Upadhyay, R.K.; Nath, S. Arsenic stress in plants. J. Agron. Crop Sci. 2010, 196, 161–174. [Google Scholar] [CrossRef]
- Quaghebeur, M.; Rengel, Z. Arsenic speciation governs arsenic uptake and transport in terrestrial plants. Microchim. Acta 2005, 151, 141–152. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Logoteta, B.; Beone, G.M.; Cagnin, M.; Cacco, G. Arsenic uptake and speciation and the effects of phosphate nutrition in hydroponically grown kikuyu grass (Pennisetum clandestinum Hochst). Environ. Sci. Pollut. Res. 2012, 19, 3046–3053. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Trivedi, P.K.; Misra, P.; Tiwari, M.; Shri, M.; Shukla, D.; Kumar, S.; Rai, A.; Pandey, A.; Nigam, D.; et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 2009, 74, 688–702. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, B.; Kaab, S.B.; Rezgui, M.; Majdoub, N.; da Silva, J.A.T.; Kaab, L.B.B. Salicylic acid alleviates arsenic and zinc toxicity in the process of reserve mobilization in germinating fenugreek (Trigonella foenum-graecum L.) seeds. S. Afr. J. Bot. 2019, 124, 235–243. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, A.; Dubey, A.K.; Ranjan, R.; Pandey, A.; Kumari, B.; Singh, G.; Mandotra, S.; Chauhan, P.S.; Srikrishna, S.; et al. GABA mediated reduction of arsenite toxicity in rice seedling through modulation of fatty acids, stress responsive amino acids and polyamines biosynthesis. Ecotoxicol. Environ. Saf. 2019, 173, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37. [Google Scholar] [CrossRef]
- Zhao, F.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef]
- Kamiya, T.; Islam, M.R.; Duan, G.; Uraguchi, S.; Fujiwara, T. Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1 is involved in As accumulation in shoots of rice. Soil Sci. Plant Nutr. 2013, 59, 580–590. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-y.; Su, Y.-h.; Mcgrath, S.P.; Zhao, F.-j. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Mikutta, C.; Kretzschmar, R. Arsenite binding to sulfhydryl groups in the absence and presence of ferrihydrite: A model study. Environ. Sci. Technol. 2014, 48, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Aftab, T.; Ansari, A.A.; Shabbir, A.; Masroor, M.; Khan, A.; Uddin, M. Arsenic exposure modulates physiological attributes and artemisinin biosynthesis in Artemisia annua L. Int. J. Herb. Med. 2019, 7, 19–26. [Google Scholar]
- Naeem, M.; Nabi, A.; Aftab, T.; Khan, M.M.A. Oligomers of carrageenan regulate functional activities and artemisinin production in Artemisia annua L. exposed to arsenic stress. Protoplasma 2019. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. Nitrate supplementation attenuates As(V) toxicity in Solanum lycopersicum L. cv Pusa Rohini: Insights into As(V) sub-cellular distribution, photosynthesis, nitrogen assimilation, and DNA damage. Plant Physiol. Biochem. 2019, 139, 44–55. [Google Scholar] [CrossRef]
- Gusman, G.S.; Oliveira, J.A.; Farnese, F.S.; Cambraia, J. Arsenate and arsenite: The toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol. Plant. 2013, 35, 1201–1209. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernandez-Zapata, J.C.; Martinez Nicolas, J.J.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Singh, R.; Jha, A.B.; Misra, A.N.; Sharma, P. Differential responses of growth, photosynthesis, oxidative stress, metals accumulation and NRAMP genes in contrasting Ricinus communis genotypes under arsenic stress. Environ. Sci. Pollut. Res. 2019, 26, 31166–31177. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Kofronova, M.; Maskova, P.; Lipavska, H. Two facets of world arsenic problem solution: Crop poisoning restriction and enforcement of phytoremediation. Planta 2018, 248, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.S.; Singh, A.K. Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolising enzymes in rice plants. Biol. Plant. 1999, 42, 233–239. [Google Scholar] [CrossRef]
- Mishra, P.; Dubey, R.S. Effect of aluminium on metabolism of starch and sugars in growing rice seedlings. Acta Physiol. Plant. 2008, 30, 265–275. [Google Scholar] [CrossRef]
- Hartley-Whitaker, J.; Ainsworth, G.; Meharg, A.A. Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001, 24, 713–722. [Google Scholar] [CrossRef]
- Kumari, S.; Khan, A.; Singh, P.; Dwivedi, S.K.; Ojha, K.K.; Srivastava, A. Mitigation of As toxicity in wheat by exogenous application of hydroxamate siderophore of Aspergillus origin. Acta Physiol. Plant. 2019, 41, 107. [Google Scholar] [CrossRef]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152. [Google Scholar] [CrossRef]
- Karam, E.A.; Keramat, B.; Asrar, Z.; Mozafari, H. Study of interaction effect between triacontanol and nitric oxide on alleviating of oxidative stress arsenic toxicity in coriander seedlings. J. Plant Interact. 2017, 12, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.P.; Van Den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef]
- Salerno, G.L.; Curatti, L. Origin of sucrose metabolism in higher plants: When, how and why? Trends Plant Sci. 2003, 8, 63–69. [Google Scholar] [CrossRef]
- Ramon, M.; Rolland, F.; Sheen, J. Sugar sensing and signaling. Arabidopsis Book 2008, 6, e0117. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, H.; Sami, F.; Hayat, S. Glucose: Sweet or bitter effects in plants—A review on current and future perspective. Carbohydr. Res. 2020, 487. [Google Scholar] [CrossRef] [PubMed]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, F.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Bot. 2005, 56, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, A.B.; Dubey, R.S. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef]
- Sanglard, L.M.V.P.; Detmann, K.C.; Martins, S.C.V.; Teixeira, R.A.; Pereira, L.F.; Sanglard, M.L.; Fernie, A.R.; Araújo, W.L.; DaMatta, F.M. The role of silicon in metabolic acclimation of rice plants challenged with arsenic. Environ. Exp. Bot. 2016, 123, 22–36. [Google Scholar] [CrossRef]
- Li, C.x.; Feng, S.l.; Shao, Y.; Jiang, L.n.; Lu, X.y.; Hou, X.l. Effects of arsenic on seed germination and physiological activities of wheat seedlings. J. Environ. Sci. 2007, 19, 725–732. [Google Scholar] [CrossRef]
- Campos, N.V.; Araújo, T.O.; Arcanjo-Silva, S.; Freitas-Silva, L.; Azevedo, A.A.; Nunes-Nesi, A. Arsenic hyperaccumulation induces metabolic reprogramming in Pityrogramma calomelanos to reduce oxidative stress. Physiol. Plant. 2016, 157, 135–146. [Google Scholar] [CrossRef]
- Tremlová, J.; Sehnal, M.; Száková, J.; Goessler, W.; Steiner, O.; Najmanová, J.; Horáková, T.; Tlustoš, P. A profile of arsenic species in different vegetables growing in arsenic-contaminated soils. Arch. Agron. Soil Sci. 2017, 63, 918–927. [Google Scholar] [CrossRef]
- Strasser, B.J. Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kofronova, M.; Hrdinova, A.; Maskova, P.; Soudek, P.; Tremlova, J.; Pinkas, D.; Lipavska, H. Strong antioxidant capacity of horseradish hairy root cultures under arsenic stress indicates the possible use of Armoracia rusticana plants for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 174, 295–304. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K.; Delong, J.M.; Hodges, D.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric anthocyanin for estimating lipid peroxidation in plant tissues containing and other interfering. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Hiner, a.N.; Rodríguez-López, J.N.; Arnao, M.B.; Lloyd Raven, E.; García-Cánovas, F.; Acosta, M. Kinetic study of the inactivation of ascorbate peroxidase by hydrogen peroxide. Biochem. J. 2000, 348, 321–328. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbato specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1987, 22, 867–880. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric acid disappearance and conversion to products of enzymic oxidation in grape must and wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar]
- Mancinelli, A.L.; Huangyang, C.P.; Lindquist, P.; Anderson, O.R.; Rabino, I. Photocontrol of Anthocyanin Synthesis III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Mishra, S.; Alfeld, M.; Sobotka, R.; Andresen, E.; Falkenberg, G.; Küpper, H. Analysis of sublethal arsenic toxicity to Ceratophyllum demersum: Subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J. Exp. Bot. 2016, 67, 4639–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.N.; Srivastava, S.; Paladi, R.; Suprasanna, P. Calcium supplementation modulates arsenic-induced alterations and augments arsenic accumulation in callus cultures of Indian mustard (Brassica juncea (L.) Czern.). Protoplasma 2012, 249, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Shayesteh, L.S.; Ghasmpour, H.; Alavi, M. Effects of arsenic on growth, photosynthetic activity, and accumulation in two new hyperaccumulating populations of Isatis cappadocica desv. J. Plant Growth Regul. 2013, 32, 823–830. [Google Scholar] [CrossRef]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef] [Green Version]
- Marin, A.R.; Pezeshki, S.R.; Masschelen, P.H.; Choi, H.S. Effect of dimethylarsenic acid (dmaa) on growth, tissue arsenic, and photosynthesis of rice plants. J. Plant Nutr. 1993, 16, 865–880. [Google Scholar] [CrossRef]
- Stoeva, N.; Berova, M.; Zlatev, Z. Physiological response of maize to arsenic contamination. Biol. Plant. 2003, 47, 449–452. [Google Scholar] [CrossRef]
- Liu, Y.; Damaris, R.N.; Yang, P. Proteomics analysis identified a DRT protein involved in arsenic resistance in Populus. Plant Cell Rep. 2017, 36, 1855–1869. [Google Scholar] [CrossRef]
- Malik, J.A.; Goel, S.; Sandhir, R.; Nayyar, H. Uptake and distribution of arsenic in chickpea: Effects on seed yield and seed composition. Commun. Soil Sci. Plant Anal. 2011, 42, 1728–1738. [Google Scholar] [CrossRef]
- Zou, J.H.; Yu, K.L.; Zhang, Z.G.; Jiang, W.S.; Liu, D.H. Antioxidant response system and chlorophyll fluorescence in chromium (VI)—Treated Zea mays L. seedlings. Acta Biol. Crac. Ser. Bot. 2009, 51, 23–33. [Google Scholar]
- Filek, M.; Kościelniak, J.; Łabanowska, M.; Bednarska, E.; Bidzińska, E. Selenium-induced protection of photosynthesis activity in rape (Brassica napus) seedlings subjected to cadmium stress. Fluorescence and EPR measurements. Photosynth. Res. 2010, 105, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Kumar, J.; Srivastava, P.K.; Bashri, G.; Prasad, S.M. PSII photochemistry, oxidative damage and anti-oxidative enzymes in arsenate-stressed Oryza sativa L. seedlings. Chem. Ecol. 2017, 33, 34–50. [Google Scholar] [CrossRef]
- Tewari, A.; Singh, R.; Singh, N.K.; Rai, U.N. Amelioration of municipal sludge by Pistia stratiotes L.: Role of antioxidant enzymes in detoxification of metals. Bioresour. Technol. 2008, 99, 8715–8721. [Google Scholar] [CrossRef]
- Sanglard, L.M.V.P.; Martins, S.C.V.; Detmann, K.C.; Silva, P.E.M.; Lavinsky, A.O.; Silva, M.M.; Detmann, E.; Araújo, W.L.; DaMatta, F.M. Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: An analysis of the key limitations of photosynthesis. Physiol. Plant. 2014, 152, 355–366. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef]
- Stoeva, N.; Berova, M.; Zlatev, Z. Effect of arsenic on some physiological parameters in bean plants. Biol. Plant. 2005, 49, 293–296. [Google Scholar] [CrossRef]
- Vezza, M.E.; Llanes, A.; Travaglia, C.; Agostini, E.; Talano, M.A. Arsenic stress effects on root water absorption in soybean plants: Physiological and morphological aspects. Plant Physiol. Biochem. 2018, 123, 8–17. [Google Scholar] [CrossRef]
- Ismail, G.S.M. Protective role of nitric oxide against arsenic-induced damages in germinating mung bean seeds. Acta Physiol. Plant. 2012, 34, 1303–1311. [Google Scholar] [CrossRef]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, K. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Wevar Oller, A.L.; Agostini, E. Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Shaw, A.K.; Azahar, I.; Adhikari, S.; Jana, S.; Roy, S.; Kundu, A.; Sherpa, A.R.; Hossain, Z. Arsenate (AsV) stress response in maize (Zea mays L.). Environ. Exp. Bot. 2016, 130, 53–67. [Google Scholar] [CrossRef]
- Gupta, M.; Ahmad, M.A. Arsenate induced differential response in rice genotypes. Ecotoxicol. Environ. Saf. 2014, 107, 46–54. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No.111: Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Pavlík, M.; Pavlíková, D.; Staszková, L.; Neuberg, M.; Kaliszová, R.; Száková, J.; Tlustoš, P. The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2010, 73, 1309–1313. [Google Scholar] [CrossRef]
- Saha, J.; Majumder, B.; Mumtaz, B.; Biswas, A.K. Arsenic-induced oxidative stress and thiol metabolism in two cultivars of rice and its possible reversal by phosphate. Acta Physiol. Plant. 2017, 39, 263. [Google Scholar] [CrossRef]
- Iannone, M.F.; Groppa, M.D.; Benavides, M.P. Cadmium induces different biochemical responses in wild type and catalase-deficient tobacco plants. Environ. Exp. Bot. 2015, 109, 201–211. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef]
- Juszczuk, I.M.; Wiktorowska, A.; Malusá, E.; Rychter, A.M. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.). Plant Soil 2004, 267, 41–49. [Google Scholar] [CrossRef]
- Dai, L.P.; Xiong, Z.T.; Huang, Y.; Li, M.J. Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ. Toxicol. 2006, 21, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V.; Sytar, O. Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 2012, 89, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.L.; Zhou, Y.; Tong, Y.P.; Mukhopadhyay, R.; Rosen, B.P.; Zhu, Y.G. A CDC25 homologue from rice functions as an arsenate reductase. New Phytol. 2007, 174, 311–321. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, L.; Agrawal, V. Glutathione and citric acid modulates lead- and arsenic-induced phytotoxicity and genotoxicity responses in two cultivars of Solanum lycopersicum L. Acta Physiol. Plant. 2017, 39, 151. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathionE: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Mylona, P.V.; Polidoros, A.N.; Scandalios, J.G. Modulation of antioxidant responses by arsenic in maize. Free Radic. Biol. Med. 1998, 25, 576–585. [Google Scholar] [CrossRef]
- Ellis, D.R.; Gumaelius, L.; Indriolo, E.; Pickering, I.J.; Banks, J.A.; Salt, D.E. A novel arsenate reductase from the arsenic hyperaccumulating fern pteris vittata. Plant Physiol. 2006, 141, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Sharma, I. Arsenic induced oxidative stress in plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Yeung, E.C.; Belmonte, M.F.; Tu, L.T.T.; Stasolla, C. Glutathione modulation of in vitro development. Cell. Dev. Biol. Plant 2005, 41, 584–590. [Google Scholar] [CrossRef]
- Xiang, C.B.; Werner, B.L.; Christensen, E.M.; Oliver, D.J. The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001, 126, 564–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Chakrabarty, D.; Mallick, S.; Pandey, V.; et al. Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front. Plant Sci. 2016, 6, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouvelot, S.; Héloir, M.-C.; Poinssot, B.T.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.X.; Yang, L.T.; Qi, Y.P.; Lu, Y.B.; Huang, Z.R.; Chen, L.S. Root iTRAQ protein profile analysis of two Citrus species differing in aluminum-tolerance in response to long-term aluminum-toxicity. BMC Genom. 2015, 16, 949. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Xu, X.Y.; Gong, Q.Q.; Xie, C.; Fan, W.; Yang, J.L.; Lin, Q.S.; Zheng, S.J. Root proteome of rice studied by iTRAQ provides integrated insight into aluminum stress tolerance mechanisms in plants. J. Proteom. 2014, 98, 189–205. [Google Scholar] [CrossRef]
- Richard, B.; Rivoal, J.; Spiteri, A.; Pradet, A. Anaerobic stress induces the transcription and tranlslation of sucrose synthase in rice. Plant Physiol. 1991, 95, 669–674. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, Š.; Pinkas, D.; Lipavská, H. Multi-Component Antioxidative System and Robust Carbohydrate Status, the Essence of Plant Arsenic Tolerance. Antioxidants 2020, 9, 283. https://doi.org/10.3390/antiox9040283
Kofroňová M, Hrdinová A, Mašková P, Tremlová J, Soudek P, Petrová Š, Pinkas D, Lipavská H. Multi-Component Antioxidative System and Robust Carbohydrate Status, the Essence of Plant Arsenic Tolerance. Antioxidants. 2020; 9(4):283. https://doi.org/10.3390/antiox9040283
Chicago/Turabian StyleKofroňová, Monika, Aneta Hrdinová, Petra Mašková, Jana Tremlová, Petr Soudek, Šárka Petrová, Dominik Pinkas, and Helena Lipavská. 2020. "Multi-Component Antioxidative System and Robust Carbohydrate Status, the Essence of Plant Arsenic Tolerance" Antioxidants 9, no. 4: 283. https://doi.org/10.3390/antiox9040283