Abstract
Background
The additional value of azathioprine concomitant treatment on infliximab pharmacokinetics in children is not well described yet.
Aims
In the present study, we aimed to describe the relationship between thiopurine metabolite levels, infliximab trough levels, anti-IFX antibody formation, and clinical and laboratory markers of disease activity in pediatric patients with Crohn’s disease, and to assess non-adherence.
Methods
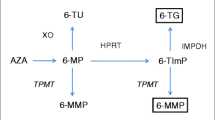
Data were collected prospectively during repeated visits from pediatric patients followed for Crohn’s disease in two Czech pediatric inflammatory bowel disease centers between January 2016 and June 2017. Thiopurine metabolites (6-thioguanine and 6-methylmercaptopurine) were measured by high-performance liquid chromatography. Infliximab trough levels and anti-IFX antibody serum levels were measured routinely by ELISA. The risk of loss of response to infliximab therapy was also assessed.
Results
A significant association between infliximab serum levels and 6-thioguanine erythrocyte levels was observed when tested as categorical variables (63 patients, 321 observations). To predict infliximab levels > 5 µg/mL, we propose a 6-thioguanine cutoff of 278 pmol/8 × 108 erythrocytes (sensitivity, 0.799; specificity, 0.347). A higher loss-of-response-to-infliximab rate (tested in a subgroup of 51 patients) was observed in patients with undetectable 6-thioguanine levels than in those with detectable levels (p = 0.026). Non-adherence to azathioprine therapy was suspected in 20% of patients.
Conclusion
Thiopurine metabolite monitoring in pediatric patients with Crohn’s disease is useful when optimizing combination therapy. Pediatric patients with undetectable 6-thioguanine levels are more likely to lose response to infliximab therapy. When targeting optimal infliximab levels, the 6-thioguanine cutoff levels in children appear to be higher than in adults.




Similar content being viewed by others
References
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95.
Van Assche G, Magdelaine-Beuzelin C, D’Haens G, Baert F, Noman M, Vermeire S, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterol. 2008;134(7):1861–8.
Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, Johanns J, et al. Clinical trial: benefits and risks of immunomodulators and maitenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30(3):210–26.
Kierkus J, Iwanczak B, Wegner A, Dadalski M, Grzybowska-Chlebowczyk U, Lazowska I, et al. Monotherapy with infliximab versus combination therapy in the maintenance of clinical remission in children with moderate to severe Crohn disease. J Pediatr Gastroenterol Nutr. 2015;60(5):580–5.
Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT, et al. 6-MP metabolite profiles provide a biochemical explanantion for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterol. 2002;122:904–15.
Schaeffeler E, Fisher C, Dierk B, Wernet D, Klaus M, Eichelbaum M, et al. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogen. 2004;14:407–17.
Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Gen. 1980;32:651–62.
Roblin X, Williet N, Peyrin-Biroulet L. Thiopurine metabolism in the era of combotherapy. Inflamm Bowel Dis. 2016;21(4):951–61.
Yarur AJ, Kubiliun MJ, Czul F, Sussman DA, Quintero MA, Jain A, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13(6):1118–24.
Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterol. 2006;130:1047–53.
Moreau AC, Paul S, Del Tedesco E, Rinaudo-Gaujous M, Baukhadra N, Genin C, et al. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory bowel disease: a meta-analysis. Inflamm Bowel Dis. 2014;20:464–71.
Dubinsky MC, Lamothe S, Ying Yang G, Targan SR, Sinnett D, Theoret Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterol. 2000;118:705–13.
Ruemmelle FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. Consensus guidelines of ECCO/ESPHGAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–207.
Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol. 2007;5:209–14.
Ansari A, Hassan C, Duley J, Marinaki A, Shobowale-Bakre EM. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1743–50.
Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795–806.
Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012;18(1):55–62.
Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A, et al. Evaluation of the pediatric Crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41(4):416–21.
Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998;44(3):551–5.
Hawwa AF, Millership JS, Colier PS, McElnay JC. Development and validation of HPLC method for the rapid and simultaneous determination of 6-mercaptopurine and four of its metabolites in plasma and red blood cells. J Pharmaceut Biomed Anal. 2009;49:401–9.
Dervieux T, Meyer G, Barham R, Matsutani M, Barry M, Boulieu R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolited in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005;51(11):2074–84.
Fangbin Z, Xiang G, Liang D, Hui L, Xueding W, et al. Prospective evaluation of pharmacogenomics and metabolite measurements upon azathioprine therapy in inflammatory bowel disease. Medicine. 2016;95(15):e3326.
Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, Van Steen K, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterol. 2015;148(7):1320–9.
Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20(11):1996–2003.
van Hoeve K, Dreesen E, Hoffman I, Van Assche G, Ferrante M, Gils A, et al. Higher infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J Crohn Colitis. 2018;12(11):1316–25.
Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterol. 2017;153(3):827–34.
Roblin X, Boschetti G, Williet N, Nancey S, Marotte H, Berger A, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open-label, prospective and randomised clinical trial. Aliment Pharmacol Ther. 2017;46(2):142–9.
Drobne D, Kurent T, Golob S, Švegl P, Rajar P, Hanžel J, et al. Optimised infliximab monotherapy is as effective as optimised combination therapy, but is associated with higher drug consumption in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49(7):880–9.
Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, et al. Concomitant use if immunomodulators affects the durability of inflixima therapy in children with Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13(10):1748–56.
van Rheen H, van Rheen PF. Long-term efficacy of anti-tumor necrosis factor agents in pediatric luminal Crohn’s disease: a systemic review or real-world evidence studies. Pediatr Gastroenterol Hepatol Nutr. 2020;23(2):121–31.
Hradsky O, Potuznikova K, Siroka J, Lerchova T, Urbanek L, Mihal V, et al. Prediction of thiopurine metabolite levels based on hematological and biochemical parameters. J Pediatr Gastroenterol Nutr. 2019;69(4):e105–10.
deBruyn JC, Jacobson K, El-Matary W, Carroll M, Wine E, Wrobel I, et al. Long-term Outcomes of Infliximab Use for Pediatric Crohn Disease: A Canadian Multicenter Clinical Practice Experience. J Pediatr Gastroenterol Nutr. 2018;66(2):268–73.
Jongsma MME, Winter DA, Huynh HQ, Norsa L, Hussey S, Kolho K-L, et al. Infliximab in young paediatric IBD patients: it is all about the dosing. Eur J Pediatr. 2020;179(12):1935–44.
Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–60.
Hemperly A, Vande CN. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57(8):929–42.
Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91(4):635–46.
Brandse JF, Mould D, Smeekes O, Ashruf Y, Kuin S, Strik A, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(4):650–60.
Jones J, Kaplan GG, Peyrin-Biroulet L, Baidoo L, Devlin S, Melmed GY, et al. Impact of concomitant immunomodulator treatment on efficacy and safety of anti-TNF therapy in Crohn’s disease: a meta-analysis of placebo controlled trials with individual patient-level data. Clin Gastroenterol Hepatol. 2015;13(3):2230–40.
Hommel KA, Davis CM, Baldassanp RN. Objective versus Subjective Assesment of Oral Medication Adherence in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis. 2009;15:589–93.
Pozler O, Chládek J, Malý J, Hroch M, Dědek P, Beránek M, et al. Steady-state of azathioprine during initiation treatement of pediatric inflammatory bowel disease. J Crohns Colitis. 2010;4(6):623–8.
Rahhal RM, Bishop WP. Initial clinical experience with allopurinol-thiopurine combination therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1678–82.
Gerich ME, Quiros JA, Marcin JP, Tennyson L, Henthorn M, Prindiville TP. A prospective evaluation of the impact of allopurinol in pediatric and adult IBD patients with preferentioal metabolism of 6-mercaptopurine to 6-methylmercaptopurine. J Crohns Colitis. 2010;14:546–52.
Friedman AB, Brown SJ, Bampton P, Barclay ML, Chung A, Macrae FA, et al. Randomised clinical trial: efficacy, safety and dosage of adjunctive allopurinol i azathioprine/mercaptopurine nonresponders (AAA Study). Aliment Pharmacol Ther. 2018;47:1092–102.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Ministry of Health (Czech Republic) funding for the conceptual development of research organizations [00064203, University Hospital Motol, Prague, Czech Republic, and 0098892, University Hospital, Olomouc, Czech Republic], and the OP VVV ENOCH [CZ.02.1.01/0.0/0.0/16_019/0000868].
Disclosure of potential conflicts of interest
Pospisilova K: lectures/congress fees/consultancy (outside submitted work)—MSD, Nutricia, Nestlé and Mead Johnsons; Karaskova E: lectures/congress fees/consultancy (outside submitted work)—MSD, AbbVie, Nutricia, and Nestlé; Hradsky O: lectures/congress fees/consultancy (outside submitted work)—MSD, AbbVie, Nutricia, Nestlé, Ferring, and Falk; Lerchova T: lectures/congress fees/consultancy (outside submitted work)—Nutricia, Ferring and Biocodex; Zarubova K: lectures/congress fees/consultancy (outside submitted work)—Nutricia and Nestlé; Velganova-Veghova M: congress fees/consultancy (outside submitted work)—MSD, AbbVie, Nutricia, and Nestlé; Bronsky J: lectures/congress fees/consultancy (outside submitted work)—MSD, AbbVie, Nutricia, Nestlé, Ferring, Biocodex, and Walmark; Gonsorcikova L, Francova I, Geryk M, Copova I, Mihal V, Siroka J, and Urbanek L report no conflicts of interest.
Ethics approval
The protocol for the observational study was approved by the Ethics Committees of the University Hospital Motol and the 2nd Medical Faculty of Charles University in Prague.
Informed consent
Written informed consent was obtained from legal guardians before study enrollment.
Consent for publication
Non applicable.
Study registration
The study has been registered retrospectively in the ENCePP registry (encepp.eu), it can be found under the registration number EUPAS38918. Registered on 12 January 2021.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The code is available from the corresponding author on reasonable request.
Author contributions
PK: study design, literature search, data collection, patient recruitment, thiopurine metabolite measurement, data analysis, manuscript writing; SJ: data collection, thiopurine metabolite measurement and laboratory supervision, manuscript critical revision; KE: data collection, patient recruitment, manuscript critical revision; HO: study design, data analysis and interpretation, manuscript critical revision; LT: study design, data collection, patient recruitment, manuscript critical revision, ZK: data collection, patient recruitment, manuscript critical revision; CI: data collection, patient recruitment, manuscript critical revision; GL: data collection, patient recruitment, manuscript critical revision; V-VM: data collection, patient recruitment, manuscript critical revision; FI: data collection, infliximab levels and antibodies measurement supervision, manuscript critical revision; UL: thiopurine metabolite measurement—method optimization, data collection, manuscript critical revision; GM: data collection, patient recruitment, manuscript critical revision; Mihal V: data collection, supervision, manuscript critical revision; BJ: study design and supervision, literature search, manuscript writing. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Pospisilova, K., Siroka, J., Karaskova, E. et al. Is It Useful to Monitor Thiopurine Metabolites in Pediatric Patients with Crohn’s Disease on Combination Therapy? A Multicenter Prospective Observational Study. Pediatr Drugs 23, 183–194 (2021). https://doi.org/10.1007/s40272-021-00439-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00439-1