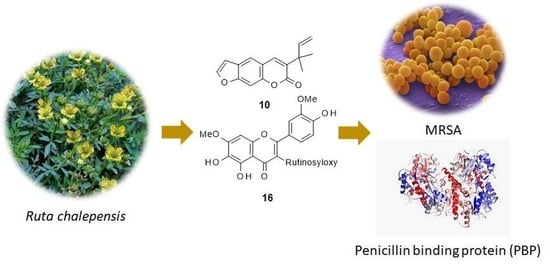
Anti-MRSA Constituents from Ruta chalepensis (Rutaceae) Grown in Iraq, and In Silico Studies on Two of Most Active Compounds, Chalepensin and 6-Hydroxy-rutin 3′,7-Dimethyl ether
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Extraction
3.4. Initial Antimicrobial Screening
3.5. Solid-Phase Extraction (SPE)
3.6. Isolation and Identification of Compounds
3.7. Resazurin Assay with Isolated Compounds
3.8. Assessment of Anti-MRSA Activity
3.9. In Silico Studies with Two Most Active Anti-MRSA Compounds from This Plant, Chalepensin (10) and 6-Hydroxy-rutin 3′,7-dimethyl Ether (16)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Livermore, D.M. Bacterial resistance: Origins, epidemiology, and impact. Clin. Infect. Dis. 2003, 36, S11–S23. [Google Scholar] [CrossRef] [Green Version]
- Moran, M. A breakthrough in R&D for neglected diseases: New ways to get the drugs we need. PloS Med. 2005, 2, e376. [Google Scholar]
- Burroughs, T.; Najafi, M.; Lemon, S.M.; Knobler, S.L. The resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment: Workshop Summary; National Academies Press: Washington, WA, USA, 2003; pp. 1–223. [Google Scholar]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. 2017, 6, 47. [Google Scholar] [CrossRef]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and antimicrobial activity of rutin and its derivatives from Ruta chalepensis (Rutaceae) growing in Iraq. Rec. Nat. Prod. 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Al-Said, M.S.; Yahia, M.T.; Ginnawi, S.R.; Ageel, A.M. Studies on Ruta chalepensis, an ancient medicinal herb still used in traditional medicine. J. Ethnopharmacol. 1990, 23, 305–312. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Abu Ghdeib, A.I. Antifungal activity of plant extracts against dermatophytes. Mycoses 1990, 42, 665–672. [Google Scholar] [CrossRef]
- Pollio, A.; De Natale, A.; Appetiti, E.; Aliotta, G.; Touwaide, A. Continuity and change in the Mediterranean medical tradition: Ruta spp. (Rutaceae) in Hippocratic medicine and present practices. J. Ethnopharmacol. 2008, 116, 469–482. [Google Scholar] [CrossRef]
- Gibbons, S.; Udo, E.E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother. Res. 2000, 14, 139–140. [Google Scholar] [CrossRef]
- Alzoreky, N.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Priya, P.S.; Sasikumar, J.; Gowsigan, G. Antibacterial activity of methanol extract of Ruta chalapensis (L), Quercus infectoria (Oliver) and Canthium parviflorum (Lam). Anc. Sci. Life 2009, 29, 28. [Google Scholar] [PubMed]
- Babu-Kasimala, M.; Tukue, M.; Ermias, R. Phytochemical screening and antibacterial activity of two common terrestrial medicinal plants Ruta chalepensis and Rumex nervosus. Bali. Med. J. 2014, 3, 116–121. [Google Scholar] [CrossRef]
- Boyd, D.R.; Sharma, N.D.; Carroll, J.G.; Loke, P.L.; O’Dowd, C.R.; Allen, C.C.R. Biphenyl dioxygenase-catalysed cis-dihydroxylation of tricyclic azaarenes: Chemoenzymatic synthesis of arene oxide metabolites and furoquinoline alkaloids. RSC Adv. 2013, 3, 10944–10955. [Google Scholar] [CrossRef] [Green Version]
- Ulubelen, A.; Terem, B.; Tuzlaci, E.; Cheng, K.; Kong, Y. Alkaloids and coumarins from Ruta chalepensis. Phytochemistry 1986, 25, 2692–2693. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Wang, J.J.; Iou, S.C.; Chang, H.C.; Chen, Y.P.; Kuo, Y.H.; Chang, Y.L.; Tenge, C.M. Cytotoxic and antiplatelet aggregation principles of Ruta graveolens. J. Chin. Chem. Soc. 2003, 50, 171–178. [Google Scholar] [CrossRef]
- Openshaw, H. Quinoline alkaloids, other than those of Cinchona. Alkaloids Chem. Physiol. 1967, 9, 223–267. [Google Scholar]
- Gaston, J.L.; Grundon, M.F.; James, K.J. Quinoline alkaloids. Part 19. Synthesis of O-methylptelefolonium iodide and (±)-dubinidine. J. Chem. Soc., Perkin Trans. 1. 1980, 24, 1136–1138. [Google Scholar] [CrossRef]
- Pal, C.; Kundu, M.K.; Bandyopadhyay, U.; Adhikari, S. Synthesis of novel heme-interacting acridone derivatives to prevent free heme-mediated protein oxidation and degradation. Bioorganic Med. Chem. Lett. 2011, 21, 3563–3567. [Google Scholar] [CrossRef]
- Um, Y.R.; Kong, C.S.; Lee, J.I.; Kim, Y.A.; Nam, T.J.; Seo, Y. Evaluation of chemical constituents from Glehnia littoralis for antiproliferative activity against HT-29 human colon cancer cells. Process. Biochem. 2010, 45, 114–119. [Google Scholar] [CrossRef]
- O’Neill, T.; Johnson, J.A.; Webster, D.; Gray, C.A. The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins. J. Ethnopharmacol. 2013, 147, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Shu Shan, D.; Zhi Wei, D.; Jing, X.; Zhu Feng, G.; Jiang Bin, F.; Kai, Y.; Li, F.; Cheng Fang, W.; Hai Yan, J.; Hai Bo, Y. Cytotoxic Constituents from the Stems of Clausena lansium (Lour.) Skeels. Molecules 2013, 18, 10768–10775. [Google Scholar]
- Richardson, J.S.M.; Sethi, G.; Lee, G.S.; Malek, S.N.A. Chalepin: Isolated from Ruta angustifolia L. Pers induces mitochondrial mediated apoptosis in lung carcinoma cells. Bmc. Complementary Altern. Med. 2016, 16, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandatmakuru, S.; Arava, V. Novel synthesis of graveoline and graveolinine. Synth. Commun. 2018, 48, 2635–2641. [Google Scholar] [CrossRef]
- Kamal, L.Z.M.; Mohd Hassan, N.; Taib, N.M.; Soe, M.K. Graveoline from Ruta angustifolia (L.) Pers. and its antimicrobial synergistic potential in erythromycin or vancomycin combinations. Sains Malays. 2018, 47, 2429–2435. [Google Scholar] [CrossRef]
- Sampaio, O.; Vieira, L.; Bellete, B.; King-Diaz, B.; Lotina-Hennsen, B.; Da Silva, M.; Veiga, T. Evaluation of alkaloids isolated from Ruta graveolens as photosynthesis inhibitors. Molecules 2018, 23, 2693. [Google Scholar] [CrossRef] [Green Version]
- Markham, K.R.; Sheppard, C.; Geiger, H. 13C-NMR studies of some naturally occurring amentoflavone and hinokifavone biflavonoids. Phytochemistry 1987, 26, 3335–3337. [Google Scholar] [CrossRef]
- Li, S.; Zhao, M.; Li, Y.; Sui, Y.; Yao, H.; Huang, L.; Lin, X. Preparative isolation of six anti-tumour biflavonoids from Selaginella doederleinii Hieron by high-speed counter-current chromatography. Phytochem. Anal. 2014, 25, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. 2002, 50, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Huang, Y.; Huang, F.; Wang, C.W.; Ou, J. Water-soluble glycosides from Ruta graveolens. J. Nat. Prod. 2001, 64, 990–992. [Google Scholar] [CrossRef]
- Wu, P.P.; Zhang, K.; Lu, Y.J.; He, P.; Zhao, S.Q. In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2014, 80, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.G.; Ko, H.J.; Chowdhury, M.A.; Lee, D.S.; Woo, E.R. A new indole glycoside from the seeds of Raphanus sativus. Arch. Pharmacal Res. 2016, 39, 755–761. [Google Scholar] [CrossRef]
- Sarker, S.D.; Kumarasamy, Y.; Nahar, L. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Réthy, B.; Zupkó, I.; Minorics, R.; Hohmann, J.; Ocsovszki, I.; Falkay, G. Investigation of cytotoxic activity on human cancer cell lines of arborinine and furanoacridones isolated from Ruta graveolens. Planta Med. 2007, 73, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Amoa Onguéné, P.; Ntie-Kang, F.; Lifongo, L.L.; Ndom, J.C.; Sippl, W.; Mbaze, L.M.A. The potential of anti-malarial compounds derived from African medicinal plants. Part I: A pharmacological evaluation of alkaloids and terpenoids. Malar. J. 2013, 12, 449. [Google Scholar] [CrossRef] [Green Version]
- Fouotsa, H.; Mbaveng, A.; Mbazoa Djama, C.; Nkengfack, A.; Farzana, S.M.; Iqbal, C.; Meyer, J.; Lall, N.; Kuete, V. Antibacterial constituents of three Cameroonian medicinal plants: Garcinia nobilis, Oricia suaveolens and Balsamocitrus camerunensis. BMC Complementary Altern. Med. 2013, 13, 81. [Google Scholar] [CrossRef] [Green Version]
- Schrodinger Suite Platform 7.0; Schrödinger: New York, NY, USA, 2021; Available online: https://www.schrodinger.com/platform (accessed on 7 February 2021).
- Kuhn, B.; Tichy, M.; Wang, L.; Robinson, S.; Martin, R.E.; Kuglstatter, A.; Benz, J.; Giroud, M.; Schirmeister, T.; Abel, R.; et al. Prospective evaluation of free energy calculations for the prioritization of cathepsin L inhibitors. J. Med. Chem. 2017, 60, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marrra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Macindoe, G.; Mavridis, L.; Venkatraman, V.; Devignes, M.-D.; Ritchie, D.W. HexServer: An FFT-based protein docking server powered by graphics processors. Nucleic Acid Res. 2010, 38, W445–W449. [Google Scholar] [CrossRef]
- Srisuknimit, V.; Qiao, Y.; Schaefer, K.; Kahne, D.; Walker, S. Peptidoglycan cross-linking preferences of Staphylococcus aureus penicillin binding proteins have implications for treating MRSA infections. J. Am. Chem. Soc. 2017, 139, 9791–9794. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M.; Ruble, C.A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993, 37, 1086–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.F.; Reith, S. Characterization of a strain of methicillin-resistant Staphylococcus aureus (EMRSA-15) by conventional and molecular methods. J. Hosp. Infect. 1993, 25, 45–52. [Google Scholar] [CrossRef]
- Nurunnnabi, T.R.; Nahar, L.; Al-Majmaie, S.; Rahman, S.M.M.; Sohrab, M.H.; Billah, M.M.; Ismail, F.M.D.; Rahman, M.M.; Sharples, G.P.; Sarker, S.D. Anti-MRSA activity of oxysporone and xylitol from the endophytic fungus Pestalotia sp. growing on the Sundarbans mangrove plant Heritiera fomes. Phytother. Res. 2018, 32, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Bacteria and Fungi | Extract | Plant Parts (MICs in mg/mL) | ||||
|---|---|---|---|---|---|---|
| Leaves | Stem | Fruits | Roots | |||
| Gram-Negative Bacteria | E. coli | n−Hexane | 6.25 × 10−1 | 6.25 × 10−1 | 3.12 × 10−1 | N/A |
| DCM | 3.12 × 10−1 | 6.25 × 10−1 | 3.12 × 10−1 | N/A | ||
| Methanol | 6.25 × 10−1 | 3.12 × 10−1 | 6.25 × 10−1 | 5 | ||
| P. aeruginosa | n−Hexane | 6.25 × 10−1 | 6.25 × 10−1 | 3.12 × 10−1 | N/A | |
| DCM | 3.12 × 10−1 | 6.25 × 10−1 | 6.25 × 10−1 | N/A | ||
| Methanol | 6.25 × 10−1 | 3.12 × 10−1 | 1.56 × 10−1 | 2.5 | ||
| Gram-Positive Bacteria | M. luteus | n−Hexane | 1.56 × 10−1 | 6.25 × 10−1 | 3.12 × 10−1 | N/A |
| DCM | 1.56 × 10−1 | 6.25 × 10−1 | 7.81 × 10−2 | N/A | ||
| Methanol | 1.95 × 10−2 | 7.81 × 10−2 | 3.90 × 10−2 | 1.25 | ||
| S. aureus | n−Hexane | 6.25 × 10−1 | 6.25 × 10−1 | 6.25 × 10−1 | N/A | |
| DCM | 6.25 × 10−1 | 6.25 × 10−1 | 6.25 × 10−1 | N/A | ||
| Methanol | 3.12 × 10−1 | 3.12 × 10−1 | 3.12 × 10−1 | 5 | ||
| Pathogenic Fungi | C. albicans | n−Hexane | 6.25 × 10−1 | 1.95 × 10−1 | 1.56 × 10−2 | N/A |
| DCM | 3.12 × 10−1 | 3.12 × 10−1 | 3.90 × 10−2 | N/A | ||
| Methanol | 1.95 × 10−2 | 7.81 × 10−2 | 3.90 × 10−2 | 2.5 | ||
| Compounds | MIC in mg/mL | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus NCTC 12981 | Escherichia coli NCTC 12241 | Pseudomonas aeruginosa NCTC 12903 | Micrococcus luteus NCTC 7508 | Candida albicans ATCC 90028 | |
| 1 | N/A | N/A | N/A | 5 × 10−1 | 5 × 10−1 |
| 3 | 5 × 10−1 | N/A | N/A | 5 × 10−1 | 5 × 10−1 |
| 6 | N/A | N/A | N/A | 5 × 10−1 | 5 × 10−1 |
| 7 | 5 × 10−1 | N/A | N/A | 6.25 × 10−2 | 5 × 10−1 |
| 8 | 5 × 10−1 | N/A | N/A | 2.5 × 10−1 | 2.5 × 10−1 |
| 9 | 1.25 × 10−1 | 5 × 10−1 | N/A | 1.25 × 10−1 | 1.25 × 10−1 |
| 10 | 1.25 × 10−1 | 1.25 × 10−1 | 5 × 10−1 | 2.5 × 10−1 | 6.25 × 10−2 |
| 11 | 1.25 × 10−1 | N/A | 5 × 10−1 | 2.5 × 10−1 | 2.5 × 10−1 |
| 12 | 5 × 10−1 | N/A | N/A | 5 × 10−1 | 5 × 10−1 |
| 13 | 2.5 × 10−1 | N/A | 5 × 10−1 | 2.5 × 10−1 | 2.5 × 10−1 |
| 14 | 2.5 × 10−1 | 2.5 × 10−1 | 2.5 × 10−1 | 2.5 × 10−1 | 2.5 × 10−1 |
| 16 | 2.5 × 10−1 | 2.5 × 10−1 | 2.5 × 10−1 | 6.25 × 10−2 | 6.25 × 10−2 |
| 18 | 2.5 × 10−1 | 5 × 10−1 | 5 × 10−1 | 1.25 × 10−1 | 6.25 × 10−2 |
| Ciprofloxacin | 9.76 × 10−4 | 1.55 × 10−2 | 1.95 × 10−3 | 9.76 × 10−4 | N/A |
| Nystatin | N/A | N/A | N/A | N/A | 9.76 × 10−4 |
| Compounds | MIC in μg/mL | |||||
|---|---|---|---|---|---|---|
| XU212 | ATCC25923 | SA1199B | EMRSA-15 | MRSA346702 | MRSA274819 | |
| 1 | − | − | − | − | − | − |
| 3 | − | − | − | − | − | 256 |
| 6 | − | − | − | − | − | − |
| 7 | − | − | − | − | − | − |
| 8 | 256 | − | 256 | − | 256 | 256 |
| 9 | − | 256 | 256 | − | − | 128 |
| 10 | 64 | 128 | − | − | 64 | 64 |
| 11 | 128 | − | 128 | − | 128 | 128 |
| 12 | − | − | − | − | − | − |
| 13 | − | − | − | − | − | 256 |
| 14 | 256 | 128 | − | − | 256 | 256 |
| 16 | 32 | 64 | − | − | 128 | 256 |
| 18 | − | 256 | − | 128 | 64 | 256 |
| Norfloxacin | 16 | 2 | 32 | 1 | 64 | 64 |
| Bioactivities | Pa (Probability to be Active) | Pi (Probability to be Inactive) | ||
|---|---|---|---|---|
| 10 | 16 | 10 | 16 | |
| Membrane integrity agonist | 0.954 | 0.973 | 0.003 | 0.002 |
| Ubiquinol-cytochrome-c reductase inhibitor | 0.863 | − | 0.013 | − |
| Fatty-acyl-CoA synthase inhibitor | 0.822 | − | 0.004 | − |
| Membrane permeability inhibitor | 0.809 | 0.962 | 0.009 | 0.002 |
| Free radical scavenger/antioxidant | 0.627 | 0.878 | 0.005 | 0.003 |
| Alcohol dehydrogenase (NADP+) inhibitor | − | 0.927 | − | 0.002 |
| Xenobiotic-transporting ATPase inhibitor | − | 0.886 | − | 0.002 |
| Lipid peroxidase inhibitor | − | 0.813 | − | 0.003 |
| Anticarcinogenic | − | 0.716 | − | 0.007 |
| Toxicity/adverse reactions | Compound 10: itchiness and eye irritation acidosis | Compound 16: metabolic acidosis | ||
| Anti-MRSA Compounds | Integrase | Tail-Anchored Proteins (TaPs) | Penicillin Binding proteins (PBPs) | Pyruvate Kinase |
|---|---|---|---|---|
| 10 | −8.933 | −11.8567 | −21.6229 | −13.615 |
| 16 | −17.331 | −11.8148 | −9.3219 | −16.237 |
| anti-MRSA Compounds | Score | Hydrogen Bonding Properties | ||
|---|---|---|---|---|
| Bond Attributes | Bond Energy | Bond Length (Å) | ||
| Integrase | ||||
| 10 | −8.933 | O39-HH11-ARG-68-B H78-O-H15-108-B O53-HE21-GCN-109-B | −0.8 | 1.80 |
| −3.3 | 2.09 | |||
| −2.6 | 4.22 | |||
| 16 | −17.331 | H84-O-ASP-107-B H79-OD1-ASP-111-B O6-HH21-ARG-16-8 | −4.7 | 2.20 |
| −3.7 | 2.23 | |||
| −4.2 | 1.77 | |||
| TaPs | ||||
| 10 | −11.8567 | H39-OE1-GLN-109-B O23-HE21-GLN-109-B | −4.1 | 1.99 |
| −4.1 | 2.01 | |||
| 16 | 11.8148 | O17-HH21-ARG-68-B O16-HE21-GLN-53-B O20-HH11-ARG-68-B O20-HH21-ARG-68-B | −6.3 | 2.02 |
| −2.3 | 2.32 | |||
| −3.7 | 2.00 | |||
| −3.5 | 2.06 | |||
| PBPs | ||||
| 10 | −21.6229 | O1-HH21-LYS-218-B O14-H-GLN-216-B | −7.3 | 2.13 |
| −7.6 | 1.70 | |||
| 16 | −9.3219 | O14-H-GLN-216-B H21-002-ASP-111- H21-001-ASP-111-B | −3.0 | 1.96 |
| −4.6 | 2.14 | |||
| −4.2 | 2.18 | |||
| Pyruvate Kinase | ||||
| 10 | −13.615 | O16-HH21-ARG-67-B | −2.1 | 2.37 |
| 16 | −16.237 | O3-HE22-GLN-53-B O16-HH11-ARG-16-B O16-HH21-ARG-16-B O19-H-ALA-13-B | −4.7 | 1.96 |
| −5.5 | 2.15 | |||
| −6.9 | 2.12 | |||
| −4.2 | 2.13 | |||
| Compds | Silicos-IT Log Sw | Silicos-IT Solubility | Silicos IT Class | GI Absorption | BBB Permeant | PGP Substrate | CYPs Inhibitor | Log Kp (cm/s) | Lipinski Violations | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/mL | Mol/L | 1A2 | 2C19 | 2C9 | 2D6 | A4 | ||||||||
| 10 | −2.93 | 3.41 × 10−1 | 1.18 × 10−3 | Soluble | High | Yes | No | No | No | No | Yes | No | −6.77 | 0 |
| 16 | −1.70 | 3.28 | 2.00 × 10−2 | Soluble | High | No | No | No | No | No | No | No | −7.29 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Majmaie, S.; Nahar, L.; Rahman, M.M.; Nath, S.; Saha, P.; Talukdar, A.D.; Sharples, G.P.; Sarker, S.D. Anti-MRSA Constituents from Ruta chalepensis (Rutaceae) Grown in Iraq, and In Silico Studies on Two of Most Active Compounds, Chalepensin and 6-Hydroxy-rutin 3′,7-Dimethyl ether. Molecules 2021, 26, 1114. https://doi.org/10.3390/molecules26041114
Al-Majmaie S, Nahar L, Rahman MM, Nath S, Saha P, Talukdar AD, Sharples GP, Sarker SD. Anti-MRSA Constituents from Ruta chalepensis (Rutaceae) Grown in Iraq, and In Silico Studies on Two of Most Active Compounds, Chalepensin and 6-Hydroxy-rutin 3′,7-Dimethyl ether. Molecules. 2021; 26(4):1114. https://doi.org/10.3390/molecules26041114
Chicago/Turabian StyleAl-Majmaie, Shaymaa, Lutfun Nahar, M. Mukhlesur Rahman, Sushmita Nath, Priyanka Saha, Anupam Das Talukdar, George P. Sharples, and Satyajit D. Sarker. 2021. "Anti-MRSA Constituents from Ruta chalepensis (Rutaceae) Grown in Iraq, and In Silico Studies on Two of Most Active Compounds, Chalepensin and 6-Hydroxy-rutin 3′,7-Dimethyl ether" Molecules 26, no. 4: 1114. https://doi.org/10.3390/molecules26041114