Abstract
Older individuals experience cardiovascular dysfunction during extended bedridden hospital or care home stays. Bed rest is also used as a model to simulate accelerated vascular deconditioning occurring during spaceflight. This study investigates changes in retinal microcirculation during a ten-day bed rest protocol. Ten healthy young males (22.9 ± 4.7 years; body mass index: 23.6 ± 2.5 kg·m–2) participated in a strictly controlled repeated-measures bed rest study lasting ten days. High-resolution images were obtained using a hand-held fundus camera at baseline, daily during the 10 days of bed rest, and 1 day after re-ambulation. Retinal vessel analysis was performed using a semi-automated software system to obtain metrics for retinal arteriolar and venular diameters, central retinal artery equivalent and central retinal vein equivalent, respectively. Data analysis employed a mixed linear model. At the end of the bed rest period, a significant decrease in retinal venular diameter was observed, indicated by a significantly lower central retinal vein equivalent (from 226.1 µm, CI 8.90, to 211.4 µm, CI 8.28, p = .026), while no significant changes in central retinal artery equivalent were noted. Prolonged bed rest confinement resulted in a significant (up to 6.5%) reduction in retinal venular diameter. These findings suggest that the changes in retinal venular diameter during bedrest may be attributed to plasma volume losses and reflect overall (cardio)-vascular deconditioning.
Similar content being viewed by others
Introduction
Bed rest has been prescribed to treat various medical conditions throughout the twentieth century1,2,3. However, in the current medical practice, daily activity and physical rehabilitation take precedence due to the revealed adverse effects of prolonged bed rest1,2,4. Nevertheless, confinement to bed is still common in care homes, where elderly or chronically ill individuals may spend extended periods bedridden for essential activities such as eating, personal care, toileting, and bathing5. Additionally, bed rest is frequently used as a model to study the physiological effects of weightlessness during spaceflight6,7,8. One widely employed approach of bedrest confinement involves 6-degree head-down tilt bed rest, which closely mimics the cephalad fluid shifts seen in microgracvity6,7,8,9,10,11.
Bed rest induces significant cardiovascular changes, increasing heart rate and reducing oxygen uptake (V̇O2 max.)12. These changes result from decreased maximal stroke volume and cardiac output12. The reduction in stroke volume is primarily attributed to decreased venous return associated with lower circulating blood volume, reduced central venous pressure, and increased venous compliance in the lower extremities12. Prolonged bed rest can lead to deconditioning and plasma volume loss, potentially causing deep vein thrombosis and orthostatic hypotension5,6,7,13. Plasma volume loss is a hallmark of extended bed rest14,15,16,17,18,19. Investigating the physiological response to bed rest is crucial for mitigating adverse effects in medical treatments, gaining insights into aging-related deconditioning, and advancing our understanding of space physiology in humans.
Traditional methods for assessing cardiovascular physiology include blood marker evaluations, chest x-rays, ECGs, echocardiogram, exercise, or stress tests, cardiac catheterization, and CT or MRI scans. Retinal vessel analysis emerged as a non-invasive physiological technique to assess cardiovascular and cerebrovascular health20,21,22,23.
The retinal blood vessels originate from the ophthalmic artery, the first branch of the internal carotid artery, and changes in retinal vessel widths are proposed as surrogate markers for evaluating systemic circulation24. Several studies have confirmed that changes in retinal vessel caliber can predict of changes in larger vessels25,26,27,28,29,30,31,32,33,34. Notably, constrictions of retinal arterioles and veins is associated with future increase in blood pressure and the development of hypertension27,28,29. Additionally, constriction of retinal arterioles and dilatation of retinal venules serves as strong predictors of coronary heart disease and clinical stroke30,31,32,33,34.
The retina, like other organs in the body, has autoregulatory mechanisms to maintain adequate blood flow and oxygen delivery. During microgravity, when blood volume is pulling upwards and mean arterial pressure decreases, the retinal vessels may respond by constricting (narrowing). This constriction is an attempt to maintain sufficient perfusion pressure and oxygen supply to the retinal tissues. This is supported by limited number of studies that are available35,36,37,38,39. The exact mechanisms behind these changes are still not fully understood.
Our ten-day horizontal bed rest study involving ten healthy young men investigated the impact of vascular unloading on the microvascular health. Our hypothesis is that bed rest-induced plasma volume loss and general vascular deconditioning will affect retinal microcirculation, resulting in observable narrowing of retinal vessel diameters.
Methodology
This horizontal bedrest study was performed at the Izola General Hospital (Izola, Slovenia). Data were collected according to the principles of good clinical practice and the WMA Declaration of Helsinki. Ethics approval was granted by the National Ethics Committee of the Slovenian Ministry of Health on July 17, 2019, under reference number 0120-304/2019/9. Participants were adequately informed about the study by the responsible hospital staff. All participants provided signed informed consent prior to study enrolment.
Participants
Ninety-three participants were initially invited to the study. Forty-six young male participants were invited for interviews, and 16 of these met all inclusion/exclusion criteria. All participants underwent a medical examination, a physical activity questionnaire (GPAQ), body composition analysis, electrocardiography (at rest and during exercise) with blood pressure measurement, medical questionnaires, functional exercise assessment, and a dietary interview. Participants were housed in standard air-conditioned hospital rooms (five participants per room) and were under constant visual monitoring with 24-h medical care, 24-h heart rate monitoring, and physical activity measurements. During bed rest, study participants performed all daily activities in bed, with no deviations from the horizontal (supine) position allowed, and both physical activity and muscle contraction tests were not allowed during the period of bed rest. All participants received hospital meals three times daily and maintained an individually controlled equicaloric diet throughout the hospital stay. Dietary energy requirements were determined for each participant by multiplying resting energy expenditure by factors of 1.2 and 1.4 in the bed rest and outpatient phases, respectively40. The macronutrient content of the diet was set at 60% carbohydrate, 25% fat, and 15% protein. Sleep time was between 10:00 pm and 7:00 am. The study details are given in the publication of Monti et al.41.
Study protocol
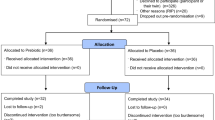
The protocol included two days of familiarization with the hospital environment and diet. Baseline data collection was performed just before the start of bed rest (BDC), followed by daily data collection during the ten days of bed rest (BR1-BR10) and one day after re-ambulation (R + 1). The simplified overview of the study flow can be seen in Fig. 1. This study was part of a larger study that also included other measurements such as anthropometric characteristics, MRI, ultrasound measurements, muscle biopsies, and cardiopulmonary exercise testing41.
Simplified overview of the study flow including study preparation (selection of the study candidates and familiarization with the study) and bed rest study itself (BDC start of bed rest (baseline); BR1-10 ten days of bed rest; R + 1 one day after re-ambulation (recovery)). Indicated are also the time points whent the retinal images were collected and analyzed.
Retinal vessel measurements
Retinal images focused on the optic disc (resolution of 1536 × 1536) were acquired from each participant's right eye by a trained person using the Optomed Aurora portable digital retinal camera (Optomed Oy, Oulu, Finland) at the same time every morning throughout the study at baseline (BDC), daily during the 10-days of bed rest (BR1 to BR10), and one day after bed rest (R + 1).
Retinal vessel analysis was conducted by a trained researcher from the Medical University of Graz, Austria, who was blinded to the study’s details. The semi-automated MONA REVA software (VITO, Mol, Belgium42) was utilized for this purpose. To minimize analysis variability, a single trained operator was responsible for this task. Before a retinal image can be analyzed, a scale ratio has to be determined. To determine the scale ratio, the distance between the centre of macula (fovea) and the centre of the optic disc (blind spot) must be measured. The distance must be measured in pixels. The scale ratio is calculated by dividing 4500 by the distance (in pixels) between the macula and blind spot43. This procedure is done in the MONA REVA software. The scale ratio, also known as the resolution number, was calculated as 6.8 um/pixel by averaging the individual resolution numbers from all retinal images. The software automatically processed retinal images, analyzing the diameters of retinal microvessels within the area between 1.5 and 5 times of the optic disc radius, ensuring consistency across all images. All vessels laying in this perifoveal area, larger than 100 µm diameter were automatically recognized and measured by the software. Subsequently, the algorithm of the software, inspired by Nguyen et al. performed automatic retinal vessel segmentation42. Post-processing included double thresholding, blob extraction, removal of small contiguous regions, and hole filling. Thereafter, the grader checked and corrected diameters and designations (arteriole or venule) using the MONA REVA vessel editing toolbox. The revised Parr-Hubbard-Knudtson formula, employing the six largest retinal arterioles and venules, was applied to calculate retinal microvascular parameters: central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE)44.
Statistical evaluation
Data were analyzed using IBM SPSS version 28.0 (Armonk, NY, USA: IBM Corp). Normality of the data was confirmed using graphical analysis of histogram and Q-Q plot, tests for kurtosis and asymmetry with a Shapiro–Wilk test. During the 12 days of measurements, missing or invalid data occasionally occurred, which was resolved using mixed linear modeling (MLM). Levene's test was used to confirm homogeneity of variance. Subjects were classified as random factors, whereas time (BDC, BR1-10, R + 1) was classified as a fixed factor. The MLM employs maximum likelihood to estimate missing data. All subjects are retained for analysis, with those having complete datasets used to estimate degrees of freedom and mean at each time point. Data from individuals with missing data are aggregated for the final averages at each data point. However, MLM compares averages of subjects with complete datasets to averages of available data from those with missing data only after confirming the randomness of the missing data. This comparison is performed at each time point. The MLM provides weighted means of the data sets that account for the effects of sample size and random/fixed factors. In addition, this model does not provide SD, only the standard error (SE) and confidence intervals (95% CI, given in Fig. 2). All statistical decisions were made at p ≤ 0.05.
Results
Ten healthy young participants (age: 22.9 ± 4.7 years; body mass index: 23.6 ± 2.5 kg·m–2) took part in the study. Normotension, normal blood glucose levels, no smoking, no medication, and no occurrence of any kind of eye disease were reported.
A significant overall change in CRVE of 6.5% (Fig. 2) was seen when the start (BDC) and the end (BR10) of the bed rest period were compared. No significant differences were found in CRAE and arteriovenous ratio (AVR), which is the ratio between CRAE and CRVE.
Discussion
The present study suggests that bed rest induces changes in microcirculation, as evidenced by retinal vessel width measurements. Bed rest led to a narrowing of retinal veins and no effect on the width of the retinal arteries (CRAE, Fig. 2). The present study hypothesizes that changes in the retinal microvascular parameters reflect changes in the cardiovascular system resulting from prolonged inactivity/bed rest5,6,7,11.
Taibbi et al. investigated the differences between a 70-day of 6° head-down tilt bed rest and 6-month space mission45. They found no changes in vessel diameter but did observe a decrease in retinal vessel density in astronauts. Additionally, they noticed a decrease in fractal dimension and vessel length in smaller (not larger) retinal vessels in astronauts46. The authors speculate that the observed decrease in vessels may be attributed to the reduction in vessel diameter, which corroborates with the results of our study in which a lower CRVE was observed. Another cross-over study examined changes in retinal vessel diameters in eleven young males (27 ± 6 years; 23.7 ± 3.0 kg m–2) during normoxic bed rest, hypoxic bed rest, and hypoxic ambulation47. The normoxic bed rest group, which most closely matches our study group observed reductions in both CRVE, consistent with our findings, and CRAE. The shorter duration of our study (10 days) compared to theirs (21 days) might explain the lack of significant results in CRAE.
Two additional studies explored short-term exposure to microgravity48,49. A study inducing microgravity through a parabolic flight revealed a 58% increase in intraocular pressure by and a 4% reduction in CRAE48. The second study, conducted by Baer and Hill, investigated the effect of passive tilting on ten healthy participants (mean age, 28.9 years; 7 men and 3 women) observed a significant 3.5% reduction in CRAE and a 3.7% increase in CRVE during head-down tilt49. However, unlike our study, both of these mentioned studies examined the acute effects of microgravity exposure (20 s of the parabolic flight and 3 min of head-down tilt). Therefore, their observed reduction in CRAE might be attributed to transient, acute physiological responses, rather than the gradual establishment of a new hemodynamic equilibrium typical for prolonged exposure to unloading, such as bed rest or space flight.
Small retinal vessels appear to play a significant role in regulating gravitationally related fluid shifts. The limited number of studies that are available suggest that microgravity may reduce retinal vascular density or retinal vessel diameter. Over the long term, the narrowing of the caliber of small peripheral vessels, such as retinal vessels, is expected as an autoregulatory response to hypovolemia during microgravity exposure (e.g. bed rest). Our study observed responses in retinal venules after a brief period of physical inactivity (10 days of bed rest). The smaller retinal vessel diameters may indicate the adaptation in the peripheral vascular system. The reduction in CRVE may be explained by the decrease in central blood volume due to bed rest-induced unloading, as suggested by previous research14,15,16,17,18,19. Enhanced peripheral vasoconstriction in individuals adapted to a microgravity environment may result from hypovolemia. Additionally, venous blood accumulation in the internal jugular vein during spaceflight could contribute to blood volume loss in the retinal vessels50,51,52,53. Another reason could be an increase in cerebral, respective ocular perfusion pressure because of fluid shifts. Increased blood flow in the retina triggers autoregulative constriction of retinal vessels to maintain constant blood flow. Furthermore, Du et al. reported heterogeneity in response across studies and different brain regions54. Additionally, reduced cerebral blood flow pulsatility during head-down tilt relative to preflight values suggests enhanced cerebral vasoconstriction, possibly due to adaptation to increased cerebral vascular pressure during microgravity55.
The present study did not observe any effects on retinal arteries. Previous studies have found a more significant impact on the arteries. Bed rest induces multifactorial physiological changes in vascular function and hemodynamics. The duration of exposure, partial pressures of O2 and CO2, metabolic tissue demands, the autonomic nervous system, and individual factors such as sex, age, or adaptability may all contribute to these physiological changes. When evaluating the effects of unloading, it is crucial to consider all potential factors that could influence the observed effects. Therefore, future studies should encompass the assessment of several other parameters to corroborate the microcirculatory findings of the present study.
Limitations
The small sample size can be named as a study limitation. However, most other bed rest studies are of similar size because of challenging operational and logistic procedures. However, the strict supervision of bed rest participants reduces the experimental variability, and the repeated measures design is a strength for the statistical analysis. Because of this study design, bed rest studies typically generate reliable results56,57,58. Another limitation is that only men participated in the study. However, previous studies have shown that physiological responses to bed rest vary by sex and should be assessed individually. Future long-term studies with larger sample sizes, including female participants, are warranted to investigate the possible effects of inactivity (e.g. bed rest or microgravity) on the microcirculation. A possible limitation of our study is the 0° tilt of the bed during bed rest, whereas the usual model used to simulate weightlessness has a tilt of − 6°. However, it can be assumed that the -6° tilt only accelerates the physiological changes caused by horizontal bed rest, which can be observed in any type of bed rest7,59. Finally, evaluating retinal images involves a highly subjective procedure that often necessitates manual fine-tuning, introducing a potential for errors that could impact the outcomes. Nevertheless, we maintain confidence in the integrity of our results, as all data analysis was meticulously carried out by a skilled and experienced grader, ensuring consistency throughout the process.
Conclusion and future directions
The present study investigated the effects of ten days of bed rest on retinal microcirculation parameters. A reduction of 6.5% (14.5 µm) in retinal venular diameter, summarized as CRVE, was observed at the end of the ten days compared to baseline measurements. No significant changes in arteriolar diameters were noted. The results suggest adaptation to bed rest exposure, which could be related to hypovolemia and cardiac deconditioning. Further research with a longer duration of bed rest is warranted to confirm and expand upon these findings.
Finally, a research gap exists regarding the effects gravitational changes have on retinal volumetric blood flow. Future research should, together, also consider assessment of volumetric blood flow along with retinal vessel diameters, taking into account the operational reliability of the measurements in bed rest experimental setups. For instance, Riva et al. reported average total volumetric flow rates of 33 ± 9.6 μL/minute for retinal arterial circulation and 34 ± 6.3 μL/minute for retinal venous circulation60, corroborating the observations of Feke et al., who noted comparable total retinal blood flow rates in both arterial and venous vessels61. Additionally, Garhofer et al. found that retinal venular blood flow was 42.1 ± 13.0 μL/minute, a value not significantly different from that observed in the arterioles at 43.3 ± 12.1 μL/minute62. Indeed future investigations of how shifts in the body fluids during central hypovolemia and/or microgravity influence retinal blood flow, using technologies such as Laser Doppler Velocimetry or Optical Coherence Tomography, could provide important information regarding retinal vasculature physiology.
Data availability
All data generated or analyzed during this study are included in this published article. For any specific additional inquiries or requests for further information, please contact the corresponding author of this manuscript.
Abbreviations
- CRAE:
-
Central retinal artery equivalent
- CRVE:
-
Central retinal vein equivalent
- AVR:
-
Artery-to-vein ratio
- V̇O2 max.:
-
Maximal oxygen uptake
- MLM:
-
Mixed linear modelling
- BDC:
-
Baseline
- BR1-BR10:
-
First to tenth day of bed rest
- R + 1:
-
One day after re-ambulation
References
Malmivaara, A. et al. The treatment of acute low back pain—Bed rest, exercises, or ordinary activity?. N. Engl. J. Med. 332(6), 351–5. https://doi.org/10.1056/NEJM199502093320602 (1995).
Waddell, G., Feder, G. & Lewis, M. Systematic reviews of bed rest and advice to stay active for acute low back pain. Br. J. Gen. Pract. 47(423), 647–52 (1997).
Thomovsky, S. A. The physiology associated with “Bed Rest” and inactivity and how it may relate to the veterinary patient with spinal cord injury and physical rehabilitation. Front. Vet. Sci. 8, 601914 (2021).
Parola, V. et al. Rehabilitation programs for bedridden patients with prolonged immobility: A scoping review protocol. Int. J. Environ. Res. Public Health 18(22), 12033 (2021).
Goswami, N., Blaber, A. P., Hinghofer-Szalkay, H. & Montani, J. P. Orthostatic intolerance in older persons: Etiology and countermeasures. Front. Physiol. https://doi.org/10.3389/fphys.2017.00803 (2017).
Goswami, N. Spaceflight meets geriatrics!. Front. Physiol. https://doi.org/10.3389/conf.fphys.2018.26.00022 (2018).
Goswami, N., Batzel, J. J. & Valenti, G. Human Systems Physiology (Generation and Applications of Extra-Terrestrial Environments on Earth. River Publishers, 2015).
Goswami, N. et al. Human physiology adaptation to altered gravity environments. Acta Astronaut. 189, 216–21 (2021).
Hargens, A. R. & Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 120(8), 891–903. https://doi.org/10.1152/japplphysiol.00935.2015 (2016).
Mulavara, A. P. et al. Physiological and functional alterations after spaceflight and bed rest. Med. Sci. Sports Exerc. 50(9), 1961–1980 (2018).
Patel, K. et al. Effect of postural changes on cardiovascular parameters across gender. Medicine (Baltimore) 95(28), e4149 (2016).
Convertino, V. A. Cardiovascular consequences of bed rest: Effect on maximal oxygen uptake. Med. Sci. Sports Exerc. 29(2), 191–196 (1997).
Blaber, A. P., Hinghofer-Szalkay, H. & Goswami, N. Blood volume redistribution during hypovolemia. Aviat. Space Environ. Med. 84(1), 59–64 (2013).
Chobanian, A. V., Lille, R. D., Tercyak, A. & Blevins, P. The metabolic and hemodynamic effects of prolonged bed rest in normal subjects. Circulation 49(3), 551–9. https://doi.org/10.1161/01.CIR.49.3.551 (1974).
Levine, B. D., Zuckerman, J. H. & Pawelczyk, J. A. Cardiac atrophy after bed-rest deconditioning. Circulation 96(2), 517–25. https://doi.org/10.1161/01.CIR.96.2.517 (1997).
Convertino, V. A. Clinical aspects of the control of plasma volume at microgravity and during return to one gravity. Med. Sci. Sports Exerc. 28(10), 45 (1996).
Fortney, S. M., Turner, C., Steinmann, L., Driscoll, T. & Alfrey, C. Blood volume responses of men and women to bed rest. J. Clin. Pharmacol. 34(5), 434–9. https://doi.org/10.1002/j.1552-4604.1994.tb04984.x (1994).
Iwasaki, K. I., Zhang, R., Perhonen, M. A., Zuckerman, J. H. & Levine, B. D. Reduced baroreflex control of heart period after bed rest is normalized by acute plasma volume restoration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287(5), R1256-62. https://doi.org/10.1152/ajpregu.00613.2002 (2004).
Convertino, V. A., Doerr, D. F., Ludwig, D. A. & Vernikos, J. Effect of simulated microgravity on cardiopulmonary baroreflex control of forearm vascular resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 266(6), R1962-9. https://doi.org/10.1152/ajpregu.1994.266.6.r1962 (1994).
Mahdy, A. et al. A pilot study: Hypertension, endothelial dysfunction and retinal microvasculature in rheumatic autoimmune diseases. J. Clin. Med. 10(18), 4067 (2021).
Marincowitz, C. et al. Vascular health assessment with flow-mediated dilatation and retinal image analysis: A pilot study in an adult population from Cape Town. Cardiovasc. J. Afr. 32(3), 133–40. https://doi.org/10.5830/CVJA-2020-046 (2021).
Louwies, T. et al. Microvascular reactivity in rehabilitating cardiac patients based on measurements of retinal blood vessel diameters. Microvasc. Res. 124, 25–29 (2019).
Hosák, L. et al. Retinal arteriolar and venular diameters are widened in patients with schizophrenia. Psychiatry Clin. Neurosci. 74(11), 619–21. https://doi.org/10.1111/pcn.13123 (2020).
Bird, B. & Stawicki, S. P. Anatomy, head and neck, ophthalmic arteries. In StatPearls, Treasure Island (FL) (eds Bird, B. & Stawicki, S. P.) (StatPearls Publishing, 2022).
De Ciuceis, C. et al. Structural alterations of subcutaneous small-resistance arteries may predict major cardiovascular events in patients with hypertension. Am. J. Hypertens. 20(8), 846–52. https://doi.org/10.1016/j.amjhyper.2007.03.016 (2007).
Rizzoni, D. et al. Prognostic significance of small-artery structure in hypertension. Circulation 108(18), 2230–5. https://doi.org/10.1161/01.CIR.0000095031.51492.C5 (2003).
Wong, T. Y. et al. Retinal arteriolar diameter and risk for hypertension. Ann. Intern. Med. 140(4), 248–55 (2004).
Ikram, M. K. et al. Retinal vessel diameters and risk of hypertension. Hypertension 47(2), 189–94. https://doi.org/10.1161/01.HYP.0000199104.61945.33 (2006).
Tanabe, Y. et al. Retinal arteriolar narrowing predicts 5-year risk of hypertension in Japanese people: The funagata study. Microcirculation 17(2), 94–102. https://doi.org/10.1111/j.1549-8719.2009.00006.x (2010).
Nguyen, T. T. & Wong, T. Y. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol. Metab. 17(7), 262–8 (2006).
Wong, T. Y. & Mitchell, P. Hypertensive retinopathy. N. Engl. J. Med. 351(22), 2310–7. https://doi.org/10.1056/NEJMra032865 (2004).
Wong, T. & Mitchell, P. The eye in hypertension. Lancet 369(9559), 425–35 (2007).
Wong, T. Y. et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: The cardiovascular health study. Arch. Intern. Med. 166(21), 2388–94. https://doi.org/10.1001/archinte.166.21.2388 (2006).
Wang, J. J. et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur. Heart J. 28(16), 1984–92. https://doi.org/10.1093/eurheartj/ehm221 (2007).
Taibbi, G. et al. Opposite response of blood vessels in the retina to 6° head-down tilt and long-duration microgravity. NPJ Microgravity 7, 38 (2021).
Vyas, R. J. et al. Decreased vascular patterning in the retinas of astronaut crew members as new measure of ocular damage in spaceflight-associated neuro-ocular syndrome. Investig. Ophthalmol. Vis. Sci. 61(14), 34 (2020).
Louwies, T. et al. Separate and combined effects of hypoxia and horizontal bed rest on retinal blood vessel diameters. Investig. Ophthalmol. Vis. Sci. 57(11), 4927–32 (2016).
Mader, T. H. et al. Intraocular pressure and retinal vascular changes during transient exposure to microgravity. Am. J. Ophthalmol. 115(3), 347–50 (1993).
Baer, R. M. & Hill, D. W. Retinal vessel responses to passive tilting. Eye (Lond.) 4(Pt 5), 751–756 (1990).
Biolo, G. et al. Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am. J. Clin. Nutr. 88(4), 950–958 (2008).
Monti, E. et al. Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J. Physiol. 599(12), 3037–61. https://doi.org/10.1113/JP281365 (2021).
Khan, A. et al. Retinal vessel multifractals predict pial collateral status in patients with acute ischemic stroke. PLoS One 17(5), e0267837. https://doi.org/10.1371/journal.pone.0267837 (2022).
De Boever, P., Louwies, T., Provost, E., IntPanis, L. & Nawrot, T. S. Fundus photography as a convenient tool to study microvascular responses to cardiovascular disease risk factors in epidemiological studies. J. Vis. Exp. 92, e51904 (2014).
Knudtson, M. D. et al. Revised formulas for summarizing retinal vessel diameters. Curr. Eye Res. 27(3), 143–149 (2003).
Taibbi, G. et al. Opposite response of blood vessels in the retina to 6° head-down tilt and long-duration microgravity. NPJ Microgravity 7, 38 (2021).
Vyas, R. J. et al. Decreased vascular patterning in the retinas of astronaut crew members as new measure of ocular damage in spaceflight-associated neuro-ocular syndrome. Investig. Ophthalmol. Vis. Sci. 61(14), 34. https://doi.org/10.1167/iovs.61.14.34 (2020).
Louwies, T. et al. Separate and combined effects of hypoxia and horizontal bed rest on retinal blood vessel diameters. Investig. Ophthalmol. Vis. Sci. 57(11), 4927–32. https://doi.org/10.1167/iovs.16-19968 (2016).
Mader, C. O. L. T. H. et al. Intraocular pressure and retinal vascular changes during transient exposure to microgravity. Am. J. Ophthalmol. 115(3), 347–50 (1993).
Baer, R. M. & Hill, D. W. Retinal vessel responses to passive tilting. Eye (Lond.) 4(Pt 5), 751–756 (1990).
Marshall-Goebel, K. et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw. Open. 2(11), e1915011 (2019).
Harris, K. et al. Search for venous endothelial biomarkers heralding venous thromboembolism in space: A qualitative systematic review of terrestrial studies. Front. Physiol. https://doi.org/10.3389/fphys.2022.885183 (2022).
Harris, K. M. et al. Pathophysiology, risk, diagnosis, and management of venous thrombosis in space: Where are we now?. NPJ Microgravity 9(1), 17 (2023).
Harris, K. M. et al. Going against the flow: Are venous thromboembolism and impaired cerebral drainage critical risks for spaceflight?. J. Appl. Physiol. 132(1), 270–273 (2022).
Du, J. et al. Alterations in cerebral hemodynamics during microgravity: A literature review. Med. Sci. Monit. 27, e928108-1-e928108-9 (2021).
Gazenko, O. G., Genin, A. M. & Egorov, A. D. Summary of medical investigations in the U.S.S.R. manned space missions. Acta Astronaut. 8(9), 907–17 (1981).
Waha, J. E. et al. Effects of exercise and nutrition on the coagulation system during bedrest immobilization. Medicine (Baltimore) 94(38), e1555 (2015).
Goswami, N. et al. Effect of computerized cognitive training with virtual spatial navigation task during bed rest immobilization and recovery on vascular function: a pilot study. Clin. Interv. Aging 10, 453–459 (2015).
Cvirn, G. et al. Bed rest does not induce hypercoagulability. Eur. J. Clin. Investig. 45(1), 63–69 (2015).
Goswami, N., van Loon, J. J. W. A., Roessler, A., Blaber, A. P. & White, O. Editorial: Gravitational physiology, aging and medicine. Front. Physiol. 10, 1338 (2019).
Riva, C. E., Grunwald, J. E., Sinclair, S. H. & Petrig, B. L. Blood velocity and volumetric flow rate in human retinal vessels. Investig. Ophthalmol. Vis. Sci. 26(8), 1124–1132 (1985).
Feke, G. T. et al. Blood flow in the normal human retina. Investig. Ophthalmol. Vis Sci. 30(1), 58–65 (1989).
Garhofer, G., Werkmeister, R., Dragostinoff, N. & Schmetterer, L. Retinal blood flow in healthy young subjects. Investig. Ophthalmol. Vis. Sci. 53(2), 698–703 (2012).
Acknowledgements
The authors thank the participants for their priceless time and effort in collecting the data. We sincerely thank the Doctoral School of Translational Molecular and Cellular Bioscience at the Medical University of Graz for their vital resources that significantly contributed to the completion of this publication.
Funding
The present study was funded by ASI, MARS-PRE, Project, n. DC-VUM-2017-006. The authors thank ASI for granting these funds to allow all the experiments to be performed.
Author information
Authors and Affiliations
Contributions
Conceptualization, B.Š., R.P., M.N. and N.G.; methodology, A.S., G.M.C., B.Š. and N.G.; contributed to data collection, G.M.C, B.S., and D.Z.; validation, B.Š., P.D.B. and N.G.; assessment and data processing, A.S., K.S.-Z., G.M.C. and B.Š.; formal analysis, A.S., and B.Š.; data curation, A.S., and G.M.C.; writing—original draft preparation, A.S.; writing—review and editing, G.M.C., D.Z., B.Š., K.S.-Z., B.S., P.M.F., B.N.N.–CH., O.Š., H.S., P.D.B, and N.G. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saloň, A., Çiftci, G.M., Zubac, D. et al. Retinal venular vessel diameters are smaller during ten days of bed rest. Sci Rep 13, 19258 (2023). https://doi.org/10.1038/s41598-023-46177-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46177-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.