Abstract
Camera-trapping and capture-recapture models are the most widely used tools for estimating densities of wild felids that have unique coat patterns, such as Eurasian lynx. However, studies dealing with this species are predominantly on a short-term basis and our knowledge of temporal trends and population persistence is still scarce. By using systematic camera-trapping and spatial capture-recapture models, we estimated lynx densities and evaluated density fluctuations, apparent survival, transition rate and individual's turnover during five consecutive seasons at three different sites situated in the Czech–Slovak–Polish borderland at the periphery of the Western Carpathians. Our density estimates vary between 0.26 and 1.85 lynx/100 km2 suitable habitat and represent the lowest and the highest lynx densities reported from the Carpathians. We recorded 1.5–4.1-fold changes in asynchronous fluctuated densities among all study sites and seasons. Furthermore, we detected high individual’s turnover (on average 46.3 ± 8.06% in all independent lynx and 37.6 ± 4.22% in adults) as well as low persistence of adults (only 3 out of 29 individuals detected in all seasons). The overall apparent survival rate was 0.63 ± 0.055 and overall transition rate between sites was 0.03 ± 0.019. Transition rate of males was significantly higher than in females, suggesting male-biased dispersal and female philopatry. Fluctuating densities and high turnover rates, in combination with documented lynx mortality, indicate that the population in our region faces several human-induced mortalities, such as poaching or lynx-vehicle collisions. These factors might restrict population growth and limit the dispersion of lynx to other subsequent areas, thus undermining the favourable conservation status of the Carpathian population. Moreover, our study demonstrates that long-term camera-trapping surveys are needed for evaluation of population trends and for reliable estimates of demographic parameters of wild territorial felids, and can be further used for establishing successful management and conservation measures.
Similar content being viewed by others
Introduction
Knowledge of demographic parameters of a population is fundamental for the successful conservation and management of many species, especially endangered ones1. Regarding large carnivores, population size estimation represents a difficult task owing to their large home ranges, low densities and cryptic nature e.g.2,3,4,5. Recent development of digital camera traps has triggered research on elusive carnivores3 and enabled conventional and spatially explicit capture–recapture modelling methods to become common tools for estimating demographic parameters of many wild felids that have unique coat patterns6.
The Eurasian lynx (Lynx lynx), an umbrella species and the flagship of predator recovery efforts throughout Europe e.g.7, represents a suitable model species for camera-trapping surveys8,9,10,11. At present, the Eurasian lynx is a fully protected species in most European countries and its conservation is further enforced by the EU’s Wild Flora and Fauna Habitats Directive, aiming for “favourable conservation status” of the population. Despite the relatively positive status of European native populations (e.g. Carelian, Baltic or Carpathian), they are likely to be threatened to varying degrees by traffic accidents, habitat fragmentation, conflicts with hunters and, to a lesser extent, with livestock breeders. These conflicts give rise to a negative attitude towards lynx conservation and often lead to retaliation in the form of illegal acts, representing the main threats for the lynx in many areas12.
Successful plans for conservation and management of lynx populations across Europe should rely on robust demographic data. Although the abundance and population density of several reintroduced populations (e.g., in the Swiss Alps, French Jura and in the Bavarian Forest) have been intensively studied8,9,10, 13, the status of their source, the Carpathian population, is based mainly on rough national estimates that are challenged by a few local studies as having been overestimated11, 14.
For twenty years (1970s–1990s), the Carpathian population became a source for successful lynx reintroductions into several areas in central, western and southern Europe15, 16. In addition, more animals are currently being captured in the Carpathians and translocated within the reinforcement and reintroduction lynx projects in Dinaric Mts, Slovenia and Palatine Forest, Germany (https://www.lifelynx.eu/ and https://snu.rlp.de/de/projekte/luchs/, respectively). This highlights the necessity to obtain robust demographic data about this native population.
Even though a noticeable lack of scientific involvement was considered the main constraint for lynx management in the Carpathians 17 years ago17, only a few studies based on short-term camera-trapping have been conducted since that time11, 18,19,20. Likewise, at the pan-European scale, most of the published density estimates are based only on short-term camera-trapping surveys conducted within one or two seasons e.g.9, 13. However, long-term studies conducted on other felids, e.g. tigers, revealed significant annual fluctuations in densities21, 22 or in the turnover rate23. Indeed, previous research of the Alpine population also suggested that lynx density can fluctuate between years24.
Species abundance can also vary in space depending on several environmental variables and also the geographical position in species distribution or historical range25. Core areas should have higher density and lower turnover compared to the edges, according to the centre distribution hypothesis26 and the centre-periphery hypothesis27. Although demographic parameters of populations often do not follow these expectations25, no study thus far has investigated demographic patterns in the continuous part of Eurasian lynx distribution range, although, e.g., lynx census in Sweden and Norway revealed a substantial variation of family group densities in Scandinavian population28.
The aim of this study was to evaluate fluctuations in the density of the Eurasian lynx at the core–edge gradient of its distribution range in the Western Carpathians, and to assess other demographic parameters—apparent survival, transition probability and the turnover of individual lynx within the studied local populations. We expected higher population densities and higher apparent survival within the core compared to the edge. However, we hypothesized that the apparent survival would be higher and the turnover and transition rate would be lower in females (due to male-biased dispersal29). This study helps to fill the gap in the knowledge of the native Carpathian lynx population and provides the first multi-seasonal population dynamics data about this elusive carnivore.
Methods
Study area
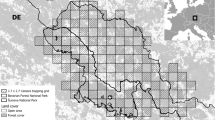
The study was conducted in the Czech-Slovak-Polish borderland at the periphery of the Western Carpathians. We chose three model study sites: Beskydy, Javorníky and Kysuce (Fig. 1). The „Beskydy” site is situated at the edge of the most western range, the site „Kysuce'' is near the core of West-Carpathian lynx distribution and breeding stronghold in Slovakia30,31,32) and the site “Javorníky” is situated in the middle of this edge-core gradient (Fig. 1). Among all study sites, altitude ranges from 350 to 1 324 m a.s.l., which causes a cold mountain climate with average year temperatures from 2 to 7 degrees. Yearly mean precipitation is 800–1 400 mm, and the ground is usually covered with snow from mid-November to late March or April33,34,35. Forests cover 70% of the whole study area (1 609 km2) and are dominated by Norway spruce (Picea abies), mainly in the form of plantations, and by beech (Fagus sylvatica). Only small areas of natural forests are present, situated primarily in protected natural reserves. The landscape in all sites is intensively used for diverse human activities. Besides forestry and hunting practices, there are also high levels of tourism and grazing activities. Human density ranges from 80 to 192 inhabitants/km2, although these values are highly irregular with most people concentrated in towns and villages36, 37. The level of landscape fragmentation by infrastructures, such as roads, railways or settlements, shows a contrasting gradient—rather remote and homogeneous mountain ranges are surrounded by intensively used valleys and basins with high human population densities38. In the Kysuce site, permanent presence and long-term reproduction of Eurasian lynx, grey wolf (Canis lupus) and brown bear (Ursus arctos) was recorded, while in the Javorníky site, only lynx and wolf reproduction was documented. In the Beskydy site, only lynx reproduction was confirmed during the study period32, author´s unpublished data.
Map of the study area, particular study sites and location of cameras with lynx detections in five seasons of systematic camera-trapping in the Czech-Slovak-Polish borderland situated at the western edge of the Carpathian Mountains. Minimum convex polygons (MCP) were enlarged by buffers resulting in a state-space in which the density of lynx was estimated. The EEA squares (10 × 10 km) in the inset show the permanent lynx distribution according to Chapron et al.62. The figure was created in ArcMap 10.7.1 (https://desktop.arcgis.com/en/arcmap/)43.
Camera-trapping
Camera-trapping was conducted throughout an 80-day winter period (November–February) and during five consecutive seasons (2015, 2016, 2017, 2018, 2019—the year means the beginning of the camera-trapping period lasting to the next year) in all study sites. The length of the camera-trapping survey was set according to the recommendations of Weingarth et al.39. Each period was divided into 16 trapping occasions of 5 days each8, 9, 39. The study sites encompassed by the outermost cameras was estimated using the minimum convex polygon (MCP40) and ranged from 811.10 to 918.49 km2 in the Beskydy site, from 223.79 to 273.35 km2 in the Javorníky site and from 320.60 to 417.88 km2 in the Kysuce site (Fig. 1). To ensure that all animals had a non-zero capture probability41, we placed cameras systematically to avoid any gap larger than the smallest home range of a female lynx in the Carpathians and set at least two cameras per female home range3. The smallest published home range size for female lynx is 124 km2 in the Carpathians42; therefore its radius (6.30 km) was used as the maximum spacing between cameras. The availability of suitable cameras (n = 16 to 60; Table 1) resulted in the average distance to the nearest neighbouring cameras (Point distance tool in ArcMap 10.7.143) from 2.08 km (standard deviation, hereafter SD, ± 1.18) to 2.37 km (± 0.95 km) in Beskydy, from 1.24 km (± 0.94) to 2.28 km (± 1.04) in Javorníky and from 1.81 km (± 1.33) to 3.29 km (± 0.69) in the Kysuce site (Fig. 1, Table S1). Thefts of camera traps in the beginning of trapping sessions caused the maximum spacing to be higher than 6.3 km in two cases during a 5-year period (Beskydy 2017 and Kysuce 2016). One camera with white flash or infrared camera (Cuddeback Ambush, Cuddeback C123, Cuddeback H20 IR, Cuddeback Green Bay, USA; Browning Spec Ops Advantage, Browning Morgan, USA) was installed at each camera-trapping site. Selection of camera sites with the highest probability of lynx detection was based on our previous knowledge obtained by snow tracking and opportunistic camera-trapping (game trails, marking sites and rocky ridges32, 44, 45).
Identification of individuals and determination of social status
Reliable identification of captured animals (see Fig. S2—Photographic database of independent lynx) was ensured by using a detailed photo-database of lynx individuals collected during the opportunistic camera-trapping (from 2009 in Beskydy and Javorníky, from 2013 in Kysuce) conducted throughout the year as well as by using data from previous deterministic surveys in all study sites32, 44,45,46. Multiple photos were obtained, especially at marking sites, allowing us to assign both body flanks to one individual. Individuals were identified by comparison of coat patterns, particularly on the hind limbs, fore limbs and flanks8, 11. At least three well-trained observers in each site were involved in the intensive identification process of identifying lynx individuals by using an online multipurpose photographic database and cross-check verification. Identification of individuals and data processing followed minimum camera-trapping standards reported by Choo et al.47.
Sex and age category of individuals was determined from clearly visible genital parts and captures of leading females with kittens in the pictures, as well as from videos gained through previous deterministic and opportunistic camera-trapping32, 44,45,46 or through genetic analyses29. Lynx individuals detected during the five seasons of deterministic camera-trapping were divided according to their social status into three different categories: adult (A)—individual older than two years that was present for at least 12 months in the study site (territorial lynx)29; subadult (S)—independent individual in the second year of life with well-known life history (known mother and birth year); not determined status (ND)—all other individuals with unknown or not determined status (Table S2).
Spatially explicit capture-recapture model
Only independent lynx individuals > 1 year old (adults and subadults) were integrated into analyses9. Multiple captures of the same individual at a particular trap site, during the same trapping occasion, were considered as a single capture8. The capture of kittens of a known leading female was considered as a capture of that female48. Lynx densities were estimated by means of spatially explicit capture-recapture analyses (SCR). For SCR analyses, we used the software SPACECAP version 1.1.049, 50 implemented within R software v. 3.6.051. To meet the used model key assumptions6, 9, we used trap response present, spatial capture-recapture model half-normal detection and Bernoulli´s encounter process with the same parameter values applied (Markov chains with 80 000 iterations, a burn-in period 40 000, thinning rate 3 and data augmentation 100) as in Kubala et al.11. The assumption of demographic population closure was tested through CloseTest52, 53. CloseTest suggested population closure in 8 out of 15 seasons (Table S4). Since results could be potentially influenced by the fact that 3 individuals moved between sites within one season, we also calculated a scenario where only captures matching the site of the first capture in that season were retained (Table S6). These changes had no significant effect on total estimates of population density so we present results where population density is estimated independently for each site with all individuals.
To find the minimum buffer width for which density estimates stabilize, we created a series of state-spaces with buffers ranging from 2 to 24 km (with increment of 2 km) around the MCP surrounding all camera traps11. The state-space was described as a grid of 576–1999 equally spaced potential home range centres (1.5 × 1.5) resulting in state-space sizes ranging between 1269 and 4497.25 km2 (Table 1 and Fig. 1). Lynx densities were estimated per 100 km2 of suitable habitat. Proportions of suitable and unsuitable habitat were derived from CORINE Land Cover 201254, where all different types of forests, shrubs and natural grasslands were considered as suitable habitat for lynx, following Kubala et al.11. Chain convergence was tested using Gelman-Rubin's test55 where values below 1.1 indicate convergence56. Finally, estimates of lynx density obtained in all study sites were compared between each pair of consecutive seasons using the calculation of the coefficient of variation and fold changes. The Kruskal–Wallis (KW) test was used to test differences in density estimates among study sites and seasons. The Spearman's rank correlation coefficient (SRCC) was used to test trends in average annual densities over the five seasons. The calculations were conducted in R51.
The multistate closed robust design
The multistate closed robust design models were run in MARK57 and estimated three parameters per site: (i) apparent survival rate (φ), which is the probability of surviving and staying in a sample site; (ii) transition probability (ψ), which represents the probability of moving from one site to another; and, (iii) capture probability (P). The modelling approach assumes that no site transitions occurred within a primary period, i.e. season58, 59. However, we acknowledge that 2.2% of the captures violated this assumption. One adjustment was made to minimize this violation, using the approach of Chabanne et al.60. If an individual was captured in two different sites within a primary period, we retained captures matching the site of the first capture recorded in that primary period. We also analysed the dataset where the captures matching site of the second captures were retained and the results were similar (same survival rate and the best model selected); thus we present only the first option here. Models were ranked using the Akaike information criterion (AICc61). The model with most support by AICc (highest AICc weight) was selected as the most parsimonious model.
Estimation of individual’s turnover
The individual’s turnover was calculated as the proportion of individuals that were recorded during a monitoring survey in the previous season but were not recorded in a consecutive season. The individual’s turnover between consecutive seasons was calculated for different sexes and age categories (all individuals vs. adults). If an individual was captured in two different sites within the same season, the calculation of turnover rate included this particular individual only in the site where it was captured for the first time (the same way as it was done in multi-state closed robust design dataset).
Ethics approval
The research used non-invasive methods only. The entry to protected areas was approved by Trenčín District Office (No. OU-TN-OSZP1-2014/49/3475) and by the agreement with the State Nature Conservancy of the Slovak Republic (No. ŠOP SR/12/2018).
Results
Camera-trapping survey
In total, we identified 53 independent lynx within 737 unique captures obtained during 44 735 effective trap days from all sites and seasons. Sex was identified for 47 individuals (29 males, 18 females), while 6 individuals remained undetermined. The age was successfully identified in 33 individuals, of which 28 were adults and 5 were subadults. For 13 individuals, we were not able to determine their social status. The status of these individuals did not change during the survey. Additionally, the age category changed for 6 individuals from subadult to adult and for one individual from undetermined to adult (Table 1, Table S2). Camera-trapping efficiency ranged from 83.8 to 99.2% among all sites and seasons. Five individuals were recorded in two different study sites—four in Beskydy and Javorníky, and one in Kysuce and Javorníky. Moreover, three out of these five individuals were recorded in two different sites within the same camera-trapping season (Table S2). Altogether, 93 pictures and videos of lynx were excluded from analyses due to their insufficient quality for lynx determination (see minimum reporting standards in Table S3).
Estimates of population density
Density estimates decreased rapidly with increasing buffer width and began to stabilize at buffer size ≥ 8 km. Stabilisation among all study sites and seasons occurred mostly in buffer size 10 and 12 km (Table S1). The posterior mean baseline encounter rate (λ0) (posterior SD) varied from 0.02 (± 0.01) to 0.22 (± 0.06) and the posterior movement parameter varied from 3.17 (± 0.69) to 9.83 (± 0.44) km among all sites and seasons (Table 2).
Overall, mean posterior densities varied between 0.26 (± 0.07) and 1.85 (± 0.35) independent lynx/100 km2 suitable habitat. In particular, posterior densities ranged between 0.26 (± 0.07) and 1.08 (± 1.58) in Beskydy (mean 0.54 lynx/100 km2), between 0.59 (± 0.16) and 1.19 (± 0.27) in Javorníky (mean 0.79 lynx/100 km2) and between 0.97 (± 0.24) and 1.85 (± 0.35) independent lynx/100 km2 suitable habitat in the Kysuce site (mean 1.41 lynx/100 km2) (Fig. 2, Table 2). Over the period of this study we recorded a 4.1-fold change in lynx density in Beskydy, a 1.9-fold change in Kysuce and a 1.5-fold change in Javorníky (Fig. 2). The coefficient of variation (CV) was the highest (58.7%) in Beskydy, followed by the Kysuce (23.9%) and the Javorníky sites (22.2%). The average annual density estimates calculated for all three sites together ranged from 0.69 to 1.20 lynx/100 km2 with no significant increase over the five seasons (SRCC, RS = 6, p = 0.23). Density estimates varied significantly between all study sites (KW test, χ2 = 9.63, p = 0.008) but not between seasons (KW test, χ2 = 2.16, p = 0.7). Bayesian P values suggesting model adequacy ranged from 0.49 to 0.88 among all sites and seasons. Gelman-Rubin diagnostics indicated convergence for all models. Values of all estimated parameters were below 1.1, except for season 2017 in Beskydy.
Estimates of apparent survival and transition probability
The best fitting model according to the AICc weight was that of constant apparent survival, constant transition rate and capture probability varied by site and season [P(site × season)]. The difference from the models where the transition rate and apparent survival varied by site or sex were not particularly high (ΔAICc ˂ 2.2), thus suggesting those models as having good support as the best one61. The best competing models are listed in Table 3, however not all model combinations converged and we were therefore limited in the number of models available.
The overall apparent survival rate was 0.63 ± 0.055 and overall transition rate 0.03 ± 0.019 according to the best model. While not significant, estimates of apparent survival rate when varying by sex was higher for males (0.67 ± 0.072) than females (0.6 ± 0.087). Lynxes with undetermined sex had the lowest survival (0.47). Survival rate also varied (non-significantly) among sites with higher apparent survival rate estimated in Beskydy (0.70 ± 0.102) and Javorníky (0.74 ± 0.092) than in Kysuce (0.52 ± 0.085). Transition rate of males was 0.05 ± 0.029 between season, in contrast to none for females (˂0.001). Transition rate of undetermined sex, however, was much higher (0.54 ± 0.00). Capture probability was significantly higher in Javorníky (0.54 ± 0.02) than in Beskydy (0.24 ± 0.02) and Kysuce (0.24 ± 0.016) (Figure S1).
Turnover and persistence of individuals
In total, the average turnover was 46.3 ± 8.06% including all independent lynx (n = 53) and 37.6 ± 4.22% for adults (n = 33). The overall turnover among all sites and seasons varied from 33.3 to 50% in males and from 37.5 to 62.5% in females. In adult males and adult females, the total average turnover reached 34.2 ± 5.44% and 42.6 ± 19.2%, respectively (Fig. 3, Table S5). Only three individuals were captured during all seasons and nine individuals in ≥ 3 seasons (Table S2).
Discussion
Density estimates
More accurate estimation of several population parameters has become possible thanks to the currently widespread use of camera traps and recent developments in spatial models63, 64. Our overall lynx density range obtained within this study (0.26–1.85 lynx/100 km2 suitable habitat) corresponds to spatial lynx density estimates reported from other areas in Europe e.g., French Jura and Vosges Mountains (0.24–0.91 lynx/100 km2)13 or Swiss Alps (1.04–1.47 lynx/100 km2)9, 48. Moreover, the mean posterior density of 0.26 lynx/100 km2 from the Beskydy site is the second lowest spatial density reported from Europe just higher than the Doubs (0.24 lynx/100 km2)13.
The average density values obtained in three study sites (Beskydy 0.54 lynx/100 km2, Javorníky 0.79 lynx/100 km2, Kysuce 1.41 lynx/100 km2) are in accordance with the centre-periphery hypothesis as well as the “abundant centre” distribution hypothesis, which assume that species reach their highest abundance in the centre of their range and decline in abundance toward the range edges26. This hypothesis is also supported by the density values reported in the previous studies from the Slovak Carpathians. In particular, lynx density from the western edge (Beskydy) was similar to density reported in the Štiavnica Mts (0.58 lynx /100 km2 suitable habitat)11, situated at the southern periphery of lynx distribution in Slovakia. The density reported from the Javorníky site was similar to the density obtained in the adjacent Strážov Mts (0.97 ± 0.25 lynx/100 km2 suitable habitat)20. The density estimated for the Kysuce site was comparable to the density values from the Muránska planina NP or the Vepor Mts (1.47 ± 0.37 and 1.20 ± 0.49 lynx/100 km2 suitable habitat, respectively), situated in the central part of Slovakia18, 19. However, our results confirmed relatively high density fluctuations in all three study sites and, thus, local densities in the central part of Slovakia may also significantly fluctuate among seasons. For example, the one-season density estimation recently reported in Velká Fatra Mts (0.81 ± 0.29 lynx/100 km2 suitable habitat)11, also situated in the centre, might represent the estimation at the lower bounds.
Non spatial and spatial capture-recapture modelling approaches have been developed and used for density estimation of populations. Similarly to Avgan et al.65, we have omitted using conventional non-spatial CR models and only the SCR model was used to estimate lynx densities. This model seems to be more reliable for lynx density estimation in comparison to standard closed CR models13, 48. Besides the model used for density estimation, there are also several other factors that might affect density estimates and make the comparison between studies disputable.
First, density estimation can be influenced by the length of the camera-trapping survey and the season in which it is conducted. We conducted our deterministic survey during an 80-day period, although the majority of studies from the Carpathians and other European populations used 60-day length e.g.9, 11, 13, 18. The extended period length in our study was set in order to obtain a sufficient number of captures and re-captures of individuals (the most crucial factor in obtaining a reliable and robust density estimation) mostly in the Beskydy, the site with the lowest density values situated at the periphery. Moreover, the additional test of demographic closure supported the 80-day period rather than 60-day (Table S4) and a longer camera-trapping survey is highly recommended to obtain sufficient data for reliable estimates of demographic parameters39, 66. Although we used an extended period length, we conducted our survey outside the mating season and dispersal period to avoid violating the demographic closure. However, in the 2017 season in Beskydy we detected no convergence in chains and a relatively high level of standard deviation of posterior density estimates. Results obtained in this particular season could be affected by several factors, such as several malfunctions of cameras, low recapture rates of individuals67 or different movement patterns among sex and social categories of lynx9, which might decrease detection probability.
Demographic changes
Substantial interannual density fluctuation and fold changes (1.5–4.1-fold change) of the native Carpathian populations recorded within our study supported previous findings of fluctuated densities observed on reintroduced lynx populations in Western Europe. Comparable fold changes (up to threefold change) of lynx densities were observed in the North Western Alps24, Swiss Jura Mts.68 and French Jura and Vosges Mts.13. However, previous long-term density estimates in Jura Mts., based mainly on telemetry research, report a fairly constant trend69. Generally, similar density fluctuations using long-term camera-trapping surveys were also reported also other territorial felids, e.g. tigers21, 22 and jaguars66. In contrast, no substantial fluctuation with relatively stable trends was recorded for cheetah70.
The overall apparent survival, which consists of true survival and permanent emigration, was 63% in all sites. The advantage of the multi-state closed robust design approach is to estimate transition rate between sites. Our transition rate among years and any site was about 3% per year, which indicates a small but consistent connection between populations of lynx from each site. No camera trap study estimated the apparent survival of Eurasian lynx, therefore limiting our comparison with only a few older studies based on radiotelemetry. Survival reached 63% in North-Eastern Poland71, 76% for adults and 53% for subadults in Swiss Jura72 and it varied by sex and age category in three study sites in Scandinavia73: survival rates ranged within 77–83% for adult males, 85–86% for adult females, 57–74% for subadult males and 43–90% for subadult females. Although our camera-trapping study did not allow for the estimation of apparent survival for adults and subadults categories separately due to limited history of all individuals, it seems our overall survival rates are among the lowest reported in Europe, also taking into account the low transition rate. Females particularly did not move among sites indicating strong female philopatry and male biased dispersal, as was also documented by the genetic analyses in our area29 and in Finland74.
We found high individual’s turnover (average for all independent lynx 46.3%, and for adults 37.6%) and low persistence of adults over the five consecutive seasons (3 out of 29 individuals). These long-term findings agree with the occasional high individual´s turnover (up to 80%) and low persistency (mean 12.7 months) of lynx individuals previously documented by pilot surveys in Štiavnica Mts. and Veľká Fatra NP11. Similarly, the high individual’s turnover was also documented in the Javorníky and Beskydy sites during our previous extensive camera-trapping survey45 and also by non-invasive genetic sampling conducted in this area29. Low individual persistence and low age of captured residents were reported during radio-tracking research in the Jura Mts.72 and most recently in the Northern Hessian subpopulation in Germany75. A high individual turnover rate (up to 89%) in combination with low persistence was reported for other felids, e.g. Geoffroy’s cats76 and tigers23.
Fluctuating densities, relatively low apparent survival and high turnover rates could be affected by several ecological (e.g. food and habitat availability, diseases, competition) and human-induced (e.g. poaching, road mortality, habitat fragmentation) factors. Here we discuss the most relevant hypotheses for observed demographic changes, starting with the least plausible one.
In a human dominated landscape, lynx distribution is shaped by a trade-off between the availability of preferred prey and the amount of human activity77,78,79. Population numbers of wild ungulates, especially roe deer as the most selected prey of lynx in Europe80, are at historical maximums in Slovakia and the Czech Republic81, 82. Therefore, it is unlikely that the observed fluctuations of lynx density have been driven by the lack of natural prey. Moreover, roe deer is more abundant in Beskydy and Javorníky than in Kysuce, while lynx density was lower in those two sites compared to the Kysuce site83. Similarly, the proportion of suitable habitat for lynx and the level of human activities are comparable among all study sites and lynx do not use all suitable habitats, especially in the Beskydy site29. Additionally, we did not observe any signs of a disease outbreak in this area. Therefore, we believe in a limited influence of the ecological factors mentioned above on asynchronous density fluctuation and high turnover rates in our study sites.
Despite the fact that a percentage of animals die naturally (diseases, intraspecific killing, aging, etc.)84, 85, we assume that a high proportion of adult mortality might be caused by anthropogenic factors as reported in other regions, e.g. Scandinavia73, Alps72 or Dinaric Mts86. We found a low survival rate and relatively high turnover of both sexes, especially adult females. This might rather indicate the influence of artificial phenomena, such as anthropogenic caused mortality, e.g. poaching87, 88 or road mortality84. Several cases of lynx poaching (n = 5), collisions with vehicle/train (n = 5) or orphaned kittens (n = 2) were documented by chance in our study sites from 2002 to 2020 (authors’ unpublished data). High anthropogenic pressure (significant level of human-induced mortality) was also documented in other areas of the Western Carpathians11, 89 and Europe90. Moreover 10% of Czech hunters surveyed in the study by88 admitted that they personally killed lynx. Occasional dips in survival caused by human-induced mortality are likely to cause drops in recruitment in subsequent years, depressing the population size as observed in Beskydy and Kysuce between seasons 2017—2018 (Fig. 2). Subsequent rebound of survival due to habitat and prey availability may result in an increased population size in following years, as seen in Beskydy and Kysuce in season 2019. Although the average annual density in our study sites over the five consecutive seasons showed a slightly increasing trend (Fig. 2), we have not observed lynx expansion westwards into other surrounding areas within the lynx historical range91. Only occasional lynx dispersals have been documented in the Moravian region over the last decade32. This underlines a poor dispersing ability of lynxes, especially females92, 93. On the other hand, human interventions might also play a significant role in limiting population expansion29, 94.
Contrary to our expectations, apparent survival rate was not higher in the core (Kysuce) and females did not have overall higher survival. Differences were not significant, but the opposite trend could be partly explained by lower capture probabilities in Kysuce than in Javorníky (Fig. S1). Another reason could be that higher population density causes higher intra-specific competition, which is reflected as depressed apparent survival rates23. More lynxes leads to higher encounter rates with hunters who perceive lynx negatively, which could also increase the social conflict and probability of illegal killing. However, these factors need more detailed investigation. In many species, demographic traits do not follow centre-peripheral hypothesis and local ecological effects may be more influential than the position of population within the range25. In other words, geographically peripheral populations are not necessarily ecologically marginal.
Conclusions for management and conservation
Average annual density estimates for the whole region (all three sites together) varied between 0.69 and 1.20 lynx/100 km2 suitable habitat and showed substantial variation in lynx density over the five seasons of systematic camera-trapping. Based on average annual densities obtained within this study, and using 28 090 km2 of suitable lynx habitat occupied by lynx in Slovakia according to Kubala et al.11, we estimate lynx population size in Slovakia to vary between 193 and 337 individuals. The lowest value (season 2015) is very similar to 197 individuals estimated by Kubala et al.11 in 2014/15 and may represent the population minimum. Estimates from season 2017 and 2019 (337 and 303 individuals, respectively) reached a similar level as the most recent estimate (280 individuals) reported by Kubala et al.20. This indicates that lynx population numbers varied within slightly lower values than those officially reported by the State Nature Conservancy for European Commission during 2013–2018 (300–400 individuals)95.
A multi-seasonal camera-trapping survey conducted in three study sites situated at the centre-periphery gradient enabled the first robust density estimation for lynx in the Western Carpathians. Since the density estimates varied greatly between consecutive seasons, our study demonstrates that long-term camera-trapping surveys might be needed not only for evaluation of population trends but for reliable estimates of population size as well. Special attention should be paid to the native populations, because these may serve as a source of individuals for repatriation and reinforcement purposes in the near future. Moreover, the fluctuating densities, relatively low apparent survival and high turnover rates presented in this study (and others e.g.11, 29) indicate that the West Carpathian population is facing several human-induced factors, which might negatively influence the otherwise favourable conservation status of this population. In order to maintain a favourable population status, we call for more rigorous investigation of illegal killing and for its reduction by establishing a network of wildlife forensic experts, by strengthening scene investigation and by prosecuting illegal activities through law enforcement. Poaching, as well as habitat loss from landscape fragmentation and an increasing number of lynx-vehicle collisions, seem to be the most limiting factors restricting population growth and dispersion of lynx in the human-dominated landscapes across Europe86, 87.
Data availability
The datasets analysed during the current study are not publicly available due to sensitivity of the occurrence data of endangered species but are available from the corresponding author on reasonable request.
References
Hayward, M. W. et al. FORUM: Ecologists need robust survey designs, sampling and analytical methods. J. Appl. Ecol. 52, 286–290 (2015).
Karanth, K. U. Estimating tiger Pantheratigris populations from camera-trap data using capture-recapture models. Biol. Conserv. 71, 333–338 (1995).
O’Connell, A. F., Nichols, J. D. & Katranth, K. U. Camera Traps in Animal Ecology: Methods and Analyses (Springer, 2011).
Molinari-Jobin, A. et al. Monitoring in the presence of species misidentification: The case of the Eurasian lynx in the Alps. Anim. Conserv. 15, 266–273 (2012).
López-Bao, J. V. et al. Toward reliable population estimates of wolves by combining spatial capture-recapture models and non-invasive DNA monitoring. Sci. Rep. 8, 2177 (2018).
Foster, R. J. & Harmsen, B. J. A critique of density estimation from camera-trap data. J. Wildl. Manage. 76, 224–236 (2012).
Rozylowicz, L., Popescu, V. D., Pǎtroescu, M. & Chişamera, G. The potential of large carnivores as conservation surrogates in the Romanian Carpathians. Biodivers. Conserv. 20, 561–579 (2011).
Weingarth, K. et al. First estimation of Eurasian lynx (Lynx lynx) abundance and density using digital cameras and capture-recapture techniques in a German national park. Anim. Biodivers. Conserv. 35, 197–207 (2012).
Pesenti, E. & Zimmermann, F. Density estimations of the Eurasian lynx (Lynx lynx) in the Swiss Alps. J. Mammal. 94, 73–81 (2013).
Blanc, L., Marboutin, E., Gatti, S. & Gimenez, O. Abundance of rare and elusive species: Empirical investigation of closed versus spatially explicit capture-recapture models with lynx as a case study. J. Wildl. Manage. 77, 372–378 (2013).
Kubala, J. et al. Robust monitoring of the Eurasian lynx Lynxlynx in the Slovak Carpathians reveals lower numbers than officially reported. Oryx 53, 548–556 (2019).
Kaczensky, P. et al. Status, Management and Distribution of Large Carnivores: Bear, Lynx, Wolf & Wolverine—in Europe (European Commission, 2013).
Gimenez, O. et al. Spatial density estimates of Eurasian lynx (Lynx lynx) in the French Jura and Vosges Mountains. Ecol. Evol. 9, 11707–11715 (2019).
Okarma, H. et al. Status of Carnivores in the Carpathian Ecoregion. Report of the Carpathian Ecoregion Initiative (2000).
Stehlík, J. Znovuvysazení rysa ostrovida Lynx lynx L. v některých evropských zemích v letech 1970–1976. Poľovnícky zborník—Folia venatoria 9, 255–265 (1979).
Červený, J. & Bufka, L. Lynx (Lynx lynx) in south-western Bohemia. Acta. Sci. Nat. Brno 30, 16–33 (1996).
Salvatori, V. et al. Hunting legislation in the Carpathian Mountains: Implications for the conservation and management of large carnivores. Wildlife Biol. 8, Pagination missing-please provide (2002).
Smolko, P. et al. Lynx monitoring in the Muránska planina NP, Slovakia and its importance for the national and European management and conservation of the species. Technical report (2018).
Kubala, J. et al. Monitoring rysa ostrovida (Lynx lynx) vo Veporských vrchoch a jeho význam pre národný a európsky manažment a ochranu druhu. Technická správa. (In Slovak) (2019).
Kubala, J. et al. Monitoring rysa ostrovida (Lynx lynx) v Strážovských vrchoch a jeho význam pre národný a európsky manažment a ochranu druhu. Technická správa. (In Slovak) (2020).
Duangchantrasiri, S. et al. Dynamics of a low-density tiger population in Southeast Asia in the context of improved law enforcement. Conserv. Biol. 30, 639–648 (2016).
Karanth, K. U., Nichols, J. D., Kumar, N. S. & Hines, J. E. Assessing tiger population dynamics using photographic capture-recapture sampling. Ecology 87, 2925–2937 (2006).
Bisht, S., Banerjee, S., Qureshi, Q. & Jhala, Y. Demography of a high-density tiger population and its implications for tiger recovery. J. Appl. Ecol. 56, 1725–1740 (2019).
Zimmermann F. et al. Abundanz und Dichte des Luchses in den Nordwestalpen : Fang-Wiederfang-Schätzung mittels Fotofallen im K-VI im Winter 2015 / 16, Vol. 41 (2016).
Pironon, S. et al. Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biol. Rev. 92, 1877–1909 (2017).
Sagarin, R. D. & Gaines, S. D. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule?. Ecol. Lett. 5, 137–147 (2002).
Eckert, C. G., Samis, K. E. & Lougheed, S. C. Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol. Ecol. 17, 1170–1188 (2008).
López-Bao, J. V. et al. Eurasian lynx fitness shows little variation across Scandinavian human-dominated landscapes. Sci. Rep. 9, 8903 (2019).
Krojerová-Prokešová, J. et al. Genetic constraints of population expansion of the Carpathian lynx at the western edge of its native distribution range in Central Europe. Heredity (Edinb). 122, (2019).
Ján, K. & Štefan, D. Mammals of Slovakia distribution, bionomy and protection. (VEDA, 2012).
Kubala, J. et al. The coat pattern in the Carpathian population of Eurasian lynx has changed: a sign of demographic bottleneck and limited connectivity. Eur. J. Wildl. Res. 66, 2 (2019).
Kutal, M. et al. Occurrence of large carnivores—Lynx lynx, Canis lupus, and Ursus arctos—and of Felis silvestris in the Czech Republic and western Slovakia in 2012–2016 (Carnivora). Lynx, new Ser. 48, 93–107.
Galvánek, J., Pietorová, E. & Matejová, M. Hodnotenie abiotických zložiek vybranej ekologicko-funkčnej jednotky. in Ochrana prírody Kysuckého regiónu a spolupráca na jeho trvalo udržateľnom rozvoji. (1996).
Tolasz, R., Miková, T., Valeriánová, A. & Voženílek, V. Atlas podnebí Česka. (2007).
Bochníček, O. Climate Atlas of Slovakia. (Slovak Hydrometeorological Institute, 2015).
Czech Statistical Office. Statistical Yearbook of the Czech Republic 2017. Accessed 9 Nov 2020. https://www.czso.cz/csu/czso/statistical-yearbook-of-the-czech-republic (2017).
Statistical Office of the Slovak Republic. Statistical Yearbook of the Slovak Republic 2017. Accessed 9 Nov 2020. https://slovak.statistics.sk:443/wps/portal?urile=wcm:path:/obsah-en-pub/publikacie/vsetkypublikacie/f3dc4a81-06ac-4fea-93b7-e0ff45a9fff6 (2017).
Romportl, D., Zyka, V. & Kutal, M. Connectivity Conservation of Large Carnivores’ Habitats in the Carpathians. in 5th European Congress of Conservation Biology (2018). https://doi.org/10.17011/conference/eccb2018/107837.
Weingarth, K. et al. Hide and seek: extended camera-trap session lengths and autumn provide best parameters for estimating lynx densities in mountainous areas. Biodivers. Conserv. 24, 2935–2952 (2015).
Mohr, C. O. Table of equivalent populations of north american small mammals. Am. Midl. Nat. 37, 223–249 (1947).
Karanth, K. U. & Nichols, J. D. Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79, 2852–2862 (1998).
Okarma, H., Sniezko, S. & Smietana, W. Home ranges of Eurasian lynx Lynxlynx in the Polish Carpathian Mountains. Wildlife Biol. 13, 481–487 (2007).
ESRI. ArcGIS Desktop. (2019).
Duľa, M., Drengubiak, P., Kutal, M., Trulík, V. & Hrdý, Ľ. Monitoring lynx in Kysuce PLA, Slovakia. (2015).
Kutal, M., Váňa, M., Bojda, M., Kutalová, L. & Suchomel, J. Camera trapping of the Eurasian lynx in the Czech-Slovakian borderland. (2015).
Duľa, M. et al. Recentný výskyt a reprodukcia rysa ostrovida (Lynx lynx) v CHKO Kysuce a NP Malá Fatra. in 75–78 (2017).
Choo, Y. R. et al. Best practices for reporting individual identification using camera trap photographs. Glob. Ecol. Conserv. 24, e01294 (2020).
Zimmermann, F., Breitenmoser-Würsten, C., Molinari-Jobin, A. & Breitenmoser, U. Optimizing the size of the area surveyed for monitoring a Eurasian lynx (Lynx lynx) population in the Swiss Alps by means of photographic capture-recapture. Integr. Zool. 8, 232–243 (2013).
Gopalaswamy, A. M. et al. Program SPACECAP: Software for estimating animal density using spatially explicit capture-recapture models. Methods Ecol. Evol. 3, 1067–1072 (2012).
Gopalaswamy, A. et al. SPACECAP: An R package for estimating animal density using spatially explicit capture-recapture models. (2014).
Team, R. C. R software. (2020).
Stanley & Burnham_1999. A closure test for capture data.Env&EcolStats.pdf.
Stanley, T. & Richards, J. CloseTest: A program for testing capture–recapture data for closure [Software Manual]. (2004).
Copernicus Programme. CORINE Land Cover 2012. http://land.copernicus.e/an-europea/orine-land-cove/lc-2012.Google Scholar (2012).
Gelman, A., Carlin, J., Stern, H. & DB, R. Bayesian data analysis.2nd edn. (2004).
Gelman, A. & Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models (Cambridge Univ, 2006).
White, G. C. & Burnham, K. P. Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139 (1999).
Arnason, A. N. Parameter estimates from mark-recapture experiments on two populations subject to migration and death. Res. Popul. Ecol. (Kyoto) 13, 97–113 (1972).
Arnason, A. N. The estimation of population size, migration rates and survival in a stratified population. Res. Popul. Ecol. (Kyoto) 15, 1–8 (1973).
Chabanne, D. B. H., Pollock, K. H., Finn, H. & Bejder, L. Applying the multistate capture–recapture robust design to characterize metapopulation structure. Methods Ecol. Evol. 8, 1547–1557 (2017).
Burnham, K. P. & Anderson, D. R. A practical information-theoretic approach. Model Sel. multimodel inference 2, (2002).
Chapron, G. et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346, 1517–1519 (2014).
Royle, J., Chandler, R. B., Sollmann, R. & Gardner, B. Spatial Capture-Recapture (Academic Press, 2014).
Rovero, F. & Zimmermann, F. Introduction. in Camera Trapping for Wildlife Research 1–7. (2016).
Avgan, B., Zimmermann, F., Güntert, M., Arikan, F. & Breitenmoser, U. The first density estimation of an isolated Eurasian lynx population in southwest Asia. Wildlife Biol. 20, 217–221 (2014).
Harmsen, B. J., Foster, R. J. & Quigley, H. Spatially explicit capture recapture density estimates: Robustness, accuracy and precision in a long-term study of jaguars (Pantheraonca). PLoS ONE 15, e0227468 (2020).
Sollmann, R., Gardner, B. & Belant, J. L. How does spatial study design influence density estimates from spatial capture-recapture models?. PLoS ONE 7, e34575 (2012).
Zimmermann, F. et al. Abondance et densité du lynx dans le Sud du Jura suisse : estimation par capture-recapture photographique dans le compartiment I , durant l ’ hiver 2014 / 15, Vol. 41 (2015).
Breitenmoser-Würsten, C. et al. Spatial and Social stability of a Eurasian lynx Lynxlynx population: an assessment of 10 years of observation in the Jura Mountains. Wildlife Biol. 13, 365–380 (2007).
Fabiano, E. C. et al. Trends in cheetah Acinonyxjubatus density in north-central Namibia. Popul. Ecol. 62, 233–243 (2020).
Jedrzejewski, W. et al. Population dynamics (1869–1994), demography, and home ranges of the lynx in Bialowieza Primeval Forest (Poland and Belarus). Ecography (Cop.) 19, 122–138 (1996).
Breitenmoser-Würsten, C., Vandel, J.-M., Zimmermann, F. & Breitenmoser, U. Demography of lynx Lynxlynx in the Jura Mountains. Wildlife Biol. 13, 381–392 (2007).
Andren, H. et al. Survival rates and causes of mortality in Eurasian lynx (Lynx lynx) in multi-use landscapes. Biol. Conserv. 131, 23–32 (2006).
Herrero, A. et al. Genetic analysis indicates spatial-dependent patterns of sex-biased dispersal in Eurasian lynx in Finland. PLoS ONE 16, e0246833 (2021).
Port, M. et al. Rise and fall of a Eurasian lynx (Lynx lynx) stepping-stone population in central Germany. Mammal Res. 66, 45–55 (2021).
Pereira, J. A. et al. Population density of Geoffroy’s cat in scrublands of central Argentina. J. Zool. 283, 37–44 (2011).
Breitenmoser, U. et al. Conservation of the lynx Lynxlynx in the Swiss Jura Mountains. Wildlife Biol. 13, 340–355 (2007).
Basille, M. et al. What shapes Eurasian lynx distribution in human dominated landscapes: selecting prey or avoiding people?. Ecography (Cop.) 32, 683–691 (2009).
Filla, M. et al. Habitat selection by Eurasian lynx (Lynx lynx) is primarily driven by avoidance of human activity during day and prey availability during night. Ecol. Evol. 7, 6367–6381 (2017).
Nowicki, P. Food habit and diet of the lynx (Lynx lynx) in Europe. J. Wildl. Res. 2, (1997).
Statistical Office of the Slovak Republic. Spring stock and hunting of game. Accessed 9 Nov 2020. http://datacube.statistics.sk/#!/view/en/VBD_SLOVSTAT/pl2006rs/v_pl2006rs_00_00_00_en (2019).
Czech Statistical Office. Number and hunting of selected game species 2010 - 2019. Accessed 9 Nov 2020. https://www.czso.cz/documents/10180/122461942/1000052006e.pdf/3cd18662-1691-45df-a398-040ecdeeef00?version=1.1 (2020).
Kutal, M., Váňa, M., Suchomel, J., Chapron, G. & Lopez-Bao, J. Trans-boundary edge effects in the western carpathians: the influence of hunting on large carnivore occupancy. PLoS ONE 11, e0168292 (2016).
Schmidt-Posthaus, H., Breitenmoser-Würsten, C., Posthaus, H., Bacciarini, L. & Breitenmoser, U. Causes of mortality in reintroduced Eurasian lynx in Switzerland. J. Wildl. Dis. 38, 84–92 (2002).
Mattisson, J. et al. Lethal male-male interactions in Eurasian lynx. Mamm. Biol. 78, 304–308 (2013).
Sindičić, M. et al. Mortality in the Eurasian lynx population in Croatia over the course of 40 years. Mamm. Biol. 81, 290–294 (2016).
Heurich, M. et al. Illegal hunting as a major driver of the source-sink dynamics of a reintroduced lynx population in Central Europe. Biol. Conserv. 224, 355–365 (2018).
Červený, J., Krojerová-Prokešová, J., Kušta, T. & Koubek, P. The change in the attitudes of Czech hunters towards Eurasian lynx: Is poaching restricting lynx population growth?. J. Nat. Conserv. 47, 28–37 (2019).
Kalaš, M. Contribution on the collisions of the European Lynx (Lynx lynx) with car traffic. in Migration corridors in the Western Carpathians: Malá Fatra—Kysucké Beskydy—Moravskoslezské Beskydy—Javorníky (ed. Kutal, M.) (2013).
Boitani, L. et al. Key actions for Large Carnivore populations in Europe. Report to DG Environment. Contract no. 07.0307/2013/654446/SER/B3 (European Commission, Bruxelles, 2015).
Kratochvil, J. et al. History of the distribution of the lynx in Europe. Acta Sci. Nat. Brno 4, 1–50 (1968).
Zimmermann, F., BreitenmoserWursten, C. & Breitenmoser, U. Natal dispersal of Eurasian lynx (Lynx lynx) in Switzerland. J. Zool. 267, 381–395 (2005).
Zimmermann, F., Breitenmoser-Würsten, C. & Breitenmoser, U. Importance of dispersal for the expansion of a Eurasian lynx Lynxlynx population in a fragmented landscape. Oryx 41, 358–368 (2007).
Kowalczyk, R., Górny, M. & Schmidt, K. Edge effect and influence of economic growth on Eurasian lynx mortality in the Białowieża Primeval Forest, Poland. 3–8. https://doi.org/10.1007/s13364-014-0203-z (2015).
Černecký, J. et al. Správa o stave biotopov a druhov európskeho významu za obdobie rokov 2013–2018 v Slovenskej republike. (2020).
Acknowledgements
We would like to express our gratitude to M. Dušková, T. Flajs, M. Gendiar, B. Machciník, M. Kalaš, T. Krajča, M. Králik., M. Kraus, M. Kudlák, M. Špilák, Z. Tyller, V. Trulík, G. Váňová and volunteers of “Lynx and Wolf Patrols” for their help collecting data and fieldwork. We are also grateful to the Nature Conservation Agency of the Czech Republic PLA Administration Beskydy, State Nature Conservancy of The Slovak Republic PLA Administration Kysuce, PLA Administration Malá Fatra, PLA Administration Horná Orava, Institute of Vertebrate Biology of the Czech Academy of Sciences, local foresters and hunters for cooperation and project support. The lynx monitoring was conducted within several projects supported by EEA Grants (No. EHP-CZ02- OV-1-063-01-2014), Interreg V-A Slovak Republic-Czech Republic (No. 304021D016) and Interreg Danube Transnational Programme (No. DTP1-187-3.1 “TRANSGREEN”). We also give our thanks to Martina Dušková and Alisa Royer for the revision of English text and two anonymous reviewers for valuable suggestions that improved the quality of the manuscript.
Funding
EEA Grants (No. EHP-CZ02- OV-1-063-01-2014), Interreg V-A Slovak Republic-Czech Republic (No. 304021D016) and Interreg Danube Transnational Programme (No. DTP1-187-3.1 “TRANSGREEN”).
Author information
Authors and Affiliations
Contributions
M.D.: Collecting data, Field-work coordination, Investigation, Conceptualization, Analyses, Writing—Original Draft; M.K.: Collecting data, Investigation, Analyses, Writing—Review and Editing, Supervision; J.K.P., T.O.: Analyses, Writing—Review and Editing; J.K., P.S..: Writing—Review and Editing; M.B., M.V., L.M., P.D., Ľ.H., J.L.: Collecting data, Field-work coordination and Investigation, D.C.: Analyses, Review and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duľa, M., Bojda, M., Chabanne, D.B.H. et al. Multi-seasonal systematic camera-trapping reveals fluctuating densities and high turnover rates of Carpathian lynx on the western edge of its native range. Sci Rep 11, 9236 (2021). https://doi.org/10.1038/s41598-021-88348-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88348-8
This article is cited by
-
Using heterogeneous camera-trapping sites to obtain the first density estimates for the transboundary Eurasian lynx (Lynx lynx) population in the Dinaric Mountains
Biodiversity and Conservation (2023)
-
The unique case of marking behaviour in juvenile lynx
acta ethologica (2023)
-
The first insight into hunting and feeding behaviour of the Eurasian lynx in the Western Carpathians
Mammal Research (2023)
-
Demography of a Eurasian lynx (Lynx lynx) population within a strictly protected area in Central Europe
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.