Abstract
Resistance to African trypanosomes in humans relies in part on the high affinity targeting of a trypanosome lytic factor 1 (TLF1) to a trypanosome haptoglobin-hemoglobin receptor (HpHbR). While TLF1 avoidance by the inactivation of HpHbR contributes to Trypanosoma brucei gambiense human infectivity, the evolutionary trade-off of this adaptation is unknown, as the physiological function of the receptor remains to be elucidated. Here we show that uptake of hemoglobin via HpHbR constitutes the sole heme import pathway in the trypanosome bloodstream stage. T. b. gambiense strains carrying the inactivating mutation in HpHbR, as well as genetically engineered T. b. brucei HpHbR knock-out lines show only trace levels of intracellular heme and lack hemoprotein-based enzymatic activities, thereby providing an uncommon example of aerobic parasitic proliferation in the absence of heme. We further show that HpHbR facilitates the developmental progression from proliferating long slender forms to cell cycle-arrested stumpy forms in T. b. brucei. Accordingly, T. b. gambiense was found to be poorly competent for slender-to-stumpy differentiation unless a functional HpHbR receptor derived from T. b. brucei was genetically restored. Altogether, we identify heme-deficient metabolism and disrupted cellular differentiation as two distinct HpHbR-dependent evolutionary trade-offs for T. b. gambiense human infectivity.
Similar content being viewed by others
Introduction
Through the combination of human infections and livestock trypanosomiasis, the neglected tropical diseases caused by African trypanosomes belonging to Trypanosoma brucei sensu lato (s.l.) have a significant impact on sub-Saharan rural development. Two sub-species of T. brucei proliferate in humans: T. brucei gambiense is responsible for chronic and slowly progressing human trypanosomiasis, while Trypanosoma b. rhodesiense causes an acute form of the disease1. When injected by the blood feeding insect vector (tsetse fly; Glossina spp.) into the human tissue, animal-infecting T. brucei brucei is rapidly killed by a potent arm of the innate immune system, represented by trypanosome lytic factors (TLF) 1 and 22,3. Both factors are high-density lipoprotein complexes containing haptoglobin-related protein4 and apolipoprotein L-15. In addition, TLF2 contains IgM molecules through which its uptake is mediated6,7. TLF1 targets the parasites efficiently by engaging the haptoglobin-hemoglobin receptor (HpHbR), one of the few cell surface receptors known to date in the kinetoplastid flagellates8,9,10.
The principal function of HpHbR, which was named after its only ligand, is heme uptake8. The HpHb complexes are formed by binding of haptoglobin (Hp) and extracellular hemoglobin (Hb) resulting from intravascular hemolysis11. Indeed, same as other trypanosomes, T. brucei s.l. are heme auxotrophs12, who in the mammalian-infective bloodstream stage (BS) acquire external heme via HpHbR8. The procyclic stage (PS) in the tsetse fly midgut contains more hemoproteins and obtains the ancient and omnipresent heme cofactor using another dedicated transporter, TbHrg13,14. The TbHrg transcript is developmentally regulated with the highest expression in the PS cells residing in the tsetse posterior midgut, gradually decreasing in the subsequent life cycle stages13.
Nevertheless, HpHbR is not essential for the proliferation of BS since the monomorphic T. b. brucei knock-out for HpHbR shows only a mildly affected growth phenotype in vitro8,15. Moreover, despite being more sensitive to the host’s oxidative stress, this cell line kills its rodent host before the first wave of immunoglobulin-based immunity develops8. Curiously, the capacity of T. b. gambiense to survive in human blood and cause infection is partially based on a point mutation in HpHbR that dramatically reduces its affinity for both TLF1 and HpHb15,16,17,18. Decreased uptake of TLF1 into T. b. gambiense is additionally modulated by lower HpHbR transcript levels than the corresponding receptors in T. b. brucei and T. b. rhodesiense19.
HpHbR expression contributes to T. b. brucei’s fitness in its animal reservoir hosts, providing positive selection pressures for the conservation of this receptor8,20. Still, HpHbR acquired critical mutations that allowed T. b. gambiense to enter a new niche, the human host, despite the parallel attenuation in the primary animal hosts. The cost of this loss of HpHbR function was tolerable, although it potentially brought a reduced capacity for cyclical transmission by the tsetse fly when compared with the closely related T. b. brucei and T. b. rhodesiense21.
Here we confirm that T. b. brucei HpHbR KO does not uptake heme and provide new evidence that this leads to the loss of ability to fuel hemoproteins with this cofactor. Moreover, the loss of this receptor is seemingly associated with a reduced ability to undergo differentiation in the mammalian host. In the absence of HpHbR, the fast dividing long slender forms of the bloodstream stage (BS-SL) do not transform into the quiescent, transmission-competent stumpy forms of the bloodstream stage (BS-ST). As a natural mutant for HpHbR, the human pathogenic T. b. gambiense is poorly capable of importing heme and generating BS-ST, while both of these key features are restored by heterologous expression of the T. b. brucei-derived HpHbR.
Results
Heme-free trypanosomes
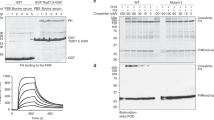
T. brucei s. l. lost the capacity to synthesize heme and therefore has to acquire it from its hosts12. First, we confirmed previous fluorimetric measurements of cellular heme content8 by a more sensitive method. Wild type (WT) T. b. brucei contains 100 pmol heme/109 cells, contrary to the T. b. brucei HpHbR KO cells, which exhibit only a trace amount of heme at the detection limit (Fig. 1a, b). For the first time, we established the amount of heme in WT T. b. gambiense, where a trace of heme was observed (6 pmol/109 cells), comparable to HpHbR KO cells (Fig. 1a, b).
a Heme b extracted from 1 × 109 cells was separated by HPLC and detected by a diode array detector. Representative chromatogram from wild type T. b. brucei, T. b. brucei knock-out for HpHbR and wild type T. b. gambiense. b Graph showing quantification of total heme b content in the same cell lines as in (a); (n = 3). c Schematic representation of the experimental design used to measure the activity of the N-terminally V5-tagged human catalase (hCAT) in the bloodstream T. brucei. d Western blot analysis with V5 antibody detecting human catalase (hCAT) in WT and HpHbR knock-out (KO) of T. b. brucei and WT T. b. gambiense overexpressing hCAT. Enolase was used as a loading control. e Immunofluorescence of hCAT detected with V5 antibody (green) in the same cell lines as in (d). DNA in the nucleus (N) and kinetoplast (k) was stained with DAPI (blue). Scale bar, 5 μm. f Measurement of human catalase activity using Oroboros oxygraph in T. b. brucei WT and HpHbR KO (left) and T. b. gambiense (right); (n = 3). Visual verification of measured activity via the O2 production in the form of bubbles after the addition of 3% H2O2 is shown in the insets. Error bars indicate ±SD. Source data are provided as a Source Data file.
Next, we studied how hemoproteins function under these conditions. Therefore, a V5-tagged human catalase (hCAT), a potent heme-dependent enzyme, is absent in the genome of T. brucei s. l.22, was expressed in three cell lines, namely WT T. b. brucei, the derived HpHbR KO, and WT T. b. gambiense (Fig. 1c, d). The ectopically-expressed catalase, which showed a uniform distribution in the cytosol (Fig. 1e), is used as a sensor for cytosolic heme. Catalase activity was readily monitored via the production of molecular oxygen, forming visible bubbles in the cell suspension (Fig. 1f; inset) upon the addition of H2O2 as a substrate (Fig. 1c). This assay showed that catalase was active in WT T. b. brucei while inactive in both T. b. brucei HpHbR KO and T. b. gambiense (Fig. 1f). These results are best explained by the failure of the latter trypanosomes to import heme due to the absence of a functional HpHbR. In addition, we also demonstrated that when taken up by HpHbR, heme is delivered into the cytosol, where it is freely available for hemoproteins as a cofactor.
Endogenous hemoprotein activity depends on HpHbR
Being involved in the sterol metabolism23,24, the endogenous hemoprotein CYP51 is considered to be essential and, therefore, a promising drug target against different trypanosomatids25,26,27. Ketoconazole is one of the broadly used compounds, which binds to the active site of CYP51 and inhibits its activity28. The genome of T. brucei s. l. encodes a single copy of CYP51, which is highly transcribed in PS but is barely detectable in BS-SL and BS-ST (Fig. 2a). RNAi in PS led to an efficient reduction of CYP51 mRNA (Supplementary Fig. 1A) followed by an almost complete absence of the corresponding protein 5 days post-induction (Supplementary Fig. 1B), as also illustrated by reduced α-CYP51 immunostaining in the RNAi-induced cells (Supplementary Fig. 1C). The gradual loss of CYP51 was associated with a significant growth reduction of the RNAi-interfered PS cells (Supplementary Fig. 1D). Of note, in PS we were unable to generate CYP51 KO cell lines, suggesting this protein is essential for this life cycle stage. In contrast to the insect stage parasites, we were able to generate CYP51 KO in the BS cells by homologous recombination of both alleles with hygromycin and phleomycin expression cassettes (Supplementary Fig. 1E). The growth phenotype of BS lacking CYP51 was only slightly affected, indicating that this protein is dispensable in this life stage (Fig. 2b).
a Northern blot analysis showing stage-specific expression of CYP51 mRNA. Total RNA from long slender (SL) and stumpy (ST) bloodstream stage, and procyclic stage (PS) was hybridized with the TbCYP51 DNA probe. The staining of ribosomal (r)RNA was used as a loading control. b Growth curves of WT T. b. brucei (black dots and line) and CYP51 knock-out (CYP51 KO) (blue dots and line) for 6 days. c The depletion of CYP51 confers insensitivity to low doses of ketoconazole. WT (black dots and line) and CYP51 KO cells (blue dots and line) were grown under the same conditions. A light gray zone indicates concentrations of ketoconazole with an inhibitory effect on CYP51, and a dark gray zone indicates a concentration range with an off-target effect (n = 3). Sidak’s multiple comparisons test *** p < 0.001; **** p < 0.0001. d Schematic representation of the experimental design showing the inhibition of hemoprotein CYP51 activity by ketoconazole. e Deficiency in the haptoglobin-hemoglobin (HpHb) uptake results in mild growth phenotype and insensitivity to the CYP51 inhibitor. WT T. b. brucei and HpHbR knock-out (KO) were incubated for 48 h with 0 to 5 μM of ketoconazole (n = 2). Sidak’s multiple comparisons test ***p < 0.001; ****p < 0.0001. f T. b. rhodesiense Etat 1.2R cells freshly isolated from mice and transferred to in vitro culture conditions with either normal human serum containing HpHb (Hp+/+), human serum lacking Hp (anhaptoglobinemic) (Hp−/−), or anhaptoglobinemic serum complemented with purified human Hp (Hp−/− + Hp). Their growth rate was determined after incubation for 48 h with 0 to 5 μM of ketoconazole (n = 3). Dunnet’s multiple comparison test *p < 0.1; **p < 0.01; ***p < 0.001. Error bars indicate ±SD. Source data are provided as a Source Data file.
We evaluated the sensitivity of WT and CYP51 KO cells to ketoconazole, a specific inhibitor of CYP51 (Fig. 2c). In agreement with the previously published IC50 values29, there was no statistically significant difference between the parental WT and CYP51 KO cells (Fig. 2c). Still, concentrations of ketoconazole ranging from 2 to 8 µM affect the WT and CYP51 KO cells differentially, since the former cells reduced their growth rate, and the latter remained unaffected (note the biphasic behavior of the dose-response curve) (Fig. 2c). Hence, CYP51 activity can be selectively inhibited with low doses of ketoconazole, discriminating between the cells with and without CYP51. In contrast, when higher doses of ketoconazole are applied, we do not see significant differences in the growth of the WT and CYP51 mutant cells, and the action of the drug should be assigned to the off-target effect.
HpHb uptake deficiency confers insensitivity to CYP51 inhibition
The pharmacological conditions described above allow assessing the activity of the hemoprotein CYP51 in the BS cells under the conditions of defective HpHb uptake (Fig. 2d). First, we exposed the HpHbR KO cells to ketoconazole and showed they are insensitive to low doses of the drug (Fig. 2e), mimicking the phenotype observed for the CYP51 KO trypanosomes (Fig. 2c).
Next, the HpHbR pathway-dependent CYP51 activity was evaluated in the WT T. b. rhodesiense resistant to the lysis by the human serum independently of HpHb uptake30. Under different cultivation conditions, we were able to modulate their access to the receptor’s cognate ligand. When grown in the Hp-containing human serum, in which the heterodimeric HpHb ligand is formed, T. b. rhodesiense is sensitive to micromolar concentrations of ketoconazole (Fig. 2f). In contrast, when grown in the anhaptoglobinemic serum31,32, i.e., in the absence of Hp, cells exhibit reduced growth and become insensitive to the drug. Yet, complementation of the anhaptoglobinemic serum with purified human Hp reverted this phenotype (Fig. 2f), proving a positive correlation between the operational HpHb uptake and the CYP51 activity. Combined, we showed that CYP51 is a genuine hemoprotein and a downstream acceptor of the HpHb complex.
Artificial expression of HpHbR in stumpy forms does not interfere with life cycle progression
Due to RNA polymerase I-mediated polycistronic transcription, in trypanosomes, most regulation occurs post-transcriptionally33. It was shown previously that the HpHbR expression is downregulated in the early phase of the BS-SL to BS-ST differentiation34. We noticed that HpHbR is located at the very end of the polycistronic transcription unit. Therefore, we modified pleomorphic WT T. b. brucei to a cell line, where the distance of HpHbR from the end of the polycistron was artificially increased by the insertion of a 10 kb-long luciferase gene in front of the procyclin gene (HpHbR-Luc) (Supplementary Fig. 2A). By following the expression of both HpHbR and luciferase genes during in vitro differentiation, we detected that the luciferase mRNA was continuously formed (Supplementary Fig. 2B), which was verified by luciferase activity measurements (Supplementary Fig. 2C). In contrast, the HpHbR transcript was downregulated in the HpHbR-Luc flagellates to the same extent as in the WT cells during the BS to PS differentiation (Supplementary Fig. 2D). This data suggests that the distance from the end of the polycistronic unit is not a critical factor in orchestrating the transcription efficiency during life cycle progression.
It is well known that the 3′ untranslated regions (UTRs) have a critical role in the regulation of transcript stability in trypanosomes33. We forced the expression of HpHbR in BS-ST by fusing the HpHbR open reading frame to the 3′ UTR of PAD1 gene (Supplementary Fig. 2E), the product of which is exclusively expressed in BS-ST35. We assumed that the artificial expression of HpHbR and subsequent continuous import of heme into cell cycle-arrested BS-ST could be detrimental.
In this HpHbR-3′PAD1 cell line, the HpHbR expression is maintained until the in vitro differentiation into the PS flagellates, which express 5x more HpHbR mRNA when compared to the WT PS (Fig. 3a). The capacity of the HpHbR-3′PAD1 cell line to differentiate in vivo from BS-SL to BS-ST was evaluated in mice, where the typical parasitic wave characteristic for the WT trypanosomes was produced (Fig. 3b). Moreover, ex vivo cells collected on day 4 were examined morphologically, and the functionality of HpHbR was verified by the uptake of the fluorescently-labeled HpHb complexes (Fig. 3c, d). As expected, 75% of the WT cells transformed into the BS-ST trypanosomes, which were exclusively Hp-free. The HpHbR-3’PAD1 cells also retained the ability to form BS-ST (~60% by day 4), all of which were Hp-positive due to the artificial expression of the receptor (Fig. 3c, d).
a The expression of HpHbR was assessed as in (b) in the WT T. b. brucei and HpHbR-3’PAD1 trypanosomes. RNA was extracted from the BS, 2 h after differentiation was triggered by cis-aconitate (cis-aco) and the procyclic stage (PS). Tukey’s multiple comparison test, n = 3. Error bars indicate ±SD. b In vivo infectivity of WT T. b. brucei and HpHbR-3’PAD1 cells was evaluated by infecting mice with 1 × 104 cells. The parasitemia was counted daily till day 7. c A representative WT T. b. brucei and HpHbR-3’PAD1 cells were isolated from the blood of mice; the latter ones internalized the fluorescently-labeled HpHb complex (green). Scale bar, 3 μm. d Based on morphology and the HpHb complex uptake, the same cells as in (e) were categorized as slender (SL) or stumpy (ST), the latter with the HpHb complex internalized (Hp-Alexa 488+) or not (Hp-Alexa 488−). e The capacity to establish midgut infection in tsetse flies was determined for WT T. b. brucei and HpHbR-3′PAD1 cells isolated from the blood of mice. Bloodstream stage parasites were administrated in the fly’s first bloodmeal without (-GSH) or with 10 mM glutathione (+GSH) supplementation. Source data are provided as a Source Data file.
Since the ability of the HpHbR-3′PAD1 cell line to differentiate has been tested only in the mammalian host, we decided to also evaluate its capacity for transmission via tsetse flies. The midguts of flies fed with blood containing either the WT or HpHbR-3’PAD1 BS parasites were dissected 10 days post-feeding, showing no significant difference in the infection rates (Fig. 3e). Therefore, we added reduced L-glutathione to the blood meal, enhancing the establishment of trypanosomes in the tsetse midgut36. Indeed, the infection rate reached up to 90%, but we did not observe a significant difference between the ability of WT and HpHbR-3’PAD1 cells to transform into PS and establish the tsetse midgut infection (Fig. 3e). Altogether, these results show that trypanosomes with the prolonged expression of HpHbR still differentiate into BS-ST and subsequent PS and retain the ability to infect tsetse flies. It also suggests that reducing heme influx into the cell (due to a missing HpHbR) does not represent a signal for differentiation into BS-ST.
T. b. brucei HpHbR KO does not produce the stumpy form
The BS trypanosomes undergo extensive cellular differentiation in preparation for an abrupt transmission from the mammalian blood into tsetse fly37. As the intensity of infection increases through the rapid proliferation of BS-SL, a parasite-derived stumpy induction factor accumulates, promoting morphological transformation into BS-ST38.
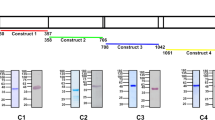
In order to test whether HpHbR plays any role in this critical phase of the life cycle, the HpHbR KO cells have also been generated in the pleomorphic 90-13T. b. brucei by replacing both alleles with the puromycin or phleomycin cassette (Supplementary Fig. 3A, B). Moreover, we have created addback cell lines with the T. b. brucei HpHbR (ABb18S; Supplementary Fig. 3A, C) and T. b. gambiense HpHbR (ABg18S; Supplementary Fig. 3A, C) restored from the 18S rRNA locus. In another cell line, a single HpHbR allele was restored in situ (ABbin situ) with more physiological levels of T. b. brucei HpHbR (Supplementary Fig. 3A, C).
First, the functionality of HpHbR in WT and the genetically engineered T. b. brucei cell lines was examined by fluorescence microscopy of Hp labeled with a green fluorochrome Hp-Alexa 488. As expected, the uptake of the HpHb complex was disrupted entirely in the HpHbR KO cell line, while 80% of the WT cells were Hp-positive (Fig. 4a, b). Both above-described addback cell lines showed the re-establishment of the Hp uptake, although to a different extent. Overexpression of HpHbR from the 18S rRNA locus almost reached the WT values (70%), whereas only 20% of ABbin situ cells were labeled (Fig. 4a, b).
a The uptake of fluorescently-labeled haptoglobin (Hp) was monitored in WT T. b. brucei (n = 3), HpHbR knock-out (HpHbR KO; n = 2), as well as in add-back cells, in which HpHbR was expressed either from the 18S rRNA locus (ABb18S; n = 3), or the in situ locus (ABbin situ; n = 3). All cells were harvested 4 days post-infection and mixed and incubated for 2 h with Hp-A488. Dunnet’s multiple comparison test, ****p < 0.0001. b Fluorescence microscopy of cell lines as in (a), with labeled Hp (green) and DNA visualized by DAPI (magenta). Note the absence of Hp uptake in HpHbR KO (scale bar, 10 μM). c In vitro differentiation of cell lines as in (a). Cells were placed in a DTM medium with 3 mM sodium citrate/cis-aconitate at 27 °C and procyclic cells were counted on day 3; (n = 10). Dunn’s multiple comparison test **p < 0.01; ****p < 0.0001. d Examination for procyclin expression on day1 and day 2 Left panel: Percentage of procyclin positive cells (procyclin+) established by fluorescence microscopy; (n = 3). Mann–Whitney test **p < 0.01; ***p < 0.001. Right panel: Representative pictures for each cell line stained with procyclin antibody (green) and DAPI (magenta). e In vivo infectivity of WT T. b. brucei, HpHbR KO, and the ABbin situ was evaluated by infecting mice with 1 × 104 cells. The parasitemia was counted daily till day 7 when the experiment was terminated. Representative growth curves (n = 3; animals per group) out of three biological repeats are shown. Parasites from infections were harvested on day 7 post-infection and separated from blood using the DEAE column. f Indirect immunofluorescence with PAD1 antibody (red), Left panel: Low magnification of the purified cells (scale bar, 10 μM); Right panel: Individual cells at high magnification (scale bar, 5 μM), with discernible DAPI-stained nucleus (N) and kinetoplast (k). g Quantification of PAD1-positive and PAD1-negative cells (ex vivo) in WT T. b. brucei, HpHbR KO, the ABbin situ, and ABg18S. In the columns, the stumpy form is shown in red. n = 3 biologically independent repeats (at least 200 cells counted per group). h Western blot analysis with PAD1 antibody of cell lines described in (g). Enolase antibody was used as a loading control. i Morphological characterization of cell lines, containing the PAD1-positive ST forms (red dots) along with the PAD1-negative (SL) cells (black dots). Left panel: cell area WT T. b. brucei (n = 40 cells), HpHbR KO (n = 35 cells), and the ABbin situ (n = 44 cells); Right panel: distance between the nucleus (N) and the kinetoplast (k) WT T. b. brucei (n = 66 cells), HpHbR KO (n = 51 cells), and the ABbin situ (n = 68 cells). Results were analyzed using the Mann–Whitney test (*p < 0.05; ***p < 0.001, ****p < 0.0001). Error bars indicate ±SD. Source data are provided as a Source Data file.
Next, the capacity to undergo differentiation in vitro was analyzed by exposing the individual cell lines to cis-aconitate and a temperature decrease to 27 °C, which is known to trigger the BS to PS transformation39,40,41. We followed their ability to proliferate and express the procyclin coat as a hallmark of PS (Fig. 4c, d). Under these conditions, the majority of WT cells (70%) became procyclin-positive by day 2 and reached high densities (mean value 6 × 106 cells/ml) by day 3 (Fig. 4c, d). In contrast, the HpHbR KO cells differentiated into only a few PS cells that did not divide and eventually died (Fig. 4c, d). Both AB cell lines showed a pretty decent capacity to differentiate in vitro, with 70% of cells covered by procyclin coat by day 2 (Fig. 4d). Although the cell numbers were not reaching the WT values by day 3, the cell line with higher expression of HpHbR (ABb18S) did not statistically differ from WT (Fig. 4c).
Individual cell lines were analyzed for their capacity to differentiate in vivo. We followed their growth in the mouse model, where the HpHbR KO parasites initially proliferated somewhat slower as compared to WT (Fig. 4e). However, on day 6 the infection rapidly accelerated, achieving a high parasitemia of ~3 × 108 cells/ml, leading to the termination of the experiment on the following day (Fig. 4e). In contrast, the WT (90–13) parasitemia declined on day 6, whereas the ABbin situ cells showed intermediate phenotypes, reaching a plateau on day 7 (Fig. 4e). Notably, the addbacks driven from the 18S locus gave different parasitemic profiles. When compared with each other, ABb18S caused a relatively low and delayed parasitic wave, while the infection with ABg18S resulted in a fast progression of the infection resembling the HpHbR KO phenotype (Supplementary Fig. 4A).
The blood-harvested parasites were further examined for PAD1 expression using immunofluorescence microscopy and western blot analysis (Fig. 4f–i). Since the PAD1 protein is specifically expressed on the surface of BS-ST and is prominently absent from BS-SL, it is used as a molecular marker for the former morphotype42. At the same time, we followed characteristic morphological features, such as the cell volume and the distance between the kinetoplast and the nucleus (Fig. 4i). We detected only a negligible number of PAD1 positives (1%) among the HpHbR KO trypanosomes, which is in line with their exclusive BS-SL morphology (Fig. 4f, g). On the contrary, 97% of the WT cells were PAD1-positive (Fig. 4g, h) and had the characteristic BS-ST morphology, associated with larger cell volume and shorter distance between the kinetoplast and the nucleus (Fig. 4i). Additionally, ABbin situ also expressed the PAD1 protein, although to a lesser extent (30%) (Fig. 4g, h), which was accompanied by the intermediate and BS-ST phenotype (Fig. 4f). On the contrary, the T. b. gambiense HpHbR addback (ABg18S) failed to rescue HpHbR KO (Fig. 4g, h; Supplementary Fig. 4A), further supporting the importance of a fully functional receptor for the stumpy differentiation.
Overexpression of T. b. brucei HpHbR in T. b. gambiense increases the stumpy formation
We studied the consequences of a restored HpHbR expression in T. b. gambiense for its life cycle progression in the mammalian host. At first, we used the laboratory strain of T. b. gambiense (LiTat1.3 strain) and T. b. gambiense expressing T. b. brucei HpHbR from the 18S rRNA locus (T. b. gambiense + b18S), as described previously18. Both cell lines were injected into the BALB/c mice and the resulting parasitemia was followed on a daily basis. The T. b. gambiense parasites emerged in the bloodstream between days 2 and 3 and sustained a rather mild infection (maximum of 3 × 107 cells/ml was reached on day 4), and on day 5 no trypanosomes were observed in the blood smears (Fig. 5a). T. b. gambiense expressing HpHbR of T. b. brucei triggered a yet significantly weaker infection, with cells detectable only until day 4, when they peaked at 5 × 106 cells/ml (Fig. 5a).
a In vivo infectivity of T. b. gambiense LiTat 1.3 (T. b. gambiense; green line) and T. b. gambiense expressing T. b. b. HpHbR (T. b. g. + b18S; red line) and T.b. g. HpHbR (T. b. g. + g18S; black line) from the 18S rRNA locus was evaluated by infecting mice with 3 × 106 cells (n = 3). The parasitemia was counted daily till day 6 when the experiment was terminated. b Indirect immunofluorescence with PAD1 antibody (red), specific for BS-ST in cell lines described in (a). DNA in the nucleus (N) and kinetoplast (k) was stained with DAPI (blue). Scale bar, 5 μm. c Quantification of PAD1-positive and PAD1-negative cells (ex vivo), day 4 p.i.) in cell lines described in (a) (n = 3). In the columns, the stumpy form is shown in red. d Morphological characterization of T. b. gambiense and T. b. g. + b18S cell lines containing the PAD1-negative cells (black dots) or PAD1-positive cells (red dots). Left panel: the distance between the nucleus (N) and the kinetoplast (k). T. b. gambiense (n = 30 cells), T. b. g. + b18S (n = 25 cells); Right panel: cell area. T. b. gambiense (n = 30 cells), T. b. g. + b18S (n = 25 cells). Results were analyzed using the Mann–Whitney test (ns, p < 0.05; ****, p < 0.0001). e In vivo infectivity of T. b. gambiense Bosendja strain. The parasitemia was counted daily till day 4, when the experiment was terminated (n = 3). f Immunofluorescence using PAD1 antibody, revealing a fraction of the PAD1-positive cells. DNA in the nucleus (N) and kinetoplast (k) was stained with DAPI (blue). Scale bar, 5 μm. g Quantification of PAD1-positive and PAD1-negative cells in the T. b. gambiense Bosendja strain ex vivo days 2-4 p.i. h Western blot analysis of WT T. b. brucei and ABbin situ, as well as T. b. gambiense Bosendja strain with PAD1 antibody. Enolase antibody was used as a loading control. Error bars indicate ±SD. Source data are provided as a Source Data file.
In T. b. gambiense, we detected only a negligible amount of the PAD1-expressing cells, while the picture was strikingly different for T. b. gambiense + b18S trypanosomes. At the peak of the infection on day 4, about 25% of cells were PAD1-positive (Fig. 5b, c), and this was associated with a significant repositioning of the nucleus towards the kinetoplast (Fig. 5d). In contrast, there was no pronounced increase in the cell volume (Fig. 5d), a feature typical for the PAD1-expressing BS-ST T. b. brucei.
Recent observations showed that stress from drug treatment and/or gene expression changes might trigger pathways mimicking the canonical differentiation to BS-ST43. The overexpression of T. b. brucei HpHbR in T. b. gambiense (T. b. gambiense + b18S; Supplementary Fig. 3D) produces a rather artificial amount of the protein (50× higher than the WT level)18. To rule out the possibility that the increased expression of PAD1 is caused by stress, we generated a cell line in T. b. gambiense, where its genuine HpHbR is overexpressed (T. b. gambiense + g18S). This cell line overexpresses T. b. gambiense HpHbR approximately 80x more than the parental strain (Supplementary Fig. 3d), hence with values similar to the T. b. gambiense + b18S parasites. When we compared both overexpressing cell lines in vivo, they showed the same profile with a low parasitemic wave peaking on day 4 (Fig. 5a). Importantly, a morphological examination on day 4 revealed only negligible presence of PAD1-positive T. b. gambiense + g18S (Fig. 5c), indicating that only T. b. brucei HpHbR enhances the stumpy formation in T. b. gambiense.
Variable expression of stumpy marker genes in T. b. gambiense group
Finally, we wondered whether the T. b. gambiense field isolate Bosendja sustains the ability to produce waves of parasitemia. Due to its limited viability in the axenic culture, Bosendja cells were injected directly from the blood stabilates into the BALB/c mice. The parasites proliferated rapidly in the bloodstream, reaching over 3 × 108 cells/ml on day 4, when the experiment was terminated (Fig. 5e). Careful morphological inspection categorized most trypanosomes as BS-SL, with a pronounced undulating membrane (Fig. 5f). Although in a very few cells (0.1%), the expression of PAD1 was detected on day 4 by immunofluorescence (Fig. 5f, g), the low amount of this marker protein remained undetectable by western blot analysis, where the T. b. brucei WT and the ABbin situ cells served as positive controls (Fig. 5h).
To further investigate whether the reduced stumpy formation is a general feature of the T. b. gambiense group, a laboratory strain Litat 1.3 and two field isolates Bosendja and PA were compared to T. b. brucei BS-LS and BS-ST controls. We followed by qPCR the expression of several genes known to be up-or downregulated44 in BS-ST (Supplementary Fig. 5). The analysis revealed that the picture is rather complex since Bosendja strain matched more BS-SL profile, unlike LiTat 1.3 and PA strains, which resembled BS-ST. Therefore, we conclude that T. b. gambiense produces bloodstream cells that do not fully correspond to either of these developmental stages, with the appearance of the stumpy-like cells being rather strain-dependent.
Discussion
The heme group is needed to sustain the life of almost all eukaryotic organisms analyzed so far45,46. Nevertheless, it has remained unclear whether trypanosomes still require heme while residing in the mammalian host. Indeed, many hemoproteins are missing or are downregulated in the BS T. b. brucei. To provide heme for its hemoproteins, such as the subunits of respiratory complexes and enzymes involved in the sterol synthesis24, the BS and PS T. b. brucei scavenge heme from their hosts via the HpHbR and TbHrg receptors, respectively8,13.
In T. brucei s. l. HpHbR has undergone a remarkable set of adaptations, likely triggered by coevolution with their distinct hosts. HpHbR of T. b. gambiense acquired specific mutations that decreased the affinity towards its ligand15,16,18. Its lower affinity for TLF compared to HpHb led to the proposal that the cells maintain the HpHb uptake while resisting lysis17. The closely related Trypanosoma congolense and T. vivax use HpHbR to obtain Hb rather than HpHb from the digested blood47. Moreover, while in T. brucei s. l. HpHbR is confined to the flagellar pocket, where it was shown to be downregulated during the stumpy formation34, it is present on the cell surface of the T. congolense epimastigotes48 at a density ~1000-fold higher than in the BS T. b. brucei47.
Following confirmation of the lack of heme b detected in the BS T. b. brucei devoid of HpHbR8, here we have shown that BS T. b. gambiense also contains minimal if any heme. We imply that the BS trypanosomes can tolerate a very limited amount of heme, suggesting a lack of essentiality of this ancient cofactor in this life cycle stage. As we reported earlier, heme is also dispensable for the plant trypanosomatid parasite Phytomonas serpens, which when grown without heme incorporates lanosterol into its membranes instead of ergosterol, overcoming the need for hemoprotein CYP5149. In this study, we focused on the hemoprotein CYP51 in T. b. brucei, where we have shown that CYP51 is not essential for the BS parasites and represents an acceptor of the HpHbR-imported heme. We have further evaluated the HpHbR-dependent CYP51 inhibition in T. b. rhodesiense by modulating its access to the receptor’s cognate ligand and proved a positive correlation between an operational HpHb uptake and the cytokinetic CYP51 activity. Moreover, to have a readout system for potent heme-dependent activity, we have overexpressed human catalase50 in the WT and HpHbR KO T. b. brucei, as well as in the WT T. b. gambiense. The data conclusively showed that in the BS T. b. brucei HpHbR internalizes heme, which is subsequently incorporated into the cytosolic hemoprotein.
Under physiological conditions, human blood is low on free Hb. Lysed erythrocytes release an excess of Hb that binds to Hp with a picomolar affinity and forms a complex internalized by macrophages51. Trypanosomes imitate this process via their HpHbR, which competes for the ligand52. Impaired erythrocytes and their clearance eventually cause anemia, representing the primary pathological hallmark of animal trypanosomiasis53. Intriguingly, reports of severe infection-associated anemia are missing for human T. b. gambiense infections, which can be even asymptomatic54,55. The reasons behind the clearance of T. b. gambiense from the bloodstream are not entirely clear, but the parasites were reported to invade other tissues even more efficiently than T. b. brucei55,56.
The erythrocytes from mice infected with trypanosomes exhibit an enhanced osmotic fragility and altered fatty acid membrane composition57 caused by host immune response and parasite-derived factors58. It was also proposed that during the acute phase of mouse infection, T. b. brucei releases extracellular vesicles that fuse with erythrocytes and consequently increase their fragility and clearance59. Thus, we propose that the excess of free hemoglobin released by trypanosome infection possibly modulates heme uptake and, subsequently, parasitic waves.
One of the main processes associated with parasitemia control in the mammalian host is the transition from the dividing BS-SL to the quiescent BS-ST60. Several proteins and effector molecules involved in this complex process have been described38,61,62. In the absence of HpHbR, the key BS-SL to BS-ST transition is disrupted, suggesting that heme uptake may be an additional player in this life cycle progression. Our observation further backs this assumption since T. b. gambiense has a poor capability to generate typical BS-ST. Importantly, a mere replacement of the T. b. gambiense HpHbR by its functional T. b. brucei variant restores the capacity to progress into this life cycle stage. The accelerated pathogenicity for mice of T. b. brucei devoid of HpHbR is likely caused by the fast division of BS-SL and the failure to develop into the non-dividing BS-ST, which would otherwise lead to protracted progress of the infection.
HpHbR is known to be a gateway for the internalization of TLF, which results in the lysis of T. b. brucei by human serum52. Here we show that in T. b. gambiense, this receptor also effectively prevents the import of heme with consequences for the heme-requiring cellular processes. While this would be lethal for a typical aerobic eukaryote that depends on external heme, trypanosomatids such as the abovementioned Phytomonas have a unique capacity to tolerate its complete deficiency46.
Changes in T. b. gambiense HpHbR seem to have far-reaching consequences. We suggest that heme dispensability may be associated with lower pathogenicity and possibly result in the chronic form of the disease. However, unless experimentally tested, this will remain speculation backed by the lower pathogenicity of the HpHbR KO T. b. brucei for both the Hp-carrying and Hp-lacking mice8. Significantly, trypanosomes with defective HpHbR struggle to progress into BS-ST, which was considered the only stage of T. brucei s. l. capable of transmission to tsetse flies62. However, this postulation has been challenged recently, showing that BS-SL and BS-ST T. b. brucei are equally competent in infecting tsetse flies, at least via artificial membrane feeding63. While BS-SL is likely capable of a short-cut transformation into PS, BS-ST seems to be better pre-adapted for survival in the hostile environment of the tsetse fly midgut. It is worth noting that the addition of glutathione and other antioxidants to the infective bloodmeal may mitigate the effect of oxidative bursts and result in an increased establishment of PS in the insect midgut36. Notably, the closely related zoonotic T. congolense infects tsetse flies without producing BS-ST64, although these parasites generate cell-cycle arrested forms akin to stumpy forms of T. brucei, albeit without significant morphological transformation65.
The importance or even mere existence of BS-ST for the transmissibility of T. b. gambiense remains controversial. Few studies proposed that BS-SL can differentiate directly or via the stumpy form into PS, directly in the tsetse midgut66,67. Moreover, high variability in the proportion of BS-SL to BS-ST was described in different field strains of T. b. gambiense68,69, which supports our observation of variable stumpy marker expressions among T. b. gambiense isolates. Additionally, the proportion of BS-ST in T. b. gambiense did not correlate with the transmissibility to tsetse flies69. The effectivity of transmission is low even under controlled experimental conditions and makes the topic even more elusive68,70.
Even though T. b. gambiense seems to be less prone than T. b. brucei to produce stumpy forms in the bloodstream, this difference may not occur in other tissues. There is convincing evidence that the skin-dwelling stumpy forms are transmissible55. However, the data is available primarily for T. b. brucei, known to have a relatively high proportion of stumpy forms in the skin. Interestingly, T. b. gambiense showed even higher skin parasitemia than T. b. brucei, with cells morphologically resembling BS-LS56. Indeed, T. b. gambiense was documented in human skin, yet the identity of the stages was not examined71. In this context, we point out that the T. b. gambiense stumpy forms we analyzed differ from the prototypical stumpy forms of T. b. brucei by having an unaltered cell volume, potentially making them more elusive during the examination of infected hosts.
In summary, we provided the first direct evidence for heme-based metabolism in bloodstream trypanosomes and their independence on this cofactor. We newly documented the unique depletion of heme in T. b. gambiense cells due to the attenuated haptoglobin-hemoglobin receptor. The loss of functional heme receptor in some trypanosomes results in the reduced occurrence of transmission-competent BS-ST cells from their life cycle and further affects the resulting parasitemia in the vertebrate host.
Methods
Cell growth and differentiation
Bloodstream T. b. brucei 90-13 (328-114 single marker), T. b. rhodesiense EtTat1.2R and T. b. gambiense LiTat 1.3 were routinely cultivated at 37 °C in HMI-9 medium (Thermo Fisher), supplemented with 20% heat-inactivated fetal bovine serum. Bloodstream T. b. brucei 427 was grown in the same medium with 10% fetal bovine serum. Field isolates of T. b. gambiense (Bosendja; AnTAR 6; MHOM/ZR/KIN001 and PA; AnTAR 6; MHOM/ZR/KIN001, both isolated from men in 1972 in the Equateur Province, Democratic Republic of Congo) were kept by passaging in mice. Cell densities were measured using the Z2 Coulter counter (Beckman Coulter) or by hemocytometer.
Transgenic cell lines
Procyclic T. b. brucei 29-13 CYP51 RNAi and HpHbR KO (328-114) cell lines were generated by us previously8,72. T. b. brucei CYP51-KO (328-114) and 90-13 HpHbR-KO cell lines were generated by successive deletion of alleles with pPuro-KO or pHygro-KO, and pPhleo-KO constructs18. The pTSArib TbHpHbR and TgHpHbR constructs used to complement the 90-13 HpHbR-KO cells were obtained by subcloning T. b. brucei HpHbR and T. b. gambiense HpHbR open reading frame into pTSARib Ble (blasticidin resistance) plasmid30, resulting in the addback cell lines ABb18S and ABg18S, respectively. The same constructs were used to generate T. b. gambiense (LiTat 1.3 strain) overexpressing TbHpHbR (18; T. b. gambiense + b18S) or TgHpHbR (T. b. gambiense + g18S) from the 18S rRNA locus. The construct designed to endogenously modulate the level of HpHbR was generated from the pET-in situ construct73 after replacing hygromycin with phleomycin (Ble) resistance. PCR-amplified DNA fragments were assembled and cloned into pET-in situ Ble plasmid by recombination (InFusion, Clontech) and the resulting cell line was named ABbin situ. The constructs are depicted in Supplementary Figs. 2A, E, 3A. Trypanosomes were transfected with linearized plasmid DNA (10 μg) or with gel-purified PCR products (5–10 μg) using a similar protocol published elsewhere74. Briefly, a total of 1 × 107 BS or 1 × 108 PS cells was harvested by centrifugation at 1000 × g at 4 °C for 10 min and washed 1 time with PBS. Cells were resuspended in 100 μl of AMAXA Human T-cell solution and electroporated with AMAXA Nucleofactor apparatus. Predefined program X-001 and X-014 was used for BS and PS cells, respectively. Selection markers were applied 6 h post-transfection, and clones were generated by limited dilution.
Real-time PCR, western blot analysis, and luciferase activity measurement
Total RNA was extracted using Trizol reagent, and the cDNA was synthesized using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara) as recommended by the manufacturer with an oligo dT used instead of random primers. The qRT-PCR was performed with the cDNA using the SYBR-green stain (Takara). Primers used for qPCR are listed in the Supplementary Table 1 in the Supplementary information file.
The C1 primers were used as endogenous control75.
In order to detect protein expression in BS, lysates from 5 × 106 cells were separated on a 12% SDS-polyacrylamide gel, transferred to a PVDF membrane and probed with the monoclonal anti-V5 and anti-α tubulin antibodies (Sigma) at 1:2000 and 1:5000 dilutions, respectively. Alternatively, the samples were prepared the same way (except the lysates were not boiled but heated to 37 °C) and probed with anti-PAD1 and anti-enolase antibodies at 1:1000 and 1: 10,000 dilutions, respectively. The images were detected with Biorad Image Lab software.
For full uncropped gels and blots see the Source Data file and Supplementary Information. 2 × 106 cells were centrifuged, lysed, and labeled by the Luciferase Assay kit as recommended by the manufacturer (Promega). The read-out was performed for 10 s with the Luminoskan TL Plus instrument (Labsystems).
Overexpression and activity assay for catalase
The construct for expression of human catalase (hCAT, accession number: NP_001743.1; https://www.ncbi.nlm.nih.gov/protein/NP_001743.1) was modified from the construct generated by us previously50 to be constitutively expressed. The obtained constructs were linearized by NotI, electroporated into BS T. b. brucei and T. b. gambiense and selected using 1 μg/ml hygromycin (Thermo Fisher).
The activity of catalase was monitored by respirometry using Oxygraph-2K (Oroboros) as a tool to measure the O2 concentration produced after the addition of H2O2 and analyzed using the Oroboros DatLab Software. T. b. brucei 90–13 and T. b. gambiense LiTat 1.3 were used to establish the background of the respirometry experiment. Briefly, 1 × 106 of BS cells resuspended in 2 ml of HMI-9 medium were treated with 20 μl of 882 mM (3%) H2O2. Alternatively, 5 × 106 parasites were resuspended in 10 μl phosphate buffered saline (PBS) and placed on a microscopic slide. The same volume of 3% H2O2 was added to the cells, mixed, and the formation of O2 visible as macroscopic bubbles were monitored as a read-out for the catalase activity. All measurements and statistics were calculated from three independent biological replicates.
Indirect immunofluorescence assay
For immunofluorescence analysis, 1 × 106 to 1 × 107 cells were fixed with 4% paraformaldehyde and settled on microscopic slides. After 10 min incubation at room temperature, they were washed with PBS and permeabilized with 100% ice-cold methanol for 20 min. Cells were incubated with 5% fat-free milk in PBS-Tween (0.05%) for 1 h, followed by incubation with primary anti-V5, anti-PAD1 or anti-CYP51 at 1:1000 dilution and secondary Alexa Fluor-488 anti-mouse and Alexa Fluor-488 or Alexa Fluor-555 anti-rabbit IgG antibodies (Thermo Fisher) at 1:1000 dilution for 1 h at room temperature. After the last washing step, cells were stained with DAPI, mounted with an anti-fade reagent (Thermo Fisher) and visualized using a fluorescent microscope Zeiss Axioplan 2 (Carl Zeiss) and documented with Olympus cellSens Standard Imaging Software.
High-performance liquid chromatography
A total of 5 × 108 BS cells was harvested by centrifugation at 1000 × g at 4 °C for 10 min and washed 3 times with PBS on ice. Cells were resuspended in 60 μl H2O, extracted with 400 μl acetone/0.2% HCl, and the supernatant was collected after centrifugation at 1000 × g at 4 °C for 5 min. The pellet was resuspended in 200 μl acetone/0.2% HCl and centrifuged as described above. Both supernatants were combined, and 150 μl of each sample was immediately injected into a high-performance liquid chromatography system (Infinity 1200, Agilent Technologies) and separated using a reverse-phase column (4 μm particle size, 3.9 × 75 mm) (Waters) with 0.1% trifluoroacetic acid and acetonitrile/0.1% trifluoroacetic acid as solvents A and B, respectively. Heme b was eluted with a linear gradient of solvent B (30–100% in 12 min) followed by 100% of B at a flow rate of 0.8 ml/min at 40 °C. Heme was detected by diode array detector (Infinity 1200, Agilent Technologies) and identified by retention time and absorbance spectra according to commercially available standard (Sigma-Aldrich).
Fly and mouse infections
Teneral tsetse flies (Glossina morsitans morsitans) were fed 24–48 h after emergence with T. b. brucei Antat 1.1 WT and HpHbR-3’PAD1 strains-infected blood meal, either or not supplemented with 10 mM reduced L-glutathione to increase the establishment of infection. Parasites were harvested from the blood of cyclophosphamide-immunosuppressed mice (Endoxan) at 6–7 days post-infection and mixed with defibrinated horse blood (E&O Laboratories). Flies were further fed every 2–3 days on uninfected defibrinated horse blood. Then, flies were dissected on day 10 after the first blood meal to assess the presence of parasites in the midgut (i.e., establishment of a PS midgut infection).
For each biological repeat, five female BALB/c mice(four to six-week-old purchased from AnLab, Czech Republic or Janvier Labs, Belgium) were intraperitoneally injected with 1 × 104 BS cells (T. b. brucei strains) or 3 × 106 (T. b. gambiense strains). The infection was followed daily by diluting tail snip blood in TrypFix buffer (3.7% formaldehyde, 1×SSC buffer) and manual counting of trypanosomes in a Neubauer hemocytometer. Mice were euthanized for the collection of parasites, which were separated from the erythrocytes on a diethylaminoethyl (DEAE) cellulose column using a standard protocol. Purified trypanosomes were washed once with PBS and subsequently used for downstream experiments.
The cell numbers in the study were documented using Excel and GraphPad Prism 8 Software.
Hp-488 preparation and labeling
Hp was conjugated with Alexa 488 using the Dylight amine-reactive kit (Thermo Fischer) as recommended by the manufacturer. The blood from mice (5 female BALB/c mice, 4–6 weeks old) was collected at different times of infection from a tail puncture with a capillary containing heparin. Following their purification, trypanosomes were subsequently incubated at 37 °C for 15 min in HMI-9 medium containing the lysosomal protease inhibitor FMK-024. Next, they were incubated with 20 µg/ml (f. c.) Alexa 488-labeled Hp for 2 h, subsequently fixed with 4% paraformaldehyde for 10 min, stained with DAPI and analyzed with a Zeiss Axioplan 2 epifluorescence microscope equipped with a Zeiss AxioCam HRm digital camera (Carl Zeiss). The resulting images were analyzed using Adobe Photoshop software and Fiji.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software Inc.). Data are presented as mean± S.D.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study are provided in the Supplementary Information and Source Data files.
References
Franco, J. R., Simarro, P. P., Diarra, A. & Jannin, J. G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 6, 257–275 (2014).
Hajduk, S. L. et al. Lysis of Trypanosoma brucei by a toxic subspecies of human high-density lipoprotein. J. Biol. Chem. 264, 5210–5217 (1989).
Raper, J., Portela, M. P., Lugli, E., Frevert, U. & Tomlinson, S. Trypanosome lytic factors: Novel mediators of human innate immunity. Curr. Opin. Microbiol. 4, 402–408 (2001).
Smith, A. B., Esko, J. D. & Hajduk, S. L. Killing of trypanosomes by the human haptoglobin-related protein. Science 268, 284–286 (1995).
Vanhamme, L. et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422, 83–87 (2003).
Raper, J., Fung, R., Ghiso, J., Nussenzweig, V. & Tomlinson, S. Characterization of a novel trypanosome lytic factor from human serum. Infect. Immun. 67, 1910–1916 (1999).
Verdi, J. et al. Inducible germline IgMs bridge Trypanosome Lytic Factor assembly and parasite recognition. Cell Host Microbe 28, 79–88.e4 (2020).
Vanhollebeke, B. et al. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science 320, 677–681 (2008).
Stødkilde, K., Torvund-Jensen, M., Moestrup, S. K. & Andersen, C. B. Structural basis for trypanosomal haem acquisition and susceptibility to the host innate immune system. Nat. Commun. 5, 5487 (2014).
Macleod, O. et al. A receptor for the complement regulator factor H increases transmission of trypanosomes to tsetse flies. Nat. Commun. 11, 1326 (2020).
Schaer, D. J., Buehler, P. W., Alayash, A. I., Belcher, J. D. & Vercellotti, G. M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121, 1276–1284 (2013).
Kořený, L., Lukeš, J. & Oborník, M. Evolution of the haem synthetic pathway in kinetoplastid flagellates: An essential pathway that is not essential after all? Int. J. Parasitol. 40, 149–156 (2010).
Horáková, E. et al. The Trypanosoma brucei TbHrg protein is a heme transporter involved in the regulation of stage-specific morphological transitions. J. Biol. Chem. 292, 6998–7010 (2017).
Cabello-Donayre, M. et al. Trypanosomatid parasites rescue heme from endocytosed hemoglobin through lysosomal HRG transporters. Mol. Microbiol. 101, 895–908 (2016).
DeJesus, E., Kieft, R., Albright, B., Stephens, N. A. & Hajduk, S. L. Single amino acid substitution in the group 1 Trypanosoma brucei gambiense haptoglobin-hemoglobin receptor abolishes TLF-1 binding. PLoS Pathog. 9, e1003317 (2013).
Symula, R. E. et al. Trypanosoma brucei gambiense group 1 is distinguished by a unique amino acid substitution in the HpHb receptor implicated in human serum resistance. PLoS Negl. Trop. Dis. 6, e1728 (2012).
Higgins, M. K. et al. Structure of the trypanosome haptoglobin hemoglobin receptor and implications for nutrient uptake and innate immunity. Proc. Natl Acad. Sci. USA 110, 1905–1910 (2013).
Uzureau, P. et al. Mechanism of Trypanosoma brucei gambiense resistance to human serum. Nature 501, 430–434 (2013).
Kieft, R. et al. Mechanism of Trypanosoma brucei gambiense (group 1) resistance to human trypanosome lytic factor. Proc. Natl Acad. Sci. USA 107, 16137–16141 (2010).
Higgins, M. K., Lane-Serff, H., MacGregor, P. & Carrington, M. A. Receptor’s tale: An eon in the life of a trypanosome receptor. PLoS Pathog. 26, 13:e1006055 (2017).
Welburn, S. C., Molyneux, D. H. & Maudlin, I. Beyond tsetse - implications for research and control of human African trypanosomiasis epidemics. Trends Parasitol. 32, 230–241 (2016).
Kraeva, N. et al. Catalase in Leishmaniinae: With me or against me? Infect. Genet. Evol. 50, 121–127 (2017).
Zhou, W., Cross, G. A. & Nes, W. D. Cholesterol import fails to prevent catalyst-based inhibition of ergosterol synthesis and cell proliferation of Trypanosoma brucei. J. Lipid Res. 48, 665–673 (2007).
Nes, C. R. et al. Novel sterol metabolic network of Trypanosoma brucei procyclic and bloodstream forms. Biochem. J. 443, 267–277 (2012).
Kessler, R. L., Soares, M. J., Probst, C. M. & Krieger, M. A. Trypanosoma cruzi response to sterol biosynthesis inhibitors: Morphophysiological alterations leading to cell death. PLoS One 8, e55497 (2013).
McCall, L. I. et al. Targeting ergosterol biosynthesis in Leishmania donovani: Essentiality of sterol 14alpha-demethylase. PLoS Negl. Trop. Dis. 9, e0003588 (2015).
Dauchy, F. A. et al. Trypanosoma brucei CYP51: Essentiality and targeting therapy in an experimental model. PLoS Negl. Trop. Dis. 10, e0005125 (2016).
Zhang, J. et al. The fungal CYP51s: Their functions, structures, related drug resistance, and inhibitors. Front. Microbiol. 10, 691 (2019).
Lepesheva, G. I. et al. Sterol 14alpha-demethylase as a potential target for antitrypanosomal therapy: Enzyme inhibition and parasite cell growth. Chem. Biol. 14, 1283–1293 (2007).
Lecordier, L. et al. Adaptation of Trypanosoma rhodesiense to hypohaptoglobinaemic serum requires transcription of the APOL1 resistance gene in a RNA polymerase I locus. Mol. Microbiol. 97, 397–407 (2015).
Koda, Y., Soejima, M., Yoshioka, N. & Kimura, H. The haptoglobin-gene deletion responsible for anhaptoglobinemia. Am. J. Hum. Genet. 62, 245–252 (1998).
Vanhollebeke, B. et al. Distinct roles of haptoglobin-related protein and apolipoprotein L-I in trypanolysis by human serum. Proc. Natl Acad. Sci. USA 104, 4118–4123 (2007).
Clayton, C. Regulation of gene expression in trypanosomatids: Living with polycistronic transcription. Open Biol. 9, 190072 (2019).
Vanhollebeke, B., Uzureau, P., Monteyne, D., Pérez-Morga, D. & Pays, E. Cellular and molecular remodeling of the endocytic pathway during differentiation of Trypanosoma brucei bloodstream forms. Eukaryot. Cell 9, 1272–1282 (2010).
MacGregor, P. & Matthews, K. R. Identification of the regulatory elements controlling the transmission stage-specific gene expression of PAD1 in Trypanosoma brucei. Nucl. Acids Res. 40, 7705–7717 (2012).
MacLeod, E. T., Maudlin, I., Darby, A. C. & Welburn, S. C. Antioxidants promote establishment of trypanosome infections in tsetse. Parasitology 134, 827–831 (2007).
Szöőr, B., Silvester, E. & Matthews, K. R. A Leap into the unknown - early events in African trypanosome transmission. Trends Parasitol. 36, 266–278 (2020).
Rojas, F. et al. Oligopeptide signaling through TbGPR89 drives trypanosome quorum sensing. Cell 176, 306–317 (2019).
Brun, R. & Schönenberger, M. Stimulating effect of citrate and cis-aconitate on the transformation of Trypanosoma brucei bloodstream forms to procyclic forms in vitro. Z. Parasitenkd. 66, 17–24 (1981).
Czichos, J., Nonnengaesser, C. & Overath, P. Trypanosoma brucei: cis-aconitate and temperature reduction as triggers of synchronous transformation of bloodstream to procyclic trypomastigotes in vitro. Exp. Parasitol. 62, 283–291 (1986).
Ziegelbauer, K., Quinten, M., Schwarz, H., Pearson, T. W. & Overath, P. Synchronous differentiation of Trypanosoma brucei from bloodstream to procyclic forms in vitro. Eur. J. Biochem. 192, 373–378 (1990).
Silvester, E., McWilliam, K. R. & Matthews, K. R. The cytological events and molecular control of life cycle development of Trypanosoma brucei in the mammalian bloodstream. Pathogens 6, 29 (2017).
Quintana, J. F., Zoltner, M. & Field, M. C. Evolving differentiation in African trypanosomes. Trends Parasitol. 37, 296–303 (2021).
Naguleswaran, A., Doiron, N. & Roditi, I. RNA-Seq analysis validates the use of culture-derived Trypanosoma brucei and provides new markers for mammalian and insect life-cycle stages. BMC Genomics 19, 227 (2018).
Hamza, I. & Dailey, H. A. One ring to rule them all: Trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 1823, 1617–1632 (2012).
Kořený, L., Oborník, M., Horáková, E., Waller, R. S. & Lukeš, J. The convoluted history of heme biosynthesis. Biol. Rev. https://doi.org/10.1111/brv.12794. (2021).
Lane-Serff, H. et al. Evolutionary diversification of the trypanosome haptoglobin-haemoglobin receptor from an ancestral haemoglobin receptor. eLife 5, e13044 (2016).
Eyford, B. A. et al. Differential protein expression throughout the life cycle of Trypanosoma congolense, a major parasite of cattle in Africa. Mol. Biochem. Parasitol. 177, 116–125 (2011).
Kořený, L. et al. Aerobic kinetoplastid flagellate Phytomonas does not require heme for viability. Proc. Natl Acad. Sci. USA 109, 3808–3813 (2012).
Horáková, E. et al. Catalase compromises the development of the insect and mammalian stages of Trypanosoma brucei. FEBS J. 287, 964–977 (2020).
Kristiansen, M. et al. Identification of the hemoglobin scavenger receptor. Nature 409, 198–201 (2001).
Vanhollebeke, B. & Pays, E. The trypanolytic factor of human serum: Many ways to enter the parasite, a single way to kill. Mol. Microbiol. 76, 806–814 (2010).
Radwanska, M., Vereecke, N., Deleeuw, V., Pinto, J. & Magez, S. Salivarian trypanosomosis: A review of parasites involved, their global distribution and their interaction with the innate and adaptive mammalian host immune system. Front. Immunol. 9, 2253 (2018).
Büscher, P. et al. Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol. 34, 197–207 (2018).
Alfituri, O. A. et al. To the skin and beyond: The immune response to African trypanosomes as they enter and exit the vertebrate host. Front. Immunol. 11, 1250 (2020).
Capewell, P. et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. eLife 5, e17716 (2016).
Cnops, J. et al. NK-, NKT- and CD8-derived IFNγ drives myeloid cell activation and erythrophagocytosis, resulting in trypanosomosis-associated acute anemia. PLoS Pathog. 11, e1004964 (2015).
Stijlemans, B., de Baetselier, P., Magez, S., van Ginderachter, J. A. & De Trez, C. African trypanosomiasis-associated anemia: The contribution of the interplay between parasites and the mononuclear phagocyte system. Front. Immunol. 9, 218 (2018).
Szempruch, A. J. et al. Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164, 246–257 (2016).
Seed, J. R. & Wenck, M. A. Role of the long slender to short stumpy transition in the life cycle of the African trypanosomes. Kinetopl. Biol. Dis. 2, 3 (2003).
Mony, B. M. et al. Genome-wide dissection of the quorum sensing signalling pathway in Trypanosoma brucei. Nature 505, 681–685 (2014).
Rojas, F. & Matthews, K. R. Quorum sensing in African trypanosomes. Curr. Opin. Microbiol. 52, 124–129 (2019).
Schuster, S. et al. Unexpected plasticity in the life cycle of Trypanosoma brucei. eLife 10, e66028 (2021).
Rotureau, B. & Van Den Abbeele, J. Through the dark continent: African trypanosome development in the tsetse fly. Front. Cell Infect. Microbiol. 3, 53 (2013).
Silvester, E., Young, J., Ivens, A. & Matthews, K. R. Interspecies quorum sensing in co-infections can manipulate trypanosome transmission potential. Nat. Microbiol. 2, 1471–1479 (2017).
Wijers, D. J. & Willett, K. C. Factors that may influence the infection rate of Glossina palpalis with Trypanosoma gambiense. II. The number and morphology of the trypanosomes present in the blood of the host at the time of the infected feed. Ann. Trop. Med. Parasitol. 54, 341–350 (1960).
Bass, K. E. & Wang, C. C. The in vitro differentiation of pleomorphic Trypanosoma brucei from bloodstream into procyclic form requires neither intermediary nor short-stumpy stage. Mol. Biochem. Parasitol. 44, 261–270 (1991).
Ravel, S., Patrel, D., Koffi, M., Jamonneau, V. & Cuny, G. Cyclical transmission of Trypanosoma brucei gambiense in Glossina palpalis gambiensis displays great differences among field isolates. Acta Trop. 100, 151–155 (2006).
Janelle, J. et al. Monitoring the pleomorphism of Trypanosoma brucei gambiense isolates in mouse: impact on its transmissibility to Glossina palpalis gambiensis. Infect. Genet. Evol. 9, 1260–1264 (2009).
Le Ray, D. Vector susceptibility to African trypanosomes. Ann. Soc. Belg. Med. Trop. 69, 165–214 (1989).
Camara, M. et al. Extravascular dermal trypanosomes in suspected and confirmed cases of gambiense Human African Trypanosomiasis. Clin. Infect. Dis. 73, 12–20 (2021).
Haubrich, B. A. et al. Discovery of an ergosterol-signaling factor that regulates Trypanosoma brucei growth. J. Lip. Res. 56, 331–341 (2015).
Kelly, S. et al. Functional genomics in Trypanosoma brucei: A collection of vectors for the expression of tagged proteins from endogenous and ectopic gene loci. Mol. Biochem. Parasitol. 154, 103–109 (2007).
Burkard, G., Fragoso, C. M. & Roditi, I. Highly efficient stable transformation of bloodstream forms of Trypanosoma brucei. Mol. Biochem. Parasitol. 153, 220–223 (2007).
Jones, N. G. et al. Regulators of Trypanosoma brucei cell cycle progression and differentiation identified using a kinome-wide RNAi screen. PLoS Pathog. 10, e1003886 (2014).
Acknowledgements
We thank Eva Kriegová and Zuzana Vavrušková (Institute of Parasitology) for their help with animal experiments. We thank Nick Van Reet (Institute of Tropical Medicine, Antwerp) for providing the field strains of T. b. gambiense. This work was supported by the ERC CZ project LL1601 to J.L., ERD Funds project OPVVV 0000759 to J.L., and Czech Grant Agency projects 20-07186S and 21-09283S to J.L.
Author information
Authors and Affiliations
Contributions
E.H., B.V., and J.L. designed the study; E.H., L.L., R.S., and B.V. developed the methodology E.H., L.L., P. Cu., R.S., P. Ch., C.J.M.L., J.V.D.A., and B.V. performed the experiments and data analysis; E.H., L.L., P. Cu., J.V.D.A., B.V., and J.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics statement
In the Czech Republic, the research was approved by the Central Commission for Animal Welfare, Biology Center (protocol No. 28/2016). All experimental procedures complied with the Czech law (Act No. 246/1992). In Belgium, the research was approved by the animal ethics committee of the Institute for Molecular Biology and Medicine and the Institute of Tropical Medicine (tsetse fly infection experiment). All mice were housed in a pathogen-free facility and the experiments were performed in compliance with the relevant laws and institutional guidelines (license LA1500474).
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horáková, E., Lecordier, L., Cunha, P. et al. Heme-deficient metabolism and impaired cellular differentiation as an evolutionary trade-off for human infectivity in Trypanosoma brucei gambiense. Nat Commun 13, 7075 (2022). https://doi.org/10.1038/s41467-022-34501-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-34501-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.