Abstract
Alterations of sympathoadrenal and sympathoneural systems have been suggested to be involved in the pathogenesis of hypertension in spontaneously hypertensive rats (SHR). To evaluate the ontogenetic changes of these systems, mRNA and protein expressions of catecholaminergic system genes were measured in adrenal glands and sympathetic ganglia, and the catecholamine levels were determined in adrenal glands, sympathetic ganglia and plasma of prehypertensive (4-week-old) and hypertensive (24-week-old) SHR. Vascular sympathetic innervation was visualized in the femoral artery by glyoxylic acid. In the adrenal glands of prehypertensive SHR, the expression of catecholamine biosynthetic enzymes Ddc, Dbh and Pnmt was lower than in aged-matched Wistar-Kyoto rats. In contrast, the adrenal content of dopamine, noradrenaline and adrenaline was higher in prehypertensive SHR (141%, 123% and 120% of Wistar-Kyoto rats, respectively, p < 0.01). In the adrenal glands of adult SHR, the expression of catecholamine biosynthetic enzymes Th, Ddc, Dbh and Pnmt was decreased along the amounts of dopamine and noradrenaline (50% and 38%, respectively, p < 0.001). The expression levels of Ddc and Dbh enzymes were also downregulated in the sympathetic ganglia of both prehypertensive and adult SHR. At both ages, the density of sympathetic innervation was twofold higher in SHR compared to Wistar-Kyoto rats (p < 0.001). In conclusion, adrenal catecholamine content was increased in prehypertensive SHR, whereas it was reduced in SHR with established hypertension. Surprisingly, downregulation of catecholamine biosynthetic enzymes was observed in both the adrenal medulla and sympathetic ganglia of SHR at both ages. Thus, this downregulation might be a compensatory mechanism that counteracts the vascular sympathetic hyperinnervation seen in SHR of both ages.
Similar content being viewed by others
Introduction
Both sympathoadrenal and sympathoneural systems play an essential role in blood pressure regulation and in the pathogenesis of hypertension [1]. In adult spontaneously hypertensive rats (SHR), excessive sympathoneural and sympathoadrenal activity has been described [2,3,4]. Sympathetic nerve activity is increased [5], and additionally, the preganglionic activity is more effectively transmitted through sympathetic ganglia [6], and the density of vascular sympathetic innervation and noradrenaline concentrations are greater [7]. The expression of tyrosine hydroxylase and catecholamine content in adrenal glands is increased [8], and adrenaline release from the adrenal gland is enhanced [9]. The levels of circulating catecholamines in adult SHR were reported to be increased [10, 11] or unchanged [12]. There is considerable information about the enhancement of the catecholamine biosynthetic pathway in the adrenals of adult SHR concerning tyrosine hydroxylase (Th, TH), DOPA decarboxylase (Ddc, DDC), dopamine βhydroxylase (Dbh, DBH) and phenylethanolamine-N-methyltransferase (Pnmt, PNMT) [13,14,15]. However, less attention has been paid to the genes involved in catecholamine storage or the filling of the catecholaminergic vesicles, i.e., chromogranins (Chga, Chgb), secretogranins (Sccg2), neuropeptide Y (Npy) and vesicular monoamine transporters (Vmat1, Vmat2). Transporters and enzymes removing catecholamines from the synaptic cleft have also been neglected, i.e., noradrenaline transporter (Net) [14, 16], monoamine oxidase (Maoa, Maob) or catechol-O-methyltransferase (Comt) [16, 17].
Spontaneous hypertension develops in SHR during prepuberty and puberty [18]. At the age of 3 weeks, there is usually no difference in blood pressure between SHR and their normotensive controls -Wistar-Kyoto (WKY) rats. Between 4 and 13 weeks of age, blood pressure increases steeply in SHR [19,20,21]. During the same developmental period, sympathetic nerve activity rises more rapidly in SHR compared to WKY rats [5]. Sympathectomy or adrenal demedullation in prehypertensive SHR attenuates the development of hypertension but the blood pressure difference between SHR and WKY rats still persists [22, 23]. It was found that there is an interplay between sympathoadrenal and sympathoneural systems [24], and therefore only a combination of sympathectomy and adrenal demedullation can fully abolish the blood pressure differences between SHR and WKY rats [25]. Moreover, this intervention has to be performed in young SHR (<6 weeks old) otherwise the effect is limited [23]. Taken together, there is evidence that sympathoadrenal and sympathoneural systems are essential in the early development of hypertension and cardiovascular changes in SHR.
The aim of this study was to describe ontogenetic differences in sympathoneural and sympathoadrenal systems between SHR and WKY rats. A comparison of prehypertensive and hypertensive animals (4 and 24 weeks of age) may reveal the abnormalities underlying the development of hypertension. Therefore, the expression of genes of the catecholaminergic system (genes related to catecholamine synthesis, catecholamine vesicles and catecholamine reuptake and degradation) was evaluated in the sympathetic ganglia and adrenal medulla, which are closely related tissues. Furthermore, the levels of catecholamines in adrenal glands and sympathetic ganglia were measured, and catecholamines were visualized in vascular sympathetic nerve endings. Finally, the plasma levels of catecholamines and metanephrines were determined.
Materials and methods
Animals
Experiments were performed in male normotensive Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHR) aged 4 and 24 weeks. One group (n = 8) of rats from both strains was used for blood pressure measurement. The second group (n = 8) was utilized only for the collection of samples to avoid stress by surgery and blood pressure measurement in these animals. The rats were housed under standard laboratory conditions (temperature 23 ± 1 °C, 12 h light–dark cycle, Altromin pellet diet (0.2% Na+, 1% K+) and tap water ad libitum). All care and experimental protocols were approved by the Ethical Committee of the Institute of Physiology, Czech Academy of Sciences and conform to the European Convention on Animal Protection and Guidelines on Research Animal Use.
Surgery and blood pressure measurement
One day before blood pressure measurement, catheters were inserted into the left carotid artery and jugular vein under isoflurane. The measurement was performed in conscious rats kept in small transparent cages (partially restrained, 30 min after the placement into cages). Blood pressure was measured using the PowerLab system (ADInstruments Pty Ltd, Bella Vista, NSW, Australia) between 08:00 AM and 11:30 AM [21].
Tissue sampling
Rats were anesthetized by isoflurane approximately 3 min before blood sampling began, which took approximately 30 s. Rats were sacrificed by exsanguination, and the death was verified by checking for cardiac and respiratory arrest. Blood was collected in tubes containing EDTA (Sarstedt AG & Co. KG, Nümbrecht, Germany) and centrifuged for 10 min (3000 × g, 4 °C); plasma was frozen in liquid nitrogen and stored at −80 °C for later use. One adrenal gland was taken for protein and catecholamine measurements. Adrenal medulla from the contralateral adrenal gland and superior cervical ganglia were used for mRNA measurements. All samples were quickly transferred to 1.5 ml tubes and then frozen in liquid nitrogen and stored at −80 °C for later use. Femoral arteries were cleaned of fat tissue and were immediately used for histochemical visualization of monoamines with the SPG method (see below).
RNA isolation, reverse transcription and quantitative real-time PCR
Frozen tissue was homogenized by MagNA Lyser Green Beads (Roche, Basel, Switzerland), and total RNA was isolated using a GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, St Louis, MO, USA). The quantity and purity of RNA were checked with a NanoDrop ND 1000 spectrophotometer (NanoDrop Products, Wilmington, DE, USA). The integrity of total RNA was tested using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). An RNase-Free DNase Set (Qiagen, Hilden, Germany) was used for the removal of residual DNA. The 120 ng or 150 ng of total RNA (the maximal possible amounts from the adrenal medulla or sympathetic ganglia, respectively) were transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA, USA). Gene expression was measured on the LightCycler® 480 System (Roche) using a HOT FIREPol® Probe qPCR Mix Plus (SolisBioDyne, Tartu, Estonia) and TaqMan® Gene Expression Assays (Life Technologies, Carlsbad, CA, USA). We measured the expression of genes involved in catecholamine biosynthesis (enzymes Th, Ddc, Dbh, Pnmt and cofactors quinoid dihydropteridine reductase, Qdpr and GTP cyclohydrolase 1, Gch1), genes related to catecholamine vesicles (Vmat1, Vmat2, Chga, Chgb, Scg2, Npy) and genes involved in catecholamine reuptake or degradation (Net, Maoa, Maob, Comt). The list of all TaqMan® Gene Expression Assays is available in Supplementary Table 1. Exported raw data were analyzed by software LinRegPCR (version 2013.0; [26]) for determination of Ct values (number of cycles needed to reach the threshold) and mean PCR efficiencies per amplicon (averaged efficiencies of individual samples amplified with a particular TaqMan Gene Expression Assay). The obtained values were used for relative quantification by a modified 2−∆∆CT method [27] in which PCR efficiency was used as a base of exponentiation. The data were normalized to the best combination of two reference genes (Hprt1 and Ywhaz in adrenal medulla, 18 S and Gapdh in sympathetic ganglia), which were selected by NormFinder software [28] as described in our previous work [29].
Western blot analysis
Frozen adrenal glands or sympathetic ganglia were homogenized in phosphate buffer saline (Sigma-Aldrich) with a protease inhibitor cocktail (1:100; Sigma-Aldrich) using MagnaLyser Green Beads (Roche). The homogenate was divided into two parts (the first for catecholamine measurement, the second for Western blot analysis). EDTA and sodium metabisulfite were added to the first part of the homogenate to prevent catecholamine degradation (final concentration of EDTA was 1 mM, and for sodium metabisulfite, it was 4 mM), and samples were stored at −80 °C until further use. RIPA buffer (Sigma-Aldrich) was added to the second part of the homogenate, and the tube was shaken with MagnaLyser again. The homogenate was centrifuged for 20 min at 14,000 × g and 4 °C. The total protein concentration was measured using the Lowry protein assay. The reducing SDS polyacrylamide gel electrophoresis was performed with Optiblot precast 4–20% gradient gels (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Electrophoresis was run at 100 V for 120 min. Semidry transfer of proteins to a Polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) was performed with a Tris Glycine Buffer (Bio-Rad Laboratories, Hercules, CA, USA) containing 10% methanol at 25 V for 30 min. Membranes were stained in Ponceau-S (0.1% Ponceau S in 5% acetic acid) to control the transfer efficiency. Subsequently, membranes were washed from the dye and blocked with 3% milk diluted in TBS-T (137 mM NaCl, 20 mM Trizma® base and 0.1% Tween® 20, Sigma-Aldrich) at room temperature (RT) for 1 h. The membranes were incubated with primary antibody diluted in 3% milk TBS-T at 4 °C overnight. The primary antibodies included: Anti-Tyrosine hydroxylase (1:4000, Abcam, ab112, LOT: GR265840-3), Anti-Dopamine beta hydroxylase (1:2000, Abcam, ab43868, LOT: GR110853-8), Anti-DOPA decarboxylase (1:2000, Abcam, ab3905, LOT: GR2164-10), Anti-PNMT (1:4000, Abcam, ab69579, LOT: GR129455-1), Anti-HPRT (1:5000, Abcam, ab109021, LOT: GR153613-1) and Anti-GAPDH (1:8000, Cell Signaling Technology, Inc., Danvers, MA, United States, #2118, LOT: 2118 S). The photos of the molecular weight marker and full gel for each antibody, and the pictures of the immunohistochemical staining of adrenal glands against TH, DDC, DBH and PNMT can be seen in Supplementary Figures 1 and 2. Subsequently, the membranes with adrenal gland samples were incubated with a Peroxidase-Conjugated Goat Anti-Rabbit secondary antibody (1:5000, Thermo Fisher Scientific) in 3% milk TBS-T for 1 h at RT. For the signal enhancement in samples of sympathetic ganglia, a biotinylated secondary antibody was used at a concentration of 1:4000 in 3% milk TBS-T for 1 h, and then Avidin and Biotinylated Horseradish Peroxidase 1:400 in 3% milk TBS-T for 1 h at room temperature was added (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA, USA). The detection of horseradish peroxidase was performed using a SuperSignal West Femto reagent (Thermo Fisher Scientific). The emitted light was captured with a chemiluminescence imaging analyzer LAS 1000 (Fujifilm, Tokyo, Japan). We used the same conditions for each run of a particular antibody in a given tissue (the same concentration, time with HRP-substrate, exposure time, etc.). The obtained images were analyzed using ImageJ 1.4v software [30]. Protein expression of the genes of interest was normalized to the expression of HPRT or GAPDH in adrenal glands or sympathetic ganglia, respectively.
Catecholamine measurement
The concentration of catecholamines (dopamine, noradrenaline and adrenaline) and their metabolites (normetadrenaline and metadrenaline) was measured in plasma and homogenates of adrenal glands and sympathetic ganglia. The competitive Enzyme immunoassays 3-CAT Research ELISA and 2-MET Plasma ELISA Fast Track (LDN, Nordhorn, Germany) were performed as recommended by the manufacturer. The absorbance at 450 nm was read on a microplate reader Tecan Infinite M200 (Tecan Group Ltd., Männedorf, Switzerland).
Histochemical visualization of monoamines (SPG Method)
The histochemical measurement of monoamines was performed as described previously [31] by the protocol of de la Torre and Surgeon [32]. Briefly, fresh glyoxylic acid solution (1% glyoxylic acid (Sigma-Aldrich), 236 mM KH2PO4 and 200 mM sucrose) was prepared. Cleaned femoral arteries were cut longitudinally and dipped three times in the glyoxylic acid solution. Then, the arteries were mounted on glass slides, dried by an air cooler for 5 min, and the slides were put on a hot plate (80 °C). After 5 min of heating, the slides were taken off. Then, Mineral Oil (Sigma-Aldrich) and a cover glass were added. The slides were heated again on a hot plate for 90 s. The specimens were observed using a fluorescence microscope Leica LMD6000 with a DAPI filter cube. Quantification was done using ImageJ 1.4 v software [30]. The images were converted to grayscale, and the black level was set to reduce the level of overstaining on a fluorescence image. We tested several threshold values to distinguish the areas with a fluorescent signal from the background. The threshold with the best outcomes was applied to all images, and the area of signal in the binary image was measured.
Statistics
Data are expressed as the mean ± SEM. Normality of distribution was tested using the Shapiro–Wilk test. The statistical significance of the data concerning mRNA and catecholamine content was determined using the Two-way ANOVA with Bonferronni post hoc test. The statistical significance of Western blot data were determined with Student’s t test. For a nonnormal distribution of data, the statistical significance was determined using Two-way ANOVA on Rank or by Mann–Whitney test; p < 0.05 was considered to be significant.
Results
Physiological parameters
There was no significant difference in mean arterial pressure between 4-week-old SHR and WKY rats. However, the heart rate was already higher in prehypertensive SHR compared to the age-matched WKY rats (Table 1). At an age of 24 weeks, both the mean arterial pressure and heart rate were higher in SHR. The body weights of SHR were lower compared to WKY rats at 4 and 24 weeks. The absolute weight of adrenal glands was similar in young rats of both strains, but it was higher in adult SHR than in WKY rats. The relative weight of adrenal glands was significantly greater in both prehypertensive and adult SHR compared to the age-matched WKY rats.
The expression of catecholamine biosynthetic enzymes in adrenal glands
In the adrenal glands of prehypertensive SHR, significantly lower mRNA expressions of Dbh and Pnmt were found, while the mRNA expressions of Th and Ddc were unchanged compared to the age-matched WKY rats (Fig. 1a). At the protein level, reduced expressions of DDC and DBH were observed while there was no change in TH and PNMT (Fig. 1b). In the adrenal glands of adult SHR, the mRNA expressions of Th, Ddc, Dbh and Pnmt were lower than in adult WKY rats (Fig. 1c). TH, DDC and DBH protein expressions were also reduced in adult SHR but the PNMT protein was unchanged (Fig. 1d).
The mRNA (a, c) and protein (b, d) expression of enzymes of catecholamine biosynthesis in the adrenal glands of prehypertensive 4-week-old (a, b) or hypertensive 24-week-old (c, d) SHR and age-matched WKY rats. The mRNA expression was standardized to the best combination of reference genes Hprt1 and Ywhaz. The protein expression was standardized to HPRT. Data are plotted relative to WKY rats as the mean ± SEM, n = 8 for each group. *p < 0.05 vs. age-matched WKY; †p < 0.05 vs. mRNA expression in 4-week-old animals of the same strain
The expression of other genes of the catecholaminergic system in adrenal glands
In the adrenal glands of prehypertensive SHR, a higher expression of Gch1 but lower expressions of Vmat2 and Npy compared to the age-matched WKY rats was found (Table 2). In the adrenal glands of adult SHR, the expression levels of almost all measured genes were reduced with the exception of overexpressed Maob and unchanged Comt.
Catecholamine content in adrenal glands
The amounts of dopamine, noradrenaline and adrenaline were greater in the adrenal glands of prehypertensive SHR compared to the age-matched WKY rats (Table 3). In the adrenal glands of adult SHR, the amounts of dopamine and noradrenaline were decreased while the amount of adrenaline was similar in comparison to adult WKY rats. Greater adrenal content of catecholamines in adult rats from both strains was partially caused by adrenal growth during aging. When the adrenal weight was considered, the adrenal contents of dopamine, noradrenaline and adrenaline were ~4-fold higher in adult WKY rats compared to young WKY rats. In SHR, the relative adrenal content of adrenaline was 2.5-fold greater in adults than in prehypertensive SHR, while adrenal dopamine and noradrenaline content did not change in SHR during aging.
The expression of catecholamine biosynthetic enzymes in sympathetic ganglia
In the sympathetic ganglia of prehypertensive SHR, lower mRNA and protein expression of Ddc and Dbh compared to the aged-matched WKY rats was found. The mRNA and protein expression of the Th gene was similar in the sympathetic ganglia of 4-week-old rats from both strains (Fig. 2a, b). In sympathetic ganglia of adult SHR, mRNA expression of Th was higher, whereas there was no change at the protein level. The mRNA expressions of Ddc and Dbh were lower in adult SHR compared to adult WKY rats. DDC was also underexpressed at the protein level, while there was only a trend towards decreased protein expression of DBH (Fig. 2c, d).
The mRNA (a, c) and protein (b, d) expression of enzymes of catecholamine biosynthesis in the superior cervical ganglia of prehypertensive 4-week-old (a, b) or hypertensive 24-week-old (c, d) SHR and age-matched WKY rats. The mRNA expression was standardized to the best combination of reference genes 18S and Gapdh. The protein expression was standardized to GAPDH. The data are plotted relatively to WKY rats as the mean ± SEM, n = 8 for each group. *p < 0.05 vs. age-matched WKY; †p < 0.05 vs. mRNA expression in 4-week-old animals of the same strain
The expression of other genes of the catecholaminergic system in sympathetic ganglia
In the sympathetic ganglia of prehypertensive SHR, the mRNA expressions of Qdpr and Chga were lower while the expression of Vmat1 was higher than in 4-week-old WKY rats (Table 2). In adult SHR, we found lower expressions of Vmat1 and Net while Chgb and Npy were overexpressed in comparison to adult WKY rats.
Catecholamine content in the sympathetic ganglia
The amounts of dopamine and noradrenaline were very low in the sympathetic ganglia (Table 3). There were no significant differences in dopamine and noradrenaline content between SHR and WKY rats of either age.
Histochemical visualization of monoamines in the femoral artery
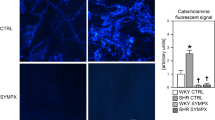
Figure 3 shows the monoamine content in sympathetic innervation of the femoral artery in SHR and WKY rats at 4 and 24 weeks visualized with glyoxylic acid staining. The fluorescent signal was ~2-fold higher in SHR of both ages compared to the age-matched WKY rats (2.16 ± 0.08 in prehypertensive SHR, 1.99 ± 0.11 in adult SHR, p < 0.001 for both ages).
Catecholamine concentrations in plasma
The concentrations of catecholamines (dopamine, noradrenaline and adrenaline) and their metabolites (normetadrenaline and metadrenaline) were measured in the plasma of SHR and WKY rats (Table 3). In prehypertensive SHR, there was a higher plasma level of noradrenaline while the levels of dopamine, adrenaline, normetadrenaline and metadrenaline were unchanged compared to the age-matched WKY rats. In adult SHR, the plasma level of dopamine was higher compared to adult WKY rats while plasma levels of noradrenaline, adrenaline, normetadrenaline and metadrenaline were similar in both rat strains.
Discussion
In this study, the ontogenetic changes of sympathoneural and sympathoadrenal systems in spontaneously hypertensive rats and their normotensive controls were characterized. In the adrenal glands of young prehypertensive SHR, the expression of catecholaminergic biosynthetic enzymes was lower, but the amounts of dopamine, noradrenaline and adrenaline in the adrenal glands were greater than in 4-week-old WKY rats. In the adrenal glands of adult SHR, we found more suppressed expression of several enzymes of catecholamine biosynthesis. In contrast to the prehypertensive SHR, the adrenal catecholamine content was lower in adult SHR with established hypertension compared to WKY rats. The expression of enzymes of catecholamine biosynthesis was downregulated, and the catecholamine content was unchanged in the sympathetic ganglia of both prehypertensive and adult SHR. In contrast, a higher density of sympathetic innervation was observed in the femoral arteries of SHR of both ages.
In the adrenal glands of prehypertensive SHR, we found a lower mRNA expression of Dbh and Pnmt but an unchanged expression of Th and Ddc in comparison to the age-matched WKY rats. These data are consistent with Friese et al. [17] who reported unchanged mRNA expression of Th and decreased expression of Dbh and Pnmt in the adrenal glands of prehypertensive SHR. Consistent with our mRNA data, the protein expression of TH was unchanged, while DDC and DBH protein expression was lower in the adrenal glands of prehypertensive SHR compared to the aged-matched WKY rats. The protein expression of PNMT was unchanged despite the decreased mRNA expression. This finding might be explained by the fact that PNMT activity (e.g., after the exposure to glucocorticoids) is regulated not only transcriptionally but also through the control of translation and enzyme degradation [33, 34]. In prehypertensive SHR, TH activity in adrenal glands was either decreased [11, 35] or increased [36]. The amount of catecholamines in the adrenal glands of young SHR was reported to be unchanged or decreased [11, 35]. We did not measure the activity of enzymes, but we found greater dopamine, noradrenaline and adrenaline content in the adrenal glands of young SHR. Thus, the increased activity of TH in prehypertensive SHR might be caused by some posttranslational mechanisms that lead to higher catecholamine content in adrenal glands.
In the adrenal glands of adult SHR, the mRNA expression of all enzymes of the catecholamine biosynthesis (Th, Ddc, Dbh and Pnmt) was decreased in comparison to the age-matched WKY rats. Grundt et al. [37] also reported reduced mRNA expression of Th in the adrenal glands of stress-naive SHR. Along with the attenuated mRNA expression in the adrenal glands of adult SHR, we found lower protein expression of the enzymes TH, DDC and DBH compared to adult WKY rats. Decreased protein expression of TH agreed with the results of the study by Moura et al. [11]. In contrast, our observation of decreased Th and Pnmt mRNA expression in the adrenal glands of adult SHR conflicted with the studies by Reja et al. [13] and Nguyen et al. [15], who reported a higher mRNA expression of Th and Pnmt in the SHR adrenals. However, the catecholaminergic system is extremely susceptible to stressful conditions. Kvetnansky et al. [38] demonstrated that a single or repeated immobilization changed the mRNA and protein expression of Th, Dbh and Pnmt genes in both the adrenal glands and sympathetic ganglia. In addition, Grundt et al. [37] reported a greater increase in the mRNA expression of Th in the adrenal glands of adult SHR after 25 min of mild stress caused by tail-cuff measurement of blood pressure, which abolished the interstrain differences present in stress-naive rats. Thus, it is important to consider that the expression of catecholaminergic system genes responds to stress differently in SHR and WKY rats and can ultimately affect the resulting observations significantly. Despite the substantially decreased mRNA expression of Pnmt in the adrenal glands of adult SHR, we observed similar PNMT protein expression in both rat strains. In the adrenal glands of adult SHR, TH activity was reported to be decreased [11] or increased [39, 40]. We observed decreased adrenal dopamine and noradrenaline content, but there was an unchanged amount of adrenaline in the adrenal glands of adult SHR. This finding fully agreed with the protein expression of respective biosynthetic enzymes revealed by our study. This result is also consistent with reduced noradrenaline [11, 41] and unchanged adrenaline [16, 25] in the adrenal glands of adult SHR. Lower adrenal noradrenaline content might also be caused by greater catecholamine secretion, which was described in the adrenal glands of adult SHR [9, 42]. Our data show that the catecholaminergic system in the adrenal glands of SHR with established hypertension is downregulated at different levels, i.e., mRNA expression, protein expression and the catecholamine content. For a comparison of our results with other studies, see Supplementary Table 2.
Compared to the age-matched WKY rats, the expression levels of Ddc and Dbh genes in both the sympathetic ganglia and the adrenal medulla of prehypertensive and adult SHR were lower. The expression of Th was unchanged in the sympathetic ganglia of prehypertensive SHR, but the mRNA expression of Th was higher in the sympathetic ganglia of adult SHR. The dopamine and noradrenaline content in the sympathetic ganglia were similar in SHR and WKY rats of both ages, which correlated with the published results of Mano et al. [43]. On the other hand, the histochemical visualization of catecholamines showed a higher density of sympathetic innervation in the femoral arteries of prehypertensive and adult SHR compared to aged-matched WKY rats. This finding is consistent with the larger amount of noradrenaline in the vascular tissue of adult SHR [43,44,45] and the increased density of sympathetic fibers in the arteries of both young and adult SHR rats [31, 46, 47] reported earlier. In general, there is a dissociation between the decreased expression of enzymes of catecholamine biosynthesis in sympathetic ganglia (which is similar to the situation in the adrenal glands) and the elevated density of vascular sympathetic innervation in both prehypertensive and adult SHR.
Finally, the plasma levels of catecholamines and their metabolites were measured. In prehypertensive SHR, a higher plasma level of noradrenaline compared to young WKY rats was observed. This result seems to be consistent with our finding of a denser sympathetic innervation of arteries in prehypertensive SHR. Other laboratories also reported increased [35, 48] or unchanged [49] plasma levels of noradrenaline and adrenaline in young SHR. In adult SHR, the only significant strain difference was the higher plasma level of dopamine. The plasma levels of noradrenaline, adrenaline, normetadrenaline and metadrenaline were unchanged in adult SHR compared to WKY rats. This finding correlated with unchanged plasma levels of noradrenaline and adrenaline in adult SHR [12, 48]. In contrast, other groups reported increased plasma levels of noradrenaline [11] or adrenaline [10] in SHR with established hypertension. These discrepancies can be explained by the influence of stress because the plasma levels of noradrenaline and adrenaline are increased more by stress in SHR than in WKY rats [50]. Indeed, we demonstrated that plasma noradrenaline was higher in adult SHR than WKY rats under stress conditions, and chemical sympathectomy abolished this strain difference [51].
In conclusion, the described ontogenetic changes of sympathoneural and sympathoadrenal systems seem to contribute to hypertension development in SHR. The expression of enzymes of catecholamine biosynthesis is downregulated in both the sympathetic ganglia and adrenal glands of adult as well as prehypertensive SHR compared to aged-matched WKY rats. This downregulation leads to lower catecholamine content in the adrenal glands of adult SHR with established hypertension but not in prehypertensive SHR. Nevertheless, there is a higher density of sympathetic innervation of blood vessels in SHR of both ages. The causes of increased adrenal catecholamine content and increased density of sympathetic innervation in prehypertensive SHR still need to be determined because these factors could be involved in the pathogenesis of high blood pressure. It cannot be excluded that the downregulation of the expression of genes of the catecholaminergic system in SHR might be a compensatory mechanism counteracting the hyperfunction of the sympathoneural and sympathoadrenal systems.
References
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Head RJ. Hypernoradrenergic innervation: its relationship to functional and hyperplastic changes in the vasculature of the spontaneously hypertensive rat. Blood Vessels. 1989;26:1–20.
de Champlain J. Pre-and postsynaptic adrenergic dysfunctions in hypertension. J Hypertens. 1990;8:S77–85.
Pintérová M, Kuneš J, Zicha J. Altered neural and vascular mechanisms in hypertension. Physiol Res. 2011;60:381–402.
Judy WV, Farrell SK. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension. 1979;1:605–14.
Magee JC, Schofield GG. Neurotransmission through sympathetic ganglia of spontaneously hypertensive rats. Hypertension. 1992;20:367–73.
Cassis LA, Stitzel RE, Head RJ. Hypernoradrenergic innervation of the caudal artery of the spontaneously hypertensive rat: an influence upon neuroeffector mechanisms. J Pharmacol Exp Ther. 1985;234:792–803.
Kumai T, Tanaka M, Watanabe M, Kobayashi S. Elevated tyrosine hydroxylase mRNA levels in the adrenal medulla of spontaneously hypertensive rats. Jpn J Pharmacol. 1994;65:367–79.
Lim DY, Jang SJ, Park DG. Comparison of catecholamine release in the isolated adrenal glands of SHR and WKY rats. Auton Autacoid Pharmacol. 2002;22:225–32.
Vlachakis ND, Alexander N, Maronde RF. Increased plasma normetanephrine in spontaneously hypertensive rats. Clin Exp Hypertens. 1980;2:309–19.
Moura E, Pinho Costa PM, Moura D, Guimarães S, Vieira-Coelho MA. Decreased tyrosine hydroxylase activity in the adrenals of spontaneously hypertensive rats. Life Sci. 2005;76:2953–64.
Kvetnansky R, McCarty R, Thoa NB, Lake CR, Kopin IJ. Sympatho-adrenal responses of spontaneously hypertensive rats to immobilization stress. Am J Physiol. 1979;236:H457–62.
Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Tyrosine hydroxylase gene expression in ventrolateral medulla oblongata of WKY and SHR: a quantitative real-time polymerase chain reaction study. Auton Neurosci. 2002;98:79–84.
Reja V, Goodchild AK, Pilowsky PM. Catecholamine-related gene expression correlates with blood pressures in SHR. Hypertension. 2002;40:342–7.
Nguyen P, Peltsch H, de Wit J, Crispo J, Ubriaco G, Eibl J, et al. Regulation of the phenylethanolamine N-methyltransferase gene in the adrenal gland of the spontaneous hypertensive rat. Neurosci Lett. 2009;461:280–4.
O’Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, et al. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–95.
Friese RS, Mahboubi P, Mahapatra NR, Mahata SK, Schork NJ, Schmid-Schönbein GW, et al. Common genetic mechanisms of blood pressure elevation in two independent rodent models of human essential hypertension. Am J Hypertens. 2005;18(5 Pt 1):633–52.
Zicha J, Kunes J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev. 1999;79:1227–82.
Albrecht I. The hemodynamics of early stages of spontaneous hypertension in rats. Part II: female study. Jpn Circ J. 1974;38:991–6.
Smith TL, Hutchins PM. Central hemodynamics in the developmental stage of spontaneous hypertension in the unanesthetized rat. Hypertension. 1979;1:508–17.
Behuliak M, Vavřínová A, Bencze M, Polgárová K, Ergang P, Kuneš J, et al. Ontogenetic changes in contribution of calcium sensitization and calcium entry to blood pressure maintenance of Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens. 2015;33:2443–54.
Lee RM, Triggle CR, Cheung DW, Coughlin MD. Structural and functional consequence of neonatal sympathectomy on the blood vessels of spontaneously hypertensive rats. Hypertension. 1987;10:328–38.
Borkowski KR. Effect of adrenal demedullation and adrenaline on hypertension development and vascular reactivity in young spontaneously hypertensive rats. J Auton Pharmacol. 1991;11:1–14.
Lee RM, Borkowski KR, Leenen FH, Tsoporis J, Coughlin M. Interaction between sympathetic nervous system and adrenal medulla in the control of cardiovascular changes in hypertension. J Cardiovasc Pharmacol. 1991;17(Suppl 2):S114–6.
Lee RM, Borkowski KR, Leenen FH, Tsoporis J, Coughlin M. Combined effect of neonatal sympathectomy and adrenal demedullation on blood pressure and vascular changes in spontaneously hypertensive rats. Circ Res. 1991;69:714–21.
Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–50.
Vavřínová A, Behuliak M, Zicha J. The importance of the selection of appropriate reference genes for gene expression profiling in adrenal medulla or sympathetic ganglia of spontaneously hypertensive rat. Physiol Res. 2016;65:401–11.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Bencze M, Behuliak M, Vavřínová A, Zicha J. Altered contractile responses of arteries from spontaneously hypertensive rat: The role of endogenous mediators and membrane depolarization. Life Sci. 2016;166:46–53.
de la Torre JC, Surgeon JW. Histochemical fluorescence of tissue and brain monoamines: results in 18min using the sucrose-phosphate-glyoxylic acid (SPG) method. Neuroscience. 1976;1:451–3.
Berenbeim DM, Wong DL, Masover SJ, Ciaranello RD. Regulation of synthesis and degradation of rat adrenal phenylethanolamine N-methyltransferase. III. Stabilization of PNMT against thermal and tryptic degradation by S-adenosylmethionine. Mol Pharmacol. 1979;16:482–90.
Wong DL, Siddall B, Wang W. Hormonal control of rat adrenal phenylethanolamine Nmethyltransferase. Enzyme activity, the final critical pathway. Neuropsychopharmacology. 1995;13:223–34.
Grobecker H, Saavedra JM, Roizen MF, Weise V, Kopin IJ, Axelrod J. Peripheral and central catecholaminergic neurons in genetic and experimental hypertension in rats. Clin Sci Mol Med. 1976;3:377s–80s.
Teitelman G, Ross RA, Joh TH, Reis DJ. Differences in utero in activities of catecholamine biosynthetic enzymes in adrenals of spontaneously hypertensive rats. Clin Sci. 1981;61(Suppl 7):227s–30s.
Grundt A, Grundt C, Gorbey S, Thomas MA, Lemmer B. Strain-dependent differences of restraint stress-induced hypertension in WKY and SHR. Physiol Behav. 2009;97:341–6.
Kvetnansky R, Micutkova L, Rychkova N, Kubovcakova L, Mravec B, Filipenko M, et al. Quantitative evaluation of catecholamine enzymes gene expression in adrenal medulla and sympathetic Ganglia of stressed rats. Ann N Y Acad Sci. 2004;1018:356–69.
Grobecker H, Saavedra JM, Weise VK. Biosynthetic enzyme activities and catecholamines in adrenal glands of genetic and experimental hypertensive rats. Circ Res. 1982;50:742–6.
Kumai T, Tanaka M, Tateishi T, Watanabe M, Nakura H, Asoh M, et al. Effects of anti-androgen treatment on the catecholamine synthetic pathway in the adrenal medulla of spontaneously hypertensive rats. Naunyn Schmiede Arch Pharmacol. 1998;357:620–4.
Korner P, Bobik A, Oddie C, Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993;22:243–52.
Miranda-Ferreira R, de Pascual R, de Diego AM, Caricati-Neto A, Gandía L, Jurkiewicz A, et al. Single-vesicle catecholamine release has greater quantal content and faster kinetics in chromaffin cells from hypertensive, as compared with normotensive, rats. J Pharmacol Exp Ther. 2008;324:685–93.
Mano M, Jeffreson S, Head RJ. Vascular, ganglia and cardiac catecholamine disposition in the spontaneously hypertensive rat and in the stroke-prone spontaneously hypertensive rat. J Vasc Res. 1992;29:8–12.
Head RJ, Cassis LA, Robinson RL, Westfall DP, Stitzel RE. Altered catecholamine contents in vascular and nonvascular tissues in genetically hypertensive rats. Blood Vessels. 1985;22:196–204.
Donohue SJ, Stitzel RE, Head RJ. Time course of changes in the norepinephrine content of tissues from spontaneously hypertensive and Wistar Kyoto rats. J Pharmacol Exp Ther. 1988;245:24–31.
Mangiarua EI, Lee RM. Increased sympathetic innervation in the cerebral and mesenteric arteries of hypertensive rats. Can J Physiol Pharmacol. 1990;68:492–9.
Scott TM, Pang SC. The correlation between the development of sympathetic innervation and the development of medial hypertrophy in jejunal arteries in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst. 1983;8:25–32.
Szemeredi K, Bagdy G, Stull R, Keiser HR, Kopin IJ, Goldstein DS. Sympathoadrenomedullary hyper-responsiveness to yohimbine in juvenile spontaneously hypertensive rats. Life Sci. 1988;43:1063–8.
Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci. 1998;62:1111–8.
McCarty R, Kvetnansky R, Lake CR, Thoa NB, Kopin IJ. Sympatho-adrenal activity of SHR and WKY rats during recovery from forced immobilization. Physiol Behav. 1978;21:951–5.
Behuliak M, Bencze M, Polgárová K, Kuneš J, Vaněčková I, Zicha J. Hemodynamic response to gabapentin in conscious spontaneously hypertensive rats. Hypertension. 2018;72:676–85.
Acknowledgements
The authors are grateful to Mr. Robert Kotanchik for his help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The work documented in this paper was made possible by institutional support from the Institute of Physiology Czech Academy of Sciences (RVO: 67985823) and research grants from the Czech Science Foundation (GACR 16-10349Y) and the Charles University Grant Agency (GAUK 1071416).
Supplementary information
Rights and permissions
About this article
Cite this article
Vavřínová, A., Behuliak, M., Bencze, M. et al. Which sympathoadrenal abnormalities of adult spontaneously hypertensive rats can be traced to a prehypertensive stage?. Hypertens Res 42, 949–959 (2019). https://doi.org/10.1038/s41440-018-0198-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0198-y
Keywords
This article is cited by
-
Development of the hypersecretory phenotype in the population of adrenal chromaffin cells from prehypertensive SHRs
Pflügers Archiv - European Journal of Physiology (2021)
-
Sympathectomy-induced blood pressure reduction in adult normotensive and hypertensive rats is counteracted by enhanced cardiovascular sensitivity to vasoconstrictors
Hypertension Research (2019)