Abstract
High-salt intake is one of the major dietary determinants of increased blood pressure and cardiovascular disease. Thus, there is scientific and medical interest in understanding the mechanistic abnormalities mediating the pressor effects of salt (salt sensitivity). According to historical theory, salt sensitivity stems from an impairment in renal function (referred to as “abnormal pressure natriuresis” or a “natriuretic handicap”), which causes salt-sensitive subjects to excrete a sodium load more slowly, and retain more of it than salt-resistant normotensive controls. However, this historical view has come under intense scrutiny because of growing awareness that in salt-sensitive subjects, acute salt loading does not usually induce greater increases in sodium balance and cardiac output than those induced by salt loading in salt-resistant normotensive controls. Here we highlight pioneering studies from Japan that challenge the historical thinking and provide insights into a contemporary theory of salt sensitivity termed the “vasodysfunction theory.” According to this theory, initiation of salt-induced hypertension usually involves abnormal vascular resistance responses to increased salt intake, not greater renal retention of a salt load in salt-sensitive subjects than in normal subjects. By shifting the focus from the historical theory to a contemporary final common pathway for the pathogenesis of salt sensitivity, research from Japan is building the scientific foundation for more effective approaches to the prevention and treatment of salt-induced hypertension. Among the most promising approaches are dietary strategies for reducing the risk for salt-induced hypertension that do not depend on reducing salt consumption in the population.
Similar content being viewed by others
Introduction
Blood pressure salt sensitivity is a common disorder associated with increased risk for hypertension [1]. Studies by Morimoto et al. [2] from the National Cardiovascular Center in Suita, Japan suggest that salt sensitivity may also be an independent risk factor for elapsed time to a cardiovascular event. Thus, in addition to increasing risk for hypertension, salt sensitivity might signify an underlying disturbance in vascular biology that influences risk for cardiovascular events beyond its effects on blood pressure per se [2]. Of the many different methods that have been explored for assessing salt sensitivity, a carefully controlled dietary protocol similar to that employed by Morimoto et al. [2], provides the highest test–retest reliability for identifying salt-sensitive subjects [3]. While there is ongoing concern about the meaning and practical utility of various methods of testing for salt sensitivity [3,4,5], the mechanistic abnormalities mediating the pressor effects of salt, and the role of dietary salt restriction in the prevention and management of hypertension, are subjects of major scientific and medical interest [1, 6,7,8,9,10,11].
Disturbances in many molecular, biochemical, neural, immunologic, and other mechanisms have been implicated in the pathogenesis of salt sensitivity [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. It has been proposed that many, if not most, of these disturbances usually mediate salt sensitivity and initiation of salt-induced hypertension through a common physiologic abnormality termed “vasodysfunction” [42, 46]. In the present review, we discuss the vasodysfunction theory of salt sensitivity and highlight pioneering studies from Japan that have been instrumental in elucidating this final common pathway through which a variety of biologic disturbances initiate salt-induced hypertension.
The physiologic abnormality that usually mediates salt sensitivity and initiation of salt-induced hypertension: an abnormal vascular resistance response to increased salt intake
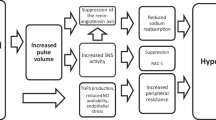
According to the vasodysfunction theory of salt sensitivity, initiation of salt-induced hypertension usually involves subnormal decreases in systemic vascular resistance (total peripheral resistance) in response to salt loading, together with normal salt-induced increases in sodium balance and cardiac output (Fig. 1) [42]. Thus, the theory holds that “vasodysfunction,” defined as a subnormal decrease in systemic vascular resistance in response to increases in salt intake, is the physiologic abnormality that initiates most instances of salt-induced hypertension. The abnormal systemic vascular resistance response to salt loading is determined at least in part by an abnormal renal vascular resistance response to salt loading (Fig. 1) [46].
The vasodysfunction theory for initiation of salt sensitivity and salt-induced hypertension. This diagram shows the usual changes in sodium balance, cardiac output, and vascular resistance that occur with initiation of increased salt intake in salt-sensitive subjects, and in salt-resistant subjects with normal blood pressure. Note that in salt-sensitive subjects, the increases in sodium balance and cardiac output that occur during initiation of salt loading are not abnormal, i.e., not greater than those that occur with salt loading in salt-resistant controls with normal blood pressure. In contrast, the salt-induced changes in vascular resistance that occur in salt-sensitive subjects are distinctly abnormal, i.e., distinctly different from those that occur in normotensive, salt-resistant controls
It is important to note that while salt-sensitive subjects undergo increases in sodium balance and cardiac output in response to acute salt loading, the vasodysfunction theory holds that those increases are usually not abnormal [42]. That is, the vasodysfunction theory holds that in most salt-sensitive subjects, increases in sodium balance and cardiac output in response to acute salt loading are not greater than those that occur with salt loading in normal controls (salt-resistant subjects with normal blood pressure) (Fig. 1). The theory holds that in contrast to salt-resistant normal controls, most salt-sensitive subjects fail to robustly vasodilate and normally reduce systemic vascular resistance in response to acute salt loading (Fig. 1) [42]. This abnormal vascular resistance response to salt loading causes systemic vascular resistance to be greater in salt-sensitive subjects than in salt-resistant normal controls. With salt loading, the abnormally high (greater) levels of systemic vascular resistance, together with normally increased levels of sodium balance and cardiac output, cause greater increases in blood pressure in salt-sensitive subjects than in salt-resistant normal controls (Fig. 1) [42]. The vasodysfunction theory can apply not only to common forms of salt sensitivity, but also to salt sensitivity that may occur in rare Mendelian forms of hypertension [47].
Questioning the historical view that subnormal sodium excretion is usually involved in the initiation of salt sensitivity and salt-induced hypertension: pioneering studies from Japan
In contrast to the contemporary vasodysfunction theory of salt sensitivity, the historical and still prevailing theory of salt sensitivity championed by Guyton, Hall, and others [48,49,50,51,52,53,54,55,56,57,58,59] incorporates the view that initiation of salt-induced hypertension usually involves an impairment in renal function (referred to as a “natriuretic handicap” or “abnormal pressure natriuresis”) which causes salt-sensitive subjects to excrete a sodium load more slowly, and retain more of it than salt-resistant subjects with normal blood pressure. This historical theory holds that in response to increased salt intake, the natriuretic handicap causes salt-sensitive subjects to undergo abnormally large increases in sodium balance, cardiac output, and therefore blood pressure. However, in careful metabolic studies conducted at Tokyo University more than 30 years ago, Ishii et al. [60] found that in response to increases in salt intake (from 100 mmol/day to ~275 mmol/day), salt-sensitive subjects usually do not excrete sodium more slowly and undergo greater increases in sodium balance than salt-resistant normal controls (salt-resistant subjects with normal blood pressure). This seminal observation from Japan was subsequently confirmed by investigators in other countries studying humans and animal models [61,62,63,64,65,66,67] and provides the foundation for a key tenet of the contemporary vasodysfunction theory: initiation of salt sensitivity does not usually involve subnormal sodium excretion and retention of greater amounts of sodium in salt-sensitive subjects than in salt-resistant normotensive controls.
Salt-sensitive Japanese and non-Japanese may often excrete a sodium load more slowly, and retain more of it when compared with salt-resistant hypertensive subjects [68, 69], but not when compared with normal subjects (salt-resistant subjects with normal blood pressure) [60,61,62,63]. Thus, contrary to historical theory, a natriuretic handicap (subnormal sodium excretion in response to salt loading) does not usually account for the initiation of most instances of salt sensitivity and salt-induced hypertension. Note that this view does not conflict with the popular teleologic interpretation of salt sensitivity which holds that in salt-sensitive subjects, increases in blood pressure in response to salt loading are “required” to excrete the salt load [1, 50, 55, 57]. The teleologic interpretation is a statement of the supposed purpose of salt-induced hypertension. It is not a statement about the mechanism of salt sensitivity, and it does not address the abnormality that usually mediates salt-induced increases in blood pressure in the first place.
The key role of the renal blood vessels in mediating salt sensitivity and abnormal vascular resistance responses to increases in salt intake
According to the vasodysfunction theory, the abnormal vascular resistance response to salt loading that usually initiates salt-induced hypertension includes impaired renal vasodilation and abnormally increased renal vascular resistance (greater renal vascular resistance in salt-sensitive subjects than in salt-loaded, salt-resistant subjects with normal blood pressure) (Fig. 1) [46]. Investigators in Japan were among the first to show that in salt-sensitive subjects, renal vascular resistance increases within a week after switching from a low-salt diet to a high-salt diet (Fig. 2) [68, 70, 71]. Salt-induced increases in renal vascular resistance have been reported to occur in both Japanese and non-Japanese subjects with salt sensitivity [68, 70,71,72,73,74,75]. These findings in humans are consistent with studies from the Department of Pharmacology, Kagawa Medical School by Tomohiro et al. [76] in conscious Dahl salt-sensitive rats (Dahl S rats) showing that salt loading induces large increases in renal vascular resistance.
Changes in renal vascular resistance, sodium excretion, and blood pressure that occur with initiation of increased salt intake in humans. These results are based on salt-loading studies in salt-sensitive subjects [63, 68, 70, 72,73,74,75] and in salt-resistant normal controls (salt-resistant subjects with normal blood pressure) [63, 74, 77,78,79]. The dotted lines indicate that with initiation of salt-induced increases in blood pressure (within the first few days of salt loading), the exact time courses for the salt-induced changes in renal vascular resistance are unknown. Adapted from Kurtz et al. [46] with permission
To determine whether the salt-induced increases in renal vascular resistance that occur in salt-sensitive subjects are abnormal, it is necessary to have an accurate understanding of the effects of salt loading on renal vascular resistance in appropriate normal controls (salt-resistant subjects with normal blood pressure). Figure 2 shows that in salt-resistant subjects with normal blood pressure, but not in salt-sensitive subjects, renal vascular resistance usually decreases within the first week of switching from a low-salt diet to a high-salt diet [74, 77,78,79]. Thus, in salt-sensitive subjects, the increases in renal vascular resistance that occur in response to salt loading appear distinctly abnormal. Further, in response to short-term salt loading, salt-induced increases in renal vascular resistance are directly correlated with salt-induced increases in blood pressure [74, 75].
As noted above, in response to salt loading, it is well established that renal vascular resistance usually increases in salt-sensitive subjects and decreases in salt-resistant normal controls (salt-resistant subjects with normal blood pressure) [46]. This raises the question: what is the usual effect of salt loading on renal vascular resistance in salt-resistant subjects with hypertension? Investigators in Japan and other countries have found that in salt-resistant subjects with hypertension, renal vascular resistance usually undergoes relatively little or no change in response to short-term increases in salt intake [68, 70,71,72,73]. Thus, within the first week of switching from a low-salt diet to a high-salt diet, renal vascular resistance increases in salt-sensitive subjects (with or without hypertension), decreases in normal subjects (salt-resistant subjects with normal blood pressure), and undergoes relatively little or no change in salt-resistant subjects with hypertension.
The usual mechanism whereby salt-induced increases in renal vascular resistance initiate salt-induced hypertension
In salt-sensitive subjects, the abnormal renal vascular resistance response to salt loading does not usually initiate hypertension by causing greater sodium retention than in salt-loaded normal controls (salt-resistant subjects with normal blood pressure). In salt-sensitive subjects, the abnormal increase in renal vascular resistance with salt loading may constrain salt-sensitive subjects from excreting more of a sodium load than normal subjects (salt-resistant subjects with normal blood pressure) [46]. However, it does not cause salt-sensitive subjects to excrete the sodium load less rapidly and retain more of it than salt-loaded normal subjects [46, 60,61,62,63]. As discussed earlier, in light of the pioneering work of Ishii and colleagues, and of confirmatory studies by others, it is apparent that salt-sensitive subjects usually do not retain more of a salt load than normal subjects, acutely or chronically [46, 60,61,62,63]. Accordingly, the vasodysfunction theory holds that in salt-sensitive subjects, the abnormal renal vascular resistance response to salt loading contributes to initiation of salt-induced hypertension by promoting greater systemic vascular resistance in salt-sensitive subjects than in salt-loaded normal subjects, not by causing greater retention of sodium than in salt-loaded normal controls (Fig. 1) [42, 46].
In salt-sensitive subjects, further research is required to precisely establish the roles of various segments of the renal circulation in determining the abnormal renal vascular resistance responses to salt loading. It has been suggested that in salt-sensitive subjects, increases in salt intake induce increases in afferent and efferent arteriolar resistance [72, 73, 75]. Salt-induced decreases in preglomerular resistance may largely account for the decreases in renal vascular resistance that occur in response to salt loading in salt-resistant normal controls [74, 79]. Furthermore, studies by Fujita and colleagues, Takeshita et al, and others suggest that in salt-sensitive subjects, salt loading may also promote increases in arterial resistance in non-renal vascular beds [28, 68, 80,81,82,83,84]. Thus, in salt-sensitive subjects, abnormal vascular resistance responses to salt loading appear to involve more than just the renal circulation.
In salt-sensitive subjects, what mechanisms mediate abnormal renal vascular resistance responses to increases in salt intake?
According to the vasodysfunction theory of salt sensitivity, the abnormal vascular resistance response to salt loading that is usually involved in initiation of salt-induced hypertension can be mediated by disturbances in a variety of molecular, biochemical, neural, immunologic, and other pathways [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45, 85]. Figure 3 depicts this final common pathway through which an assortment of mechanistic disturbances may enable salt loading to initiate hypertension. The underlying mechanistic disturbances involved in causing abnormal vascular resistance responses to salt loading and salt sensitivity may vary according to genetic, environmental, and demographic factors. Such mechanistic disturbances may cause abnormal vascular resistance responses to salt loading by increasing activity of pathways promoting vasoconstriction, impairing activity of pathways promoting vasodilation, or both. While many mechanisms can be involved in mediating abnormal vascular resistance responses to increases in salt intake, here we highlight the role of disturbances in the nitric oxide (NO) system in the vasculature, and single out key studies from Japan pertaining to this topic.
A final common pathway through which a variety of mechanistic disturbances enable increased salt intake to initiate hypertension. For a discussion of the factors that chronically maintain salt-induced increases in blood pressure, see Kurtz et al. [11]. The mechanistic disturbances mediating the abnormal vascular resistance responses to a high-salt diet that initiate hypertension may also mediate the abnormalities in vascular resistance that characterize sustained hypertension
Highlighting the role of disturbances in nitric oxide activity in mediating the vasodysfunction that initiates salt-induced hypertension
With respect to the pathogenesis of salt sensitivity, we are particularly interested in disturbances that impair activity of vasodilation pathways involved in the flow-mediated decreases in vascular resistance that normally occur in response to increased salt intake. NO plays a major role in flow-mediated vasodilation, and increased NO activity is an important determinant of the reductions in renal and systemic vascular resistance that normally occur in response to a high-salt diet [79]. While other factors besides NO can be involved in flow-induced dilation, Matic et al. [86] have suggested that in the setting of a high-salt diet, the dependence of flow-induced dilation on NO becomes particularly prominent. In 1991, Chen and Sanders [29] proposed that abnormalities in NO activity are involved in the pathogenesis of salt sensitivity and reported that in Dahl S rats, subnormal NO responses to increased salt intake may initiate disturbances in vascular resistance and hypertension.
Asymmetrical dimethylarginine as a mechanism of salt-induced disturbances in NO activity mediating renal vasodysfunction and salt sensitivity
Shortly after the landmark study by Chen and Sanders [29], investigators from Japan [40, 41, 70, 87,88,89,90] and elsewhere [91,92,93,94,95] began to explore mechanisms mediating abnormal NO responses to increased salt intake. Consistent with the hypothesis proposed by Chen and Sanders, Tolins and Shultz [94] found that inhibition of NO synthesis induces salt sensitivity in Sprague Dawley rats that are otherwise resistant to the pressor effects of a high-salt diet. Pioneering work by Japanese investigators indicated that in salt-sensitive subjects, impaired NO activity in response to a high-salt diet may be mediated by endogenous inhibitors of NO synthase and by various factors that impair NO bioavailability. Specifically, Matsuoka et al. [40] from Kurume University School of Medicine reported that the failure of Dahl S rats to normally increase NO activity in response to increases in salt intake is mediated by abnormally increased levels of asymmetrical dimethylarginine (ADMA). ADMA substantially inhibits NO activity by inhibiting NO synthase activity and by increasing oxidative stress [96,97,98,99]. Oxidative stress impairs NO bioavailability and also increases ADMA levels, and has been proposed to be a key determinant of salt sensitivity [43, 44, 99,100,101]. Suda et al. [97] from the School of Medicine of the University of Occupational and Environmental Health in Kitakyushu reported that in mice, ADMA can induce vascular oxidative stress and vascular damage in the presence or absence of endothelial nitric oxide synthase, possibly by increasing vascular levels of angiotensin-converting enzyme and activity of the vascular renin angiotensin system (RAS).
In agreement with the experimental findings in animal models, studies in both Japanese and non-Japanese humans have demonstrated that in salt-sensitive subjects, but not in salt-resistant subjects [31, 41, 63, 102,103,104], increases in salt intake: (1) reduce vascular activity of the enzyme dimethylarginine dimethylaminohydrolase (DDAH) that degrades ADMA; (2) induce increases in plasma ADMA and urinary excretion of ADMA; and (3) reduce biomarkers of NO in plasma. In key studies in hypertensive and normotensive Japanese, Fujiwara et al. [41] at Hirosaki University found that salt-induced changes in blood pressure correlated inversely with salt-induced changes in plasma nitrate/nitrite levels, which correlated inversely with salt-induced changes in plasma levels of ADMA. Based on these and other observations, the investigators concluded that “Modulation of NO synthesis by salt intake may be involved in a mechanism for salt sensitivity in human hypertension, presumably via the change in ADMA” [41]. Figure 4 illustrates the proposed involvement of ADMA in salt-induced disturbances in NO activity mediating vasodysfunction in salt-sensitive subjects.
Consistent with the role for ADMA in salt sensitivity proposed by Fujiwara et al. [41], Schmidlin et al. [63] found that in salt-sensitive African Americans, increases in plasma levels of ADMA occur within 24 h of initiating increased salt intake. In normal, salt-resistant African-American control subjects, the same salt loading does not increase ADMA levels [63]. In salt-sensitive subjects, the early salt-induced increases in ADMA levels precede the initiation of salt-induced increases in blood pressure and are not simply a consequence of salt-induced hypertension [63].
Although many mechanisms may mediate the disturbances in renal vascular resistance involved in the pathogenesis of salt sensitivity, the role of abnormal ADMA activity is of particular interest because it is one of the few mechanisms that might explain why the trait of salt-sensitivity does not always “follow the kidney” in transplantation studies [105]. As we have discussed elsewhere [42], it is conceivable that non-renal production of ADMA, or renal production of ADMA, or both could bring about salt-sensitivity. Specifically, intrarenal disturbances in NO activity in salt-sensitive animals caused by salt-induced increases in circulating ADMA from extra-renal sources could explain why transplanting a kidney from a Dahl salt-resistant rat into a bilaterally nephrectomized salt-sensitive recipient fails to correct salt-sensitivity in the recipient [105]. In addition, intrarenal disturbances in NO activity caused by salt-induced increases in renal production of ADMA and decreases in renal clearance of ADMA could account for the observation that transplantation of a kidney from a Dahl salt-sensitive donor into a bilaterally nephrectomized salt-resistant recipient induces salt-sensitivity in the recipient [105].
Additional mechanisms of salt-induced disturbances in NO activity mediating renal vasodysfunction and salt sensitivity
Many factors in addition to ADMA may be involved in mediating salt-induced disturbances in oxidative stress and NO activity that promote renal vasodysfunction and salt sensitivity [30, 43, 45, 106, 107]. For example, Kobori et al. [108, 109] reported that in Dahl S rats, increased salt intake causes a paradoxical increase in intrarenal levels of angiotensinogen and fails to normally suppress angiotensin II in the kidney. With salt loading, greater levels of angiotensin II in renal tissue in salt-sensitive rats versus salt-resistant rats could generate greater NADPH oxidase activity and abnormally high levels of renal oxidative stress that can interfere with NO bioactivity [44]. The observations of Kobori et al. in Dahl rats [108] are consistent with the clinical studies of Konishi et al. [110] and others [74, 111, 112], which suggest that abnormal responses of the intrarenal RAS to salt loading may mediate salt-induced disturbances in oxidative stress, renal vascular resistance, and salt sensitivity in humans. Because the balance between NO activity and angiotensin II activity is a key determinant of vascular tone [101, 113, 114], interactions between the NO system and the RAS should be considered when assessing the mechanisms underlying renal vasodysfunction and salt sensitivity. Figure 5 illustrates a role for the intrarenal RAS in mediating impaired NO bioavailability, abnormal renal vascular resistance, and increased blood pressure in response to salt loading.
Based on the work of Oberleithner and others [36, 39, 115,116,117,118], it has been proposed that impairment of NO-mediated vasodilation in response to a high-salt diet may also be caused by increases in endothelial cell stiffness mediated by salt-induced increases in plasma sodium concentrations, together with aberrant increases in the activity of epithelial-like sodium channels in endothelial cells (termed “EnNaCs”) [118]. Increases in EnNaC activity promote increased endothelial cell membrane stiffness by promoting sodium influx, cell swelling, and membrane actin polymerization [36, 116,117,118]. By reducing membrane deformability, increased endothelial cell stiffness may interfere with flow-mediated activation of mechanoreceptors and signaling pathways that promote increases in NO activity and vasodilation in response to increases in salt intake [36, 117, 118]. Figure 6 illustrates a role for increases in EnNaC activity and endothelial stiffness in mediating impaired NO bioavailability and abnormal vascular resistance in response to salt loading. Because increases in EnNaC activity may be caused by mineralocorticoid excess, or by certain genetic mutations, this mechanism of salt-induced vasodysfunction may be of particular importance in patients with hyperaldosteronism, the syndrome of apparent mineralocorticoid excess, Liddle syndrome, or some forms of congenital adrenal hyperplasia [47, 116, 118].
Implications of the vasodysfunction theory of salt sensitivity for prevention and treatment of salt-induced hypertension
As we have emphasized, initiation of salt-induced hypertension usually involves the combination of (1) abnormal vascular resistance responses to acute salt loading that cause renal vascular resistance to become greater in salt-sensitive subjects than in salt-resistant normal controls and (2) normal increases in sodium retention in response to acute salt loading that do not cause greater increases in sodium balance and cardiac output in salt-sensitive subjects than in salt-resistant normal controls. Thus, in salt-sensitive subjects, prevention of either the abnormal vascular resistance responses to salt loading, or the normal increases in sodium balance and cardiac output in response to salt loading, can help prevent the initiation of salt-induced hypertension.
While restriction of dietary intake of salt is routinely recommended for prevention or treatment of salt-induced hypertension, many individuals may not wish, or be able, to reduce their intake of salt to the levels recommended by medical authorities in Japan (<6 g NaCl per day) [7] or to even lower dietary targets recommended in other countries such as the United States and Germany (<3.8 g NaCl per day) [119, 120]. Thus, additional strategies are needed for prevention and management of salt sensitivity and salt-induced hypertension.
Ideally, interventions to prevent or treat salt-induced hypertension should be primarily directed at the abnormal physiologic mechanisms that usually mediate salt sensitivity, i.e., the abnormal vascular resistance responses to salt loading, including those involving the renal vasculature. In patients in whom the salt sensitivity is due to functional disturbances in vascular resistance mediated by low NO activity, excess activity of the RAS, or excess activity of other vasoconstrictors, use of angiotensin receptor blockers (ARBs), calcium channel blockers, or both may be sufficient to treat salt sensitivity. For example, in cases of salt sensitivity mediated by subnormal NO responses to increases in salt intake, interventions aimed at improving NO bioavailability in the renal vasculature would represent a targeted approach to prevention and treatment of salt-induced hypertension. However, in salt-sensitive subjects with noncompliant blood vessels (e.g. advanced arteriolar nephrosclerosis) in whom subnormal vasodilatory responses to salt loading may be mediated by structural changes in the vasculature, treatments aimed at promoting vasodilation may have limited effectiveness in reducing vascular resistance and attenuating salt-induced hypertension. Accordingly, in those subjects, salt restriction and or diuretic therapy will be required in the management of salt sensitivity.
Pharmacologic approaches to preventing salt sensitivity mediated by subnormal NO responses to a high-salt diet
In subjects with salt sensitivity mediated by subnormal NO responses to a high-salt diet, pharmacologic correction of mechanistic abnormalities that cause oxidative stress and impair NO bioactivity may attenuate salt sensitivity and reduce the risk for salt-induced hypertension. Consistent with this view, Imanishi et al. [111] proposed that in some patient subgroups, treatment with ARBs to attenuate salt-induced increases in renal oxidative stress and support NO activity may protect against salt sensitivity. Specifically, the investigators found that in diabetic patients with microalbuminuria, salt sensitivity is associated with reduced urinary excretion of NO metabolites (nitrate and nitrite, NOx) in response to salt loading [111]. Treatment with an ARB reduced renal excretion of a marker of oxidative stress (8-hydroxy-2′-deoxyguanosine), increased NOx excretion, and reduced salt sensitivity (defined by the blood pressure effects of switching NaCl intake from 60 mmol/day for one week to 180 mmol/day for one week) [111]. As noted by Imanishi et al. [111], these observations suggest that the protective effect of ARB treatment on salt sensitivity may be mediated through antioxidative mechanisms that restore NO bioavailability in the kidney.
In studies in which blood pressure has been directly measured through arterial catheters in unanesthetized animals, blockade of the RAS has also been found to significantly attenuate salt-induced hypertension in classic animal models of salt sensitivity including in Dahl S rats [121,122,123] and in rodents with reduced renal mass [67]. Recently, Hatanaka et al. [124], from Osaka University Graduate School of Medicine, speculated that in partially nephrectomized mice, the ARB azilsartan attenuates salt sensitivity by inhibiting proximal tubule reabsorption of sodium. However, in those studies, no measurements were reported of the effects of azilsartan on the changes in sodium balance induced by salt loading. Furthermore, Kanagy and Fink [67] found that in partially nephrectomized rats, the ARB losartan prevents salt-induced hypertension without attenuating salt-induced increases in sodium balance. The present discussion focuses on the possibility that RAS inhibitors attenuate salt sensitivity by inhibiting activity of the intrarenal RAS, reducing renal oxidative stress, and maintaining NO bioavailability. However, it should be noted that effects of RAS inhibitors on the central nervous system and sympathoexcitation may also mediate the capacity of these inhibitors to protect against abnormal vascular resistance responses to salt loading that initiate salt-induced hypertension [125, 126].
As previously discussed, some cases of salt sensitivity involve impaired NO activity caused by increases in endothelial cell stiffness mediated by mineralocorticoid excess, or by other factors that stimulate activity of EnNaCs [39, 118]. In those cases, pharmacologic treatment would rationally include agents that attenuate EnNaC activity (e.g., mineralocorticoid receptor (MR) blockers or epithelial sodium channel blockers) [127, 128]. Such agents might be expected to protect against salt-induced increases in blood pressure not only by ameliorating abnormal vascular resistance responses to salt loading but also by attenuating salt-induced increases in sodium balance. However, we are unaware of any published studies which have compared the effects of salt loading on sodium balance, cardiac output, and vascular resistance in salt-sensitive subjects treated with MR blockers or epithelial sodium channel blockers to those in placebo-treated salt-sensitive controls. It should also be noted that the capacity of MR blockers or epithelial sodium channel blockers to affect vascular resistance responses to salt loading and attenuate salt sensitivity may be related to effects of these drugs on central nervous system activity [129, 130].
Unfortunately, in clinical practice, we do not have efficient tests for readily identifying patients with salt sensitivity and we do not have effective tests for readily determining the primary abnormalities causing salt sensitivity in most affected individuals. Thus, from a practical point of view, it is currently difficult to target pharmacologic therapy to the primary mechanistic abnormalities mediating salt sensitivity. As we continue to gain a better understanding of the mechanisms of salt sensitivity, it may become possible to develop better tests for identifying salt-sensitive patients and for guiding the choice of pharmacologic therapy in the future.
Dietary approaches to augmenting NO activity and preventing salt sensitivity
The use of dietary approaches to prevent salt sensitivity by augmenting NO activity was originally tested in animals by Chen and Sanders [29] more than 25 years ago. In studies using Dahl S rats, the investigators found that increased intake of the NO precursor l-arginine could prevent salt-induced hypertension [29]. However, patients with endothelial dysfunction have a reduced ability to convert l-arginine to NO [131]. As an example, Higashi et al. [70], at the Hiroshima University School of Medicine, found that in Japanese in-patients with mild to moderate essential hypertension and salt sensitivity, salt loading may impair the ability of l-arginine to increase endothelial NO synthesis in the renal vasculature and decrease renal vascular resistance. For these and other reasons, we have advocated alternative dietary approaches to preventing salt-induced hypertension based on increased intake of vegetables with a high content of nitrate which can augment generation of NO without the need to increase NO synthase activity [132].
In humans and in animals, NO can be generated by reduction of nitrite derived from dietary or non-dietary sources of nitrate [133]. In addition, supplemental administration of nitrate or nitrite has been reported to reduce blood pressure in humans and animals [133]. Gao et al. [134] have suggested that the renal microvasculature is a primary target for blood pressure regulation by nitrite and nitrate because preglomerular resistance vessels are particularly sensitive to the capacity of nitrite to promote vasodilation and to inhibit vasoconstriction induced by angiotensin II. While angiotensin II is known to promote efferent arteriolar constriction, it can also increase afferent arteriolar tone [110]. According to Gao et al. [134], “nitrate and nitrite dilate renal afferent arterioles and counteract angiotensin II-induced vasoconstriction by generating NO-like bioactivity and reducing NADPH oxidase activity”.
The high concentration of nitrate in leafy green vegetables is considered to be an important determinant of the antihypertensive effect of traditional Japanese diets [135] and of the Dietary Approaches to Stop Hypertension (DASH) diet [136,137,138,139,140]. In normal subjects fed a diet containing traditional Japanese vegetables with a high nitrate content, Sobko et al. [135] from the Kyorin University School of Medicine in Tokyo found that plasma levels of nitrate and nitrite were higher, and blood pressure was lower than in subjects fed a control diet lacking those vegetables. Blood pressure of Japanese vegetarians is also lower than that of non-vegetarians [141]. Furthermore, Japanese longevity is among the highest in the world [142, 143], which might be explained, in part, by high consumption of a diet rich in green leafy vegetables associated with reduced risk of cardiovascular diseases [135, 144].
In unilaterally nephrectomized rats, supplemental dietary nitrate has been reported to attenuate salt-induced hypertension [145], and in normotensive or hypertensive humans, the DASH diet has also been found to attenuate salt-induced increases in blood pressure [146]. Vegetable-rich diets such as the DASH diet and the DASH-Japan Ube Modified diet Program (DASH-JUMP) not only can contain large amounts of nitrate, they can also contain substantial amounts of potassium, both of which could contribute to protection from salt-induced increases in blood pressure [146, 147]. In fact, potassium is a nutrient recognized by the Japanese government in the evaluation of foods with functional claims for maintaining healthy blood pressure. Although potassium may reduce blood pressure partly by increasing urinary excretion of sodium, the antihypertensive effects of potassium also appear to involve effects on the NO system [103, 148]. For example, studies in normotensive salt-sensitive humans by Fang et al. [103] indicate that the capacity of supplemental potassium to protect against salt-induced increases in blood pressure is related, at least in part, to its capacity to prevent salt-induced increases in plasma levels of ADMA and decreases in plasma levels of nitrate and nitrite. The beneficial effects of nitrate and potassium on NO activity and blood pressure provide scientific support for efforts by Japanese and other governmental agencies to encourage greater consumption of vegetables in the general population.
Summary
According to the vasodysfunction theory of salt sensitivity, the initiation of salt-induced hypertension usually involves abnormal renal vascular resistance responses to increases in salt intake, not greater renal retention of a salt load in salt-sensitive subjects than in salt-resistant normal controls. The scientific foundation of this theory is based heavily on pioneering studies from Japan that have provided critical insights into the mechanisms that normally mediate resistance to the pressor effects of a high-salt diet, and the abnormalities commonly involved in the pathogenesis of salt-induced hypertension. In addition to shifting the focus away from historical theories of salt sensitivity, research from Japan has pointed to new dietary strategies for reducing the risk for salt-induced hypertension that are based on enhancing NO activity and that do not depend on reducing salt consumption in the population.
References
Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46.
Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–7.
Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. An appraisal of methods recently recommended for testing salt sensitivity of blood pressure. J Am Heart Assoc. 2017;6:e005653
de Leeuw PW, Kroon AA. Salt and sensitivity. Hypertension. 2013;62:461–2.
Galletti F, Strazzullo P. The blood pressure-salt sensitivity paradigm: pathophysiologically sound yet of no practical value. Nephrol Dial Transplant. 2016;31:1386–91.
Tsuchihashi T, Kai H, Kusaka M, Kawamura M, Matsuura H, Miura K, et al. [Scientific statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension (3) Assessment and application of salt intake in the management of hypertension. Hypertens Res. 2013;36:1026–31.
Miura K, Ando K, Tsuchihashi T, Yoshita K, Watanabe Y, Kawarazaki H, et al. [Scientific statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension (2) Goal and strategies of dietary salt reduction in the management of hypertension. Hypertens Res. 2013;36:1020–5.
Ando K, Kawarazaki H, Miura K, Matsuura H, Watanabe Y, Yoshita K, et al. [Scientific statement] Report of the Salt Reduction Committee of the Japanese Society of Hypertension (1) Role of salt in hypertension and cardiovascular diseases. Hypertens Res. 2013;36:1009–19.
Ando K, Fujita T. Pathophysiology of salt sensitivity hypertension. Ann Med. 2012;44(Suppl 1):S119–26.
Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, et al. National Heart, Lung, and Blood Institute Working Group Report on Salt in Human Health and Sickness: building on the current scientific evidence. Hypertension. 2016;68:281–8.
Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr. The American Heart Association Scientific Statement on Salt Sensitivity of Blood Pressure: prompting consideration of alternative conceptual frameworks for the pathogenesis of salt sensitivity? J Hypertens. 2017;25:2214–25.
Brooks VL, Osborn JW. Hormonal-sympathetic interactions in long-term regulation of arterial pressure: an hypothesis. Am J Physiol. 1995;268(Pt 2):R1343–58.
Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis. J Physiol. 2015;593:3065–75.
Stocker SD, Kinsman BJ, Sved AF. Recent advances in neurogenic hypertension: dietary salt, obesity, and inflammation. Hypertension. 2017;70:474–78.
Gavras I, Gavras H. ‘Volume-expanded’ hypertension: the effect of fluid overload and the role of the sympathetic nervous system in salt-dependent hypertension. J Hypertens. 2012;30:655–9.
Mark AL. Sympathetic neural contribution to salt-induced hypertension in Dahl rats. Hypertension. 1991;17(Suppl):I86–90.
Fujita M, Fujita T. The role of CNS in the effects of salt on blood pressure. Curr Hypertens Rep. 2016;18:10.
Shimosawa T, Mu S, Shibata S, Fujita T. The kidney and hypertension: pathogenesis of salt-sensitive hypertension. Curr Hypertens Rep. 2012;14:468–72.
Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–9.
Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–49.
Blaustein MP, Chen L, Hamlyn JM, Leenen FH, Lingrel JB, Wier WG, et al. Pivotal role of alpha2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J Physiol. 2016;594:6079–103.
Liclican EL, McGiff JC, Falck JR, Carroll MA. Failure to upregulate the adenosine2A receptor-epoxyeicosatrienoic acid pathway contributes to the development of hypertension in Dahl salt-sensitive rats. Am J Physiol Ren Physiol. 2008;295:F1696–704.
Pettersen KH, Bugenhagen SM, Nauman J, Beard DA, Omholt SW. Arterial stiffening provides sufficient explanation for primary hypertension. PLoS Comput Biol. 2014;10:e1003634.
Prager-Khoutorsky M, Choe KY, Levi DI, Bourque CW. Role of vasopressin in rat models of salt-dependent hypertension. Curr Hypertens Rep. 2017;19:42.
Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ, et al. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res. 2013;113:1296–307.
Matsuguchi H, Schmid PG, Van Orden D, Mark AL. Does vasopressin contribute to salt-induced hypertension in the Dahl strain? Hypertension. 1981;3:174–81.
Hatzinikolaou P, Gavras H, Brunner HR, Gavras I. Sodium-induced elevation of blood pressure in the anephric state. Science. 1980;209:935–6.
Chamarthi B, Williams JS, Williams GH. A mechanism for salt-sensitive hypertension: abnormal dietary sodium-mediated vascular response to angiotensin-II. J Hypertens. 2010;28:1020–6.
Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–67.
Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. J Am Soc Nephrol. 2017;28:1362–9.
Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29:415–24.
Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–61.
Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens. 2010;19:385–92.
Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–15.
Bevan JA. Flow regulation of vascular tone. Its sensitivity to changes in sodium and calcium. Hypertension. 1993;22:273–81.
Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–6.
Oberleithner H. A physiological concept unmasking vascular salt sensitivity in man. Pflug Arch. 2012;464:287–93.
Kusche-Vihrog K, Oberleithner H. An emerging concept of vascular salt sensitivity. F1000 Biol Rep. 2012;4:20.
Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelium. Pflug Arch. 2014;466:851–9.
Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, et al. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension. 1997;29(Pt 2):242–7.
Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–61.
Morris RC, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;133:881–93.
Manning RD Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand. 2003;179:243–50.
Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–35.
Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–82.
Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr. The pivotal role of renal vasodysfunction in salt sensitivity and the initiation of salt-induced hypertension. Curr Opin Nephrol Hypertens. 2018;27:83–92.
Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, Morris RC. Molecular based mechanisms of Mendelian forms of salt-dependent hypertension: questioning the prevailing theory. Hypertension. 2015;65:932–41.
Guyton AC, ed. Circulatory physiology: III. Arterial pressure and hypertension. Philadelphia: W.B. Saunders; 1980.
Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259(Pt 2):R865–877.
Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis: a cause or a consequence of hypertension? Hypertension. 1990;15:547–59.
Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–41.
Hall JE. Guyton and Hall textbook of medical physiology. 13th ed. Philadelphia: Elsevier; 2015.
Hall JE. Renal dysfunction, rather than non-renal vascular dysfunction, mediates salt-induced hypertension. Circulation. 2016;133:894–907.
Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56.
Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–23.
Brands MW. Chronic blood pressure control. Compr Physiol. 2012;2:2481–94.
Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:1229–37.
Cowley AW Jr., Abe M, Mori T, O’Connor PM, Ohsaki Y, et al. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Ren Physiol. 2015;308:F179–197.
Schrier RW. Renal and electrolyte disorders. 8th ed. Philadelphia: Wolters Kluwer; 2018.
Ishii M, Atarashi K, Ikeda T, Hirata Y, Igari T, Uehara Y, et al. Role of the aldosterone system in the salt-sensitivity of patients with benign essential hypertension. Jpn Heart J. 1983;24:79–89.
Wedler B, Brier ME, Wiersbitzky M, Gruska S, Wolf E, Kallwellis R, et al. Sodium kinetics in salt-sensitive and salt-resistant normotensive and hypertensive subjects. J Hypertens. 1992;10:663–9.
Schmidlin O, Sebastian AF, Morris RC Jr. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49:1032–9.
Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–5.
Roman RJ, Osborn JL. Renal function and sodium balance in conscious Dahl S and R rats. Am J Physiol. 1987;252:R833–41.
Nakamura K, Cowley AW Jr. Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension. 1989;13:243–9.
Hu L, Manning RD Jr. Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in Dahl rats. Am J Physiol. 1995;268(Pt 2):H2375–83.
Kanagy NL, Fink GD. Losartan prevents salt-induced hypertension in reduced renal mass rats. J Pharmacol Exp Ther. 1993;265:1131–6.
Fujita T, Ando K, Ogata E. Systemic and regional hemodynamics in patients with salt-sensitive hypertension. Hypertension. 1990;16:235–44.
Fujita T, Henry WL, Bartter FC, Lake CR, Delea CS. Factors influencing blood pressure in salt-sensitive patients with hypertension. Am J Med. 1980;80:234.
Higashi Y, Oshima T, Watanabe M, Matsuura H, Kajiyama G. Renal response to L-arginine in salt-sensitive patients with essential hypertension. Hypertension. 1996;27(Pt 2):643–8.
Sanai T, Kimura G. Renal function reserve and sodium sensitivity in essential hypertension. J Lab Clin Med. 1996;128:89–97.
Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–12.
Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994;23:195–9.
van Paassen P, de Zeeuw D, Navis G, de Jong PE. Does the renin-angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension. 1996;27:202–8.
Schmidlin O, Forman A, Tanaka M, Sebastian A, Morris RC Jr. NaCl-induced renal vasoconstriction in salt-sensitive African Americans: antipressor and hemodynamic effects of potassium bicarbonate. Hypertension. 1999;33:633–9.
Tomohiro A, Kimura S, He H, Fujisawa Y, Nishiyama A, Kiyomoto K, et al. Regional blood flow in Dahl-Iwai salt-sensitive rats and the effects of dietary L-arginine supplementation. Am J Physiol. 1997;272(Pt 2):R1013–9.
Hollenberg NK, Chenitz WR, Adams DF, Williams GH. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest. 1974;54:34–42.
Redgrave J, Rabinowe S, Hollenberg N, Williams GH. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertension. J Clin Invest. 1985;75:1285–90.
Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am J Physiol. 1998;274(Pt 2):F914–23.
Van Paassen P, de Zeeuw D, de Jong JW, Navis G. Renal hemodynamics in human hypertension. Adv Organ Biol. 2000;9:369–82.
Abboud FM. Effects of sodium, angiotensin, and steroids on vascular reactivity in man. Fed Proc. 1974;33:143–9.
Mark AL, Lawton WJ, Abboud FM, Fitz AE, Connor WE, Heistad DD. Effects of high and low sodium intake on arterial pressure and forearm vascular resistance in borderline hypertension. Circ Res. 1975;36/37(Suppl 1):I-194–I-198.
Takeshita A, Imaizumi T, Ashihara T, Nakamura M. Characteristics of responses to salt loading and deprivation in hypertensive subjects. Circ Res. 1982;51:457–64.
Koolen MI, Van Brummelen P. Adrenergic activity and peripheral hemodynamics in relation to sodium sensitivity in patients with essential hypertension. Hypertension. 1984;6:820–5.
Ito K, Hirooka Y, Sunagawa K. Cardiac sympathetic afferent stimulation induces salt-sensitive sympathoexcitation through hypothalamic epithelial Na+ channel activation. Am J Physiol Heart Circ Physiol. 2015;308:H530–539.
Matic A, Jukic I, Stupin A, Baric L, Mihaljevic Z, Unfirer S, et al. High salt intake shifts the mechanisms of flow-induced dilation in the middle cerebral arteries of Sprague-Dawley rats. Am J Physiol Heart Circ Physiol. 2018. https://doi.org/10.1152/ajpheart.00097.2018 [Epub ahead of print]
Ito S. Nitric oxide in the kidney. Curr Opin Nephrol Hypertens. 1995;4:23–30.
Hayakawa H, Hirata Y, Suzuki E, Kimura K, Kikuchi K, Nagano T, et al. Long-term administration of L-arginine improves nitric oxide release from kidney in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1994;23(Pt 1):752–6.
Osanai T, Fujiwara N, Saitoh M, Sasaki S, Tomita H, Nakamura M, et al. Relationship between salt intake, nitric oxide and asymmetric dimethylarginine and its relevance to patients with end-stage renal disease. Blood Purif. 2002;20:466–8.
Miyaki K, Tohyama S, Murata M, Kikuchi H, Takei I, Watanabe K, et al. Salt intake affects the relation between hypertension and the T-786C polymorphism in the endothelial nitric oxide synthase gene. Am J Hypertens. 2005;18(Pt 1):1556–62.
Yamada SS, Sassaki AL, Fujihara CK, Malheiros DM, De Nucci G, Zatz R. Effect of salt intake and inhibitor dose on arterial hypertension and renal injury induced by chronic nitric oxide blockade. Hypertension. 1996;27:1165–72.
Fujihara CK, Michellazzo SM, de Nucci G, Zatz R. Sodium excess aggravates hypertension and renal parenchymal injury in rats with chronic NO inhibition. Am J Physiol. 1994;266(Pt 2):F697–705.
Raij L. Nitric oxide, salt sensitivity, and cardiorenal injury in hypertension. Semin Nephrol. 1999;19:296–303.
Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230–6.
Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37(Pt 2):444–8.
Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–30.
Suda O, Tsutsui M, Morishita T, Tasaki H, Ueno S, Nakata S, et al. Asymmetric dimethylarginine produces vascular lesions in endothelial nitric oxide synthase-deficient mice: involvement of renin-angiotensin system and oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1682–8.
Veresh Z, Racz A, Lotz G, Koller A. ADMA impairs nitric oxide-mediated arteriolar function due to increased superoxide production by angiotensin II-NAD(P)H oxidase pathway. Hypertension. 2008;52:960–6.
Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension. 2012;59:375–81.
Majid DS, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–52.
Kopkan L, Cervenka L. Renal interactions of renin-angiotensin system, nitric oxide and superoxide anion: implications in the pathophysiology of salt-sensitivity and hypertension. Physiol Res. 2009;58(Suppl 2):S55–67.
Cubeddu LX, Alfieri AB, Hoffmann IS, Jimenez E, Roa CM, Cubeddu R, et al. Nitric oxide and salt sensitivity. Am J Hypertens. 2000;13:973–9.
Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive Asians. Hypertension. 2006;48:724–9.
Cao Y, Mu JJ, Fang Y, Yuan ZY, Liu FQ. Impact of high salt independent of blood pressure on PRMT/ADMA/DDAH pathway in the aorta of Dahl salt-sensitive rats. Int J Mol Sci. 2013;14:8062–72.
Morgan DA, DiBona GF, Mark AL. Effects of interstrain renal transplantation on NaCl-induced hypertension in Dahl rats. Hypertension. 1990;15:436–42.
Majid DS, Prieto MC, Navar LG. Salt-sensitive hypertension: perspectives on intrarenal mechanisms. Curr Hypertens Rev. 2015;11:38–48.
Boegehold MA, Drenjancevic I, Lombard JH. Salt, Angiotensin II, superoxide, and endothelial function. Compr Physiol. 2016;6:215–54.
Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–7.
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–87.
Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, et al. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58:205–11.
Imanishi M, Okada N, Konishi Y, Morikawa T, Maeda I, Kitabayashi C, et al. Angiotensin II receptor blockade reduces salt sensitivity of blood pressure through restoration of renal nitric oxide synthesis in patients with diabetic nephropathy. J Renin Angiotensin Aldosterone Syst. 2013;14:67–73.
Underwood PC, Chamarthi B, Williams JS, Vaidya A, Garg R, Adler GK, et al. Nonmodulation as the mechanism for salt sensitivity of blood pressure in individuals with hypertension and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3775–82.
Millatt LJ, Abdel-Rahman EM, Siragy HM. Angiotensin II and nitric oxide: a question of balance. Regul Pept. 1999;81:1–10.
Patzak A, Persson AE. Angiotensin II-nitric oxide interaction in the kidney. Curr Opin Nephrol Hypertens. 2007;16:46–51.
Perez FR, Venegas F, Gonzalez M, Andres S, Vallejos C, Riquelme G, et al. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension. 2009;53:1000–7.
Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, et al. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–9.
Kusche-Vihrog K, Tarjus A, Fels J, Jaisser F. The epithelial Na+ channel: a new player in the vasculature. Curr Opin Nephrol Hypertens. 2014;23:143–8.
Warnock DG, Kusche-Vihrog K, Tarjus A, Sheng S, Oberleithner H, Kleyman TR, et al. Blood pressure and amiloride-sensitive sodium channels in vascular and renal cells. Nat Rev Nephrol. 2014;10:146–57.
Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–9.
Strohm D, Bechthold A, Ellinger S, Leschik-Bonnet E, Stehle P, Heseker H. Revised reference values for the intake of sodium and chloride. Ann Nutr Metab. 2018;72:12–17.
Jin C, O’Boyle S, Kleven DT, Pollock JS, Pollock DM, White JJ. Antihypertensive and anti-inflammatory actions of combined azilsartan and chlorthalidone in Dahl salt-sensitive rats on a high-fat, high-salt diet. Clin Exp Pharmacol Physiol. 2014;41:579–88.
Liang B, Leenen FH. Prevention of salt induced hypertension and fibrosis by angiotensin converting enzyme inhibitors in Dahl S rats. Br J Pharmacol. 2007;152:903–14.
Liang B, Leenen FH. Prevention of salt-induced hypertension and fibrosis by AT1-receptor blockers in Dahl S rats. J Cardiovasc Pharmacol. 2008;51:457–66.
Hatanaka M, Kaimori J-Y, Yamamoto S, Matsui I, Hamano T, Takabatake Y, et al. Azilsartan improves salt sensitivity by modulating the proximal tubular Na(+)-H(+) Exchanger-3 in mice. PLoS One. 2016;11:e0147786.
Leenen FH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension. 2001;37:981–4.
Huang BS, Leenen FH. Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension. 1998;32:1028–33.
Paar M, Pavenstadt H, Kusche-Vihrog K, Druppel V, Oberleithner H, Kliche K. Endothelial sodium channels trigger endothelial salt sensitivity with aging. Hypertension. 2014;64:391–6.
Toda N, Nakanishi S, Tanabe S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: therapeutic implications. Br J Pharmacol. 2013;168:519–33.
Van Huysse JW, Amin MS, Yang B, Leenen FH. Salt-induced hypertension in a mouse model of Liddle syndrome is mediated by epithelial sodium channels in the brain. Hypertension. 2012;60:691–6.
Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FH. Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience. 2016;314:90–105.
Zand J, Lanza F, Garg HK, Bryan NS. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr Res. 2011;31:262–9.
Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J Cardiol. 2018;72:42–49.
Carlstrom M, Lundberg JO, Weitzberg E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol (Oxf). 2018;224:e13080.
Gao X, Yang T, Liu M, Peleli M, Zollbrecht C, Weitzberg E, et al. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–70.
Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–40.
Lundberg JO, Feelisch M, Bjorne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–62.
Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10.
Gee LC, Ahluwalia A. Dietary nitrate lowers blood pressure: epidemiological, pre-clinical experimental and clinical trial evidence. Curr Hypertens Rep. 2016;18:17.
Khatri J, Mills CE, Maskell P, Odongerel C, Webb AJ. It is rocket science—why dietary nitrate is hard to beet! Part I: Twists and turns in the realisation of the nitrate-nitrite-NO pathway. Br J Clin Pharmacol. 2017;83:129–39.
Mills CE, Khatri J, Maskell P, Odongerel C, Webb AJ. It is rocket science—why dietary nitrate is hard to Beet! part II: Further mechanisms and therapeutic potential of the nitrate-nitrite-NO pathway. Br J Clin Pharmacol. 2017;83:140–51.
Nakamoto K, Watanabe S, Kudo H, Tanaka A. Nutritional characteristics of middle-aged Japanese vegetarians. J Atheroscler Thromb. 2008;15:122–9.
Robine JM, Cubaynes S. Worldwide demography of centenarians. Mech Ageing Dev. 2017;165(Pt B):59–67.
Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208.
Sho H. History and characteristics of Okinawan longevity food. Asia Pac J Clin Nutr. 2001;10:159–64.
Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, et al. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011;89:574–85.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D. et al. Group DA-SCR. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10.
Kawamura A, Kajiya K, Kishi H, Inagaki J, Mitarai M, Oda H, et al. Effects of the DASH-JUMP dietary intervention in Japanese participants with high-normal blood pressure and stage 1 hypertension: an open-label single-arm trial. Hypertens Res. 2016;39:777–85.
Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, et al. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci USA. 2009;106:2829–34.
Funding support
National Center for Research Resources, M0 RR-00079, US Public Health Service; National Institutes of Health/National Heart, Lung and Blood Institute grant RO1-HL64230; Praemium Academiae award of the Czech Academy of Sciences to MP; and gifts from the Saw Island Foundation, the Antel Foundation, and the Maier Family Foundation
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kurtz, T.W., DiCarlo, S.E., Pravenec, M. et al. Changing views on the common physiologic abnormality that mediates salt sensitivity and initiation of salt-induced hypertension: Japanese research underpinning the vasodysfunction theory of salt sensitivity. Hypertens Res 42, 6–18 (2019). https://doi.org/10.1038/s41440-018-0122-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0122-5
Keywords
This article is cited by
-
Role of the microbiota in hypertension and antihypertensive drug metabolism
Hypertension Research (2022)
-
Frequency of alcohol drinking modifies the association between salt intake and albuminuria: a 1-year observational study
Hypertension Research (2020)
-
High-salt intake increases TRPC3 expression and enhances TRPC3-mediated calcium influx and systolic blood pressure in hypertensive patients
Hypertension Research (2020)
-
Sodium-induced inflammation—an invisible player in resistant hypertension
Hypertension Research (2020)
-
Female Sex, a Major Risk Factor for Salt-Sensitive Hypertension
Current Hypertension Reports (2020)