Recent Advances in Chiral Analysis of Proteins and Peptides
Abstract
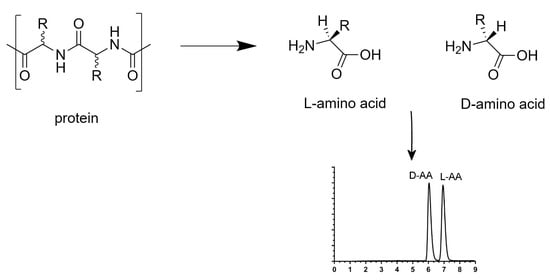
:1. Introduction
2. Determination of Chiral Protein Sequences
3. Analysis of Chiral Amino Acids
3.1. Direct Method
3.1.1. Liquid Chromatography
Polysaccharide-Based Chiral Stationary Phases
Cyclodextrin-Based Chiral Stationary Phases
Crown Ether-Based Chiral Stationary Phases
Brush-Type or Pirkle-Type Chiral Stationary Phases
Ion- and Zwitterion-Exchange-Based Chiral Stationary Phases
Macrocycle Antibiotic-Based Chiral Stationary Phases
3.1.2. Supercritical Fluid Chromatography
Polysaccharide-Based Chiral Stationary Phases
Crown Ether-Based Chiral Stationary Phases
3.1.3. Gas Chromatography
Cyclofructan-Based Chiral Stationary Phases
3.1.4. Capillary Electrophoresis
Cyclodextrin as a Chiral Selector
Crown Ether as a Chiral Selector
Ligand Exchange as a Chiral Selector
Micellar Electrokinetic Chromatography (MEKC)
3.1.5. Comparison of Different Techniques
3.2. Indirect Method
4. Concluding Remarks and Prospects
Funding
Conflicts of Interest
References
- Qi, X.; Tester, R.F. Fructose, Galactose and Glucose—In Health and Disease. Nutr. ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef]
- Qi, X.; Tester, R.F. Lactose, Maltose, and Sucrose in Health and Disease. Nutr. Food Res. 2020, 64, 1901082–1901091. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahrami, G.; Daglia, M.; Nabavi, S.M.; Naseri, R.; Farzaei, M.H. Plant-Derived Supplementary Carbohydrates, Polysaccharides and Oligosaccharides in Management of Diabetes Mellitus: A Comprehensive Review. Food Rev. Int. 2019, 35, 563–586. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- He, B.L.; Lloyd, D.K. Chiral Methods. In Specification of Drug Substances and Products, 2nd ed.; Riley, C.M., Rosanske, T.W., Reid, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 425–458. ISBN 9780081028247. [Google Scholar]
- Annavarapu, S.; Nanda, V. Mirrors in the PDB: Left-Handed α-Turns Guide Design with d-amino Acids. BMC Struct. Biol. 2009, 9, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Grieco, P.; Carotenuto, A.; Auriemma, L.; Saviello, M.R.; Campiglia, P.; Gomez-Monterrey, I.M.; Marcellini, L.; Luca, V.; Barra, D.; Novellino, E.; et al. The effect of d-amino acid substitution on the selectivity of temporin L towards target cells: Identification of a potent anti-Candida peptide. Biochim. Biophys. Acta Biomembr. 2013, 1828, 652–660. [Google Scholar] [CrossRef]
- Gößler-Schöfberger, R.; Hesser, G.; Reif, M.M.; Friedmann, J.; Duscher, B.; Toca-Herrera, J.L.; Oostenbrink, C.; Jilek, A. A stereochemical switch in the aDrs model system, a candidate for a functional amyloid. Arch. Biochem. Biophys. 2012, 522, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Fujii, N.; Fujii, N.; Kida, M.; Kinouchi, T. Influence of Lβ-, Dα- and Dβ-Asp Isomers of the Asp-76 Residue on the Properties of AA-Crystallin 70–88 Peptide. Amino Acids 2010, 39, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Saito, T. Homochirality and Life. Chem. Rec. 2004, 4, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N. d-amino Acid in Elderly Tissues. Biol. Pharm. Bull. 2005, 28, 1585–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, N.; Kawaguchi, T.; Sasaki, H.; Fujii, N. Simultaneous Stereoinversion and Isomerization at the Asp-4 Residue in βB2-Crystallin from the Aged Human Eye Lenses. Biochemistry 2011, 50, 8628–8635. [Google Scholar] [CrossRef]
- Fujji, N.; Takata, T.; Fujii, N.; Aki, K.; Sakaue, H. d-amino Acids in Protein: The Mirror of Life as a Molecular Index of Aging. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 840–847. [Google Scholar] [CrossRef]
- Miyamoto, T.; Homma, H. Detection and Quantification of d-amino Acid Residues in Peptides and Proteins Using Acid Hydrolysis. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 775–782. [Google Scholar] [CrossRef]
- Ha, S.; Kinouchi, T.; Fujii, N. Age-Related Isomerization of Asp in Human Immunoglobulin G Kappa Chain. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140410–140417. [Google Scholar] [CrossRef]
- Bastings, J.J.A.J.; van Eijk, H.M.; Olde Damink, S.W.; Rensen, S.S. d-amino Acids in Health and Disease: A Focus on Cancer. Nutients 2019, 11, 2205. [Google Scholar] [CrossRef] [Green Version]
- Kuwada, M.; Teramoto, T.; Kumagaye, K.Y.; Nakajima, K.; Watanabe, T.; Kawai, T.; Kawahami, Y.; Niidome, T.; Sawada, K.; Nishizawa, Y.; et al. ω-Agatoxin-TK Containing d-Serine at Position 46, but Not Synthetic ω-[L-Ser]Agatoxin-TK, Exerts Blockade of P-Type Calcium Channels in Cerebellar Purkinje Neurons. Mol. Pharmacol. 1994, 46, 587–593. [Google Scholar]
- Richter, G.; Egger, R.; Kreil, G. D-Alanine in the Frog Skin Peptide Dermorphin Is Derived from L-Alanine in the Precursor. Science 1987, 238, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Pavithrra, G.; Rajasekaran, R. Gramicidin Peptide to Combat Antibiotic Resistance: A Review. Int. J. Pept. Res. Ther. 2020, 26, 191–199. [Google Scholar] [CrossRef]
- Fujii, N.; Satoh, K.; Harada, K.; Ishibashi, Y. Simultaneous Stereoinversion and Isomerization at Specific Aspartic Acid Residues in OrA-Crystallin from Human Lens. J. Biochem. 1994, 116, 663–669. [Google Scholar] [CrossRef]
- Hooi, M.Y.S.; Truscott, R.J.W. Racemisation and Human Cataract. d-Ser, d-Asp/Asn and d-Thr Are Higher in the Lifelong Proteins of Cataract Lenses than in Age-Matched Normal Lenses. Age 2011, 33, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Hooi, M.Y.S.; Raftery, M.J.; Truscott, R.J.W. Accelerated Aging of Asp 58 in AA Crystallin and Human Cataract Formation. Exp. Eye Res. 2013, 106, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Julian, R.R. Two-Dimensional Identification and Localization of Isomers in Crystallin Peptides Using TWIM-MS. Analyst 2020, 145, 5232–5341. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Takata, T.; Kim, I.; Morishima, K.; Inoue, R.; Magami, K.; Matsubara, T.; Sugiyama, M.; Koide, T. Asp Isomerization Increases Aggregation of α-Crystallin and Decreases Its Chaperone Activity in Human Lens of Various Ages. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140446–140457. [Google Scholar] [CrossRef] [PubMed]
- Masuda, W.; Nouso, C.; Kitamura, C.; Terashita, M.; Noguchi, T. D-Aspartic Acid in Bovine Dentine Non-Collagenous Phophoprotein. Arch. Oral Biol. 2002, 47, 757–762. [Google Scholar] [CrossRef]
- Powell, J.T.; Vine, N.; Crossman, M. On the Accumulation of D-Aspartate in Elastin and Other Proteins of the Ageing Aorta. Atherosclerosis 1992, 97, 201–208. [Google Scholar] [CrossRef]
- Ritz-Timme, S.; Laumeier, I.; Collins, M.J. Aspartic Acid Racemization: Evidence for Marked Longevity of Elastin in Human Skin. Br. J. Dermatol. 2003, 149, 951–959. [Google Scholar] [CrossRef]
- Ishigo, S.; Negishi, E.; Miyoshi, Y.; Onigahara, H.; Mita, M.; Miyamoto, T.; Masaki, H.; Homma, H.; Ueda, T.; Hamase, K. Establishment of a Two-Dimensional HPLC-MS/MS Method Combined with DCl/d2O Hydrolysis for the Determination of Trace Amounts of d-amino Acid Residues in Proteins. Chromatography 2015, 36, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.G.; Hancock, S.E.; Raftery, M.J.; Truscott, R.J.W. Isoaspartic Acid Is Present at Specific Sites in Myelin Basic Protein from Multiple Sclerosis Patients: Could This Represent a Trigger for Disease Onset? Acta Neuropathol. Commun. 2016, 4, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Fujii, N.; Ishibashi, Y.; Satoh, K.; Fujino, M.; Harada, K. Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta 1994, 1204, 157–163. [Google Scholar] [CrossRef]
- Livnat, I.; Tai, H.-G.; Jansson, E.T.; Bai, L.; Romanova, E.V.; Chen, T.-T.; Yu, K.; Chen, S.-A.; Zhang, Y.; Wang, Z.-Y.; et al. A d-amino Acid-Containing Neuropeptide Discovery Funnel. Anal. Chem. 2016, 88, 11868–11876. [Google Scholar] [CrossRef]
- Young, G.W.; Hoofring, S.A.; Mamula, M.J.; Doyle, H.A.; Bunick, G.J.; Hu, Y.; Aswad, D.W. Protein L-Isoaspartyl Methyltransferase Catalyzes In Vivo Racemization of Aspartate-25 in Mammalian Histone H2B. J. Biol. Chem. 2005, 280, 26094–26098. [Google Scholar] [CrossRef] [Green Version]
- Ritz, S.; Turzynski, A.; Schütz, H.W.; Hollmann, A.; Rochholz, G. Identification of Osteocalcin as a Permanent Aging Constituent of the Bone Matrix: Basis for an Accurate Age at Death Determination. Forensic Sci. Int. 1996, 77, 13–26. [Google Scholar] [CrossRef]
- Kawamura, I.; Mijiddorj, B.; Kayano, Y.; Matsuo, Y.; Ozawa, Y.; Ueda, K.; Sato, H. Separation of d-amino Acid-Containing Peptide Phenylseptin Using 3,3′-Phenyl-1,1′-Binaphthyl-18-Crown-6-Ether Columns. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140429–140436. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Wolkow, C.; Wang, R.; Cotter, R.J.; Reardon, I.M.; Zürcher-Neely, H.A.; Heinrikson, R.L.; Ball, M.J.; et al. Structural Alterations in the Peptide Backbone of Beta-Amyloid Core Protein May Account for Its Deposition and Stability in Alzheimer’s Disease. J. Biol. Chem. 1993, 268, 3072–3083. [Google Scholar] [CrossRef]
- Kaneko, I.; Yamada, N.; Sakuraba, Y.; Kamenosono, M.; Tutumi, S. Suppression of Mitochondrial Succinate Dehydrogenase, a Primary Target of β-Amyloid, and Its Derivative Racemized at Ser Residue. J. Neurochem. 1995, 65, 2585–2593. [Google Scholar] [CrossRef]
- Zhang, J.; Yip, H.; Katta, V. Identification of Isomerization and Racemization of Aspartate in the Asp–Asp Motifs of a Therapeutic Protein. Anal. Biochem. 2011, 410, 234–243. [Google Scholar] [CrossRef]
- Mijiddorj, B.; Kaneda, S.; Sato, H.; Kitahashi, Y.; Javkhlantugs, N.; Naito, A.; Ueda, K.; Kawamura, I. The Role of d-Allo-Isoleucine in the Deposition of the Anti-Leishmania Peptide Bombinin H4 as Revealed by 31 P Solid-State NMR, VCD Spectroscopy, and MD Simulation. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gause, G.F. Gramicidin S review of recent work. Lancet 1946, 248, 46–47. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, H.; Fu, Y.; Jusuf, S.; Zeng, M.; Ludwig, R.; Krystek, S.R., Jr.; Chen, G.; Tao, L.; Das, T.K. Isomerization and Oxidation in the Complementarity-Determining Regions of a Monoclonal Antibody: A Study of the Modification–Structure–Function Correlations by Hydrogen–Deuterium Exchange Mass Spectrometry. Anal. Chem. 2016, 88, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Soyez, D.; Laverdure, A.-M.; Kallen, J.; van Herp, F. Demonstration of a Cell-Specific Isomerization of Invertebrate Neuropeptides. Neuroscience 1997, 82, 935–942. [Google Scholar] [CrossRef]
- Jimenéz, E.C.; Olivera, B.M.; Gray, W.R.; Cruz, L.J. Contryphan Is a D-Tryptophan-Containing Conus Peptide. J. Biol. Chem. 1996, 271, 28002–28005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erspamer, V.; Melchiorri, P.; Falconieri-Erspamer, G.; Negri, L.; Corsi, R.; Severini, C.; Barra, D.; Simmaco, M.; Kreil, G. Deltorphins: A Family of Naturally Occurring Peptides with High Affinity and Selectivity for 6 Opioid Binding Sites. Proc. Natl. Acad. Sci. USA 1989, 86, 5188–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, A.; Delfour, A.; Sagan, S.; Amiche, M.; Pradelles, P.; Rossier, J.; Nicolas, P. Isolation of Dermenkephalin from Amphibian Skin, a High-Affinity (δ-Selective Opioid Heptapeptide Containing a d-amino Acid Residue. FEBS Lett. 1989, 255, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Cloos, P.A.C.; Fledelius, C. Collagen Fragments in Urine Derived from Bone Resorption Are Highly Racemized and Isomerized: A Biological Clock of Protein Aging with Clinical Potential. Biochem. J. 2000, 345, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Kubota, I.; Takao, T.; Shimonishi, Y.; Yasuda-Kamatani, Y.; Minakata, H.; Nomoto, K.; Muneoka, Y.; Kobayashi, M. Fulicin, a Novel Neuropeptide Containing a d-amino Acid Residue Isolated from the Ganglia of Achatina Fulica. Biochem. Biophys. Res. Commun. 1991, 178, 486–493. [Google Scholar] [CrossRef]
- Kamatani, Y.; Minakata, H.; Kenny, P.T.M.; Iwashita, T.; Watanabe, K.; Funase, K.; Sun, X.P.; Yongsiri, A.; Kim, K.H.; Novales-Li, P.; et al. Achatin-I, an endogenous neuroexcitatoty tetrapeptide from Achatina fulica ferussac containing a d-amino acid residue. Biochem. Biophys. Res. Commun. 1989, 160, 1015–1020. [Google Scholar] [CrossRef]
- Lee, C.J.; Qiu, T.A.; Sweedler, J.V. D-Alanine: Distribution, Origin, Physiological Relevance, and Implications in Disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140482–140501. [Google Scholar] [CrossRef] [PubMed]
- Ayon, N.J. Features, roles and chiral analyses of proteinogenic amino acids. AIMS Mol. Sci. 2020, 7, 229–268. [Google Scholar] [CrossRef]
- Fisher, G.H.; Garcia, N.M.; Payan, I.L.; Cadilla-Perezrios, R.; Sheremata, W.A.; Man, E.H. D-aspartic acid in purified myelin and myelin basic protein. Biochem. Biophys. Res. Commun. 1986, 135, 683–687. [Google Scholar] [CrossRef]
- Lee, J.M.; Petrucelli, L.; Fisher, G.; Ramdath, S.; Castillo, J.; Di Fiore, M.; D’Aniello, A. Evidence for D-Aspartyl-β-Amyloid Secretase Activity in Human Brain. J. Neuropathol. Exp. Neurol. 2002, 61, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Oka, T. Free D-Aspartate and d-Serine in the Mammalian Brain and Periphery. Prog. Neurobiol. 1997, 52, 325–353. [Google Scholar] [CrossRef]
- Homma, H. Biochemistry of D-Aspartate in Mammalian Cells. Amino Acids 2007, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The Role and Molecular Mechanism of D-Aspartic Acid in the Release and Synthesis of LH and Testosterone in Humans and Rats. Reprod. Biol. Endocrinol. 2009, 7, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.S.; Zmora, N.; Katayama, H.; Tsutsui, N. Crustacean Hyperglycemic Hormone (CHH) Neuropeptidesfamily: Functions, Titer, and Binding to Target Tissues. Gen. Comp. Endocrinol. 2010, 166, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Strauch, R.C.; Svedin, E.; Dilkes, B.; Chapple, C.; Li, X. Discovery of a Novel Amino Acid Racemase through Exploration of Natural Variation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 11726–11731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Moriya, T.; Homma, H.; Oshim, T. Enzymatic Properties and Physiological Function of Glutamate Racemase from Thermus thermophilus. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140461–140467. [Google Scholar] [CrossRef]
- Ollivaux, C.; Soyez, D.; Toullec, J.-Y. Biogenesis of D -Amino Acid Containing Peptides/Proteins: Where, When and How? J. Pept. Sci. 2014, 20, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Fujii, N.; Momose, Y.; Ishii, N.; Takita, M.; Akaboshi, M.; Kodama, M. The Mechanisms of Simultaneous Stereoinversion, Racemization, and Isomerization at Specific Aspartyl Residues of Aged Lens Proteins. Mech. Ageing. Dev. 1999, 107, 347–358. [Google Scholar] [CrossRef]
- Fujii, N.; Sakaue, H.; Sasaki, H.; Fujii, N. A Rapid, Comprehensive Liquid Chromatography-Mass Spectrometry (LC-MS)-based Survey of the Asp Isomers in Crystallins from Human Cataract Lenses. J. Biol. Chem. 2012, 287, 39992–40002. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.R.; Kent, S.B.H.; Chu, I.C.; Merrifield, R.B. Quantitative Determination of d- and l-amino Acids by Reaction with Tert-Butyloxycarbonyl-L-Leucine N-Hydroxysuccinimide Ester and Chromatographic Separation as L,D and L,L Dipeptides. Anal. Chem. 1978, 50, 637–640. [Google Scholar] [CrossRef]
- Yan, L.; Ke, Y.; Kan, Y.; Lin, D.; Yang, J.; He, Y.; Wu, L. New Insight into Enzymatic Hydrolysis of Peptides with Site-Specific Amino Acid d-Isomerization. Bioorg. Chem. 2020, 10, 104389–104399. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Readel, E.R.; Wey, M.; Armstrong, D.W. Complete Identification of All 20 Relevant Epimeric Peptides in β-Amyloid: A New HPLC-MS Based Analytical Strategy for Alzheimer’s Research. Chem. Commun. 2020, 56, 1537–1540. [Google Scholar] [CrossRef]
- Takata, T.; Ha, S.; Koide, T.; Fujii, N. Site-Specific Rapid Deamidation and Isomerization in Human Lens AA-crystallin In Vitro. Protein Sci. 2020, 29, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Benner, T. Hydrolysis-Induced Racemization of Amino Acids: Hydrolysis-Induced Amino Acid Racemization. Limnol. Oceanogr. Methods 2005, 3, 318–325. [Google Scholar] [CrossRef]
- Danielsen, M.; Nebel, C.; Dalsgaard, T.K. Simultaneous Determination of l- and d-amino Acids in Proteins: A Sensitive Method Using Hydrolysis in Deuterated Acid and Liquid Chromatography–Tandem Mass Spectrometry Analysis. Foods 2020, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Manning, J.M. Determination of d- and l-amino Acid Residues in Peptides. Use of Tritiated Hydrochloric Acid to Correct for Racemization during Acid Hydrolysis. J. Am. Chem. Soc. 1970, 92, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Davankov, V. The Nature of Chiral Recognition: Is It a Three-Point Interaction? Chirality 1997, 9, 99–102. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Zeng, S. Chiral Mobile-Phase Additives in HPLC Enantioseparations. In Chiral Separations. Methods and Protocols, 3rd ed.; Scriba, G.K.E., Ed.; Methods in Molecular Biology; Humana: Totowa, NJ, USA; Springer: New York, NY, USA, 2019; pp. 81–91. ISBN 9781493994380. [Google Scholar]
- Chankvetadze, B. Recent Trends in Preparation, Investigation and Application of Polysaccharide-Based Chiral Stationary Phases for Separation of Enantiomers in High-Performance Liquid Chromatography. Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Mejía-Carmona, K.; da Silva Burato, J.S.; Borsatto, J.V.B.; de Toffoli, A.L.; Lanças, F.M. Miniaturization of liquid chromatography coupled to mass spectrometry: 1. Current trends on miniaturized LC columns. Trends Anal. Chem. 2020, 122, 115735–115750. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Jiang, W.; Liu, H.; Sun, B. Chiral Recognition for Chromatography and Membrane-Based Separations: Recent Developments and Future Prospects. Molecules 2021, 26, 1145. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawashima, M.; Hatada, K. Useful Chiral Packing Materials for High-Performance Liquid Chromatographic Resolution of Enantiomers: Phenylcarbamates of Polysaccharides Coated on Silica Gel. J. Am. Chem. Soc. 1984, 106, 5357–5359. [Google Scholar] [CrossRef]
- Lin, Z.; Tai, H.-C.; Zhu, C.; Fabiano, A.; Borges-Muñoz, A.; Ye, Y.K.; He, B.L. Evaluation of a Polysaccharide-Based Chiral Reversed-Phase Liquid Chromatography Screen Strategy in Pharmaceutical Analysis. J. Chromatogr. A 2021, 1645, 462085–462094. [Google Scholar] [CrossRef]
- Chankvetadze, B. Polysaccharide-Based Chiral Stationary Phases for Enantioseparations by High-Performance Liquid Chromatography: An Overview. In Chiral Separations, 3rd ed.; Scriba, G.K.E., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA; Springer: New York, NY, USA, 2019; pp. 93–126. ISBN 9781493994380. [Google Scholar]
- Mitchell, C.R.; Armstrong, D.W. Cyclodextrin-Based Chiral Stationary Phases for Liquid Chromatography. In Chiral Separation, 1st ed.; Gübitz, G., Schmid, M.G., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2004; Volume 243, pp. 31–112. ISBN 9781592596485. [Google Scholar]
- Dai, Y.; Wang, S.; Tang, W.; Ng, S.-C. Cyclodextrin-Based Chiral Stationary Phases for High-Performance Liquid Chromatography. In Modified Cyclodextrins for Chiral Separation, 1st ed.; Tang, W., Ng, S.-C., Sun, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 67–101. ISBN 9783642376481. [Google Scholar]
- Li, X.; Wang, Y. HPLC Enantioseparation on Cyclodextrin-Based Chiral Stationary Phases. In Chiral Separations, 3rd ed.; Scriba Gerhard, K.E., Ed.; Methods in Molecular Biology; Humana: Totowa, NJ, USA; Springer: New York, NY, USA, 2019; pp. 159–169. ISBN 978-1-4939-9438-0. [Google Scholar]
- Shuang, Y.; Liao, Y.; Zhang, T.; Li, L. Preparation and Evaluation of an Ethylenediamine Dicarboxyethyl Diamido-Bridged Bis(β-Cyclodextrin)-Bonded Chiral Stationary Phase for High Performance Liquid Chromatography. J. Chromatogr. A 2020, 1619, 460937–460947. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Furuno, M.; Tanaka, N.; Fukusaki, E. Fast Enantiomeric Separation of Amino Acids Using Liquid Chromatography/Mass Spectrometry on a Chiral Crown Ether Stationary Phase. J. Biosci. Bioeng. 2020, 130, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Upmanis, T.; Kažoka, H.; Arsenyan, P. A Study of Tetrapeptide Enantiomeric Separation on Crown Ether Based Chiral Stationary Phases. J. Chromatogr. A 2020, 1622, 461152–461161. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Taniguchi, M.; Fukusaki, E. High-Sensitive Liquid Chromatography-Tandem Mass Spectrometry-Based Chiral Metabolic Profiling Focusing on Amino Acids and Related Metabolites. J. Biosci. Bioeng. 2019, 127, 520–527. [Google Scholar] [CrossRef]
- Carrão, D.B.; Perovani, I.S.; de Albuquerque, N.C.P.; de Oliveira, A.R.M. Enantioseparation of Pesticides: A Critical Review. TrAC Trends Anal. Chem. 2020, 122, 115719–115734. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral Stationary Phases Based on Small Molecules: An Update of the Last 17 Years. Sep. Purif. Rev. 2017, 47, 89–123. [Google Scholar] [CrossRef]
- Kohout, M.; Hovorka, Š.; Herciková, J.; Wilk, M.; Sysel, P.; Izák, P.; Bartůněk, V.; von Baeckmann, C.; Pícha, J.; Frühauf, P. Evaluation of Silica from Different Vendors as the Solid Support of Anion-exchange Chiral Stationary Phases by Means of Preferential Sorption and Liquid Chromatography. J. Sep. Sci. 2019, 42, 3653–3661. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Ishii, C.; Furusho, A.; Hsieh, C.-L.; Shimizu, Y.; Akito, T.; Mita, M.; Okamura, T.; Konno, R.; Ide, T.; et al. Determination of Phenylalanine Enantiomers in the Plasma and Urine of Mammals and d-amino Acid Oxidase Deficient Rodents Using Two-Dimensional High-Performance Liquid Chromatography. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140540–140547. [Google Scholar] [CrossRef]
- Bäurer, S.; Ferri, M.; Carotti, A.; Neubauer, S.; Sardella, R.; Lämmerhofer, M. Mixed-Mode Chromatography Characteristics of Chiralpak ZWIX (+) and ZWIX (−) and Elucidation of Their Chromatographic Orthogonality for LC × LC Application. Anal. Chim. Acta 2020, 1093, 168–179. [Google Scholar] [CrossRef]
- Geibel, C.; Dittrich, K.; Woiwode, U.; Kohout, M.; Zhang, T.; Lindner, W.; Lämmerhofer, M. Evaluation of Superficially Porous Particle Based Zwitterionic Chiral Ion Exchangers against Fully Porous Particle Benchmarks for Enantioselective Ultra-High Performance Liquid Chromatography. J. Chromatogr. A 2019, 1603, 130–140. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, L.; Sun, X.; Wu, Y.; Wang, H.; Cheng, L.; Shen, J.; Ke, Y. Novel Chiral Stationary Phases Based on 3,5-dimethyl Phenylcarbamoylated Β-cyclodextrin Combining Cinchona Alkaloid Moiety. Chirality 2020, 32, 1080–1090. [Google Scholar] [CrossRef]
- Bajtai, A.; Ilisz, I.; Howan, D.H.O.; Tóth, G.K.; Scriba, G.K.E.; Lindner, W.; Péter, A. Enantioselective Resolution of Biologically Active Dipeptide Analogs by High-Performance Liquid Chromatography Applying Cinchona Alkaloid-Based Ion-Exchanger Chiral Stationary Phases. J. Chromatogr. A 2020, 1611, 460574–460586. [Google Scholar] [CrossRef] [PubMed]
- Horak, J.; Lämmerhofer, M. Stereoselective Separation of Underivatized and 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate Derivatized Amino Acids Using Zwitterionic Quinine and Quinidine Type Stationary Phases by Liquid Chromatography–High Resolution Mass Spectrometry. J. Chromatogr. A 2019, 1596, 69–78. [Google Scholar] [CrossRef]
- Horak, J.; Lämmerhofer, M. Derivatize, Racemize, and Analyze—An Easy and Simple Procedure for Chiral Amino Acid Standard Preparation for Enantioselective Metabolomics. Anal. Chem. 2019, 91, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Pucciarini, L.; González-Ruiz, V.; Zangari, J.; Martinou, J.-C.; Natalini, B.; Sardella, R.; Rudaz, R. Development and Validation of a Chiral UHPLC-MS Method for the Analysis of Cysteine Enantiomers in Biological Samples. J. Pharm. Biomed. Anal. 2020, 177, 112841–112849. [Google Scholar] [CrossRef]
- Seki, T.; Sato, M.; Konno, A.; Hirai, H.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H. D-Cysteine Promotes Dendritic Development in Primary Cultured Cerebellar Purkinje Cells via Hydrogen Sulfide Production. Mol. Cell. Neurosci. 2018, 93, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Tsujimura, H.; Tsuchiya, M.; Soga, S.; Ota, N.; Tanaka, A.; Kim, H. Development of a Cognitive Function Marker Based on d-amino Acid Proportions Using New Chiral Tandem LC-MS/MS Systems. Sci. Rep. 2020, 10, 804–817. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Hesaka, A.; Yoshitaka, I. Utility of d-Serine Monitoring in Kidney Disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140449–140454. [Google Scholar] [CrossRef]
- Furusho, A.; Koga, R.; Akita, T.; Mita, M.; Kimura, T.; Hamase, K. Three-Dimensional High-Performance Liquid Chromatographic Determination of Asn, Ser, Ala, and Pro Enantiomers in the Plasma of Patients with Chronic Kidney Disease. Anal. Chem. 2019, 18, 11569–11575. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Orosz, T.; Péter, A. High-Performance Liquid Chromatography Enantioseparations Using Macrocyclic Glycopeptide-Based Chiral Stationary Phases: An Overview. In Chiral Separations, 3rd ed.; Scriba, G.K.E., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA; Springer: New York, NY, USA, 2019; pp. 201–237. ISBN 9781493994380. [Google Scholar]
- Mazzoccanti, G.; Manetto, S.; Ricci, A.; Cabri, W.; Orlandin, A.; Catani, M.; Felletti, S.; Cavazzini, A.; Ye, M.; Ritchie, H.; et al. High–Throughput Enantioseparation of Nα–Fluorenylmethoxycarbonyl Proteinogenic Amino Acids through Fast Chiral Chromatography on Zwitterionic-Teicoplanin Stationary Phases. J. Chromatogr. A 2020, 1624, 461235–461246. [Google Scholar] [CrossRef]
- West, C. Recent Trends in Chiral Supercritical Fluid Chromatography. Trends Anal. Chem. 2019, 120, 115648–115657. [Google Scholar] [CrossRef]
- Kaplitz, A.; Mostafa, M.E.; Calvez, S.A.; Edwards, J.L.; Grinias, J.P. Two-dimensional Separation Techniques Using Supercritical Fluid Chromatography. J. Sep. Sci. 2021, 44, 426–437. [Google Scholar] [CrossRef]
- Jakubec, P.; Douša, M.; Nováková, L. Supercritical Fluid Chromatography in Chiral Separations: Evaluation of Equivalency of Polysaccharide Stationary Phases. J. Sep. Sci. 2020, 43, 2675–2689. [Google Scholar] [CrossRef] [PubMed]
- Lipka, E.; Dascalu, A.-E.; Messara, Y.; Tsutsqiridze, E.; Farkas, T.; Chankvetadze, B. Separation of Enantiomers of Native Amino Acids with Polysaccharide-Based Chiral Columns in Supercritical Fluid Chromatography. J. Chromatogr. A 2019, 1585, 207–212. [Google Scholar] [CrossRef]
- Miller, L.; Yue, L. Chiral Separation of Underivatized Amino Acids in Supercritical Fluid Chromatography with Chiral Crown Ether Derived Column. Chirality 2020, 32, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Schurig, V. Gas Chromatographic Enantioseparation of Derivatized α-Amino Acids on Chiral Stationary Phases—Past and Present. J. Chromatogr. B 2011, 879, 3122–3140. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-M.; Chen, X.-X.; Zhang, J.-H.; Yuan, L.-M. Gas Chromatographic Separation of Enantiomers on Novel Chiral Stationary Phases. Trends Anal. Chem. 2020, 124, 115808–115828. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirino, J.P. Chiral Selectors in Capillary Electrophoresis: Trends during 2017–2018. Molecules 2019, 24, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koster, N.; Clark, C.P.; Kohler, I. Past, Present, and Future Developments in Enantioselective Analysis Using Capillary Electromigration Techniques. Electrophoresis 2021, 42, 38–57. [Google Scholar] [CrossRef]
- Konjaria, M.-L.; Scriba, G.K.E. Enantioseparation of Analogs of the Dipeptide Alanyl-Phenylalanine by Capillary Electrophoresis Using Neutral Cyclodextrins as Chiral Selectors. J. Chromatogr. A 2020, 1623, 461158–461165. [Google Scholar] [CrossRef]
- Konjaria, M.-L.; Scriba, G.K.E. Enantioseparation of Alanyl-Phenylalanine Analogs by Capillary Electrophoresis Using Negatively Charged Cyclodextrins as Chiral Selectors. J. Chromatogr. A 2020, 1632, 461585. [Google Scholar] [CrossRef] [PubMed]
- Greño, M.; Castro-Puyana, M.; Marina, M.L. Enantiomeric Separation of Homocysteine and Cysteine by Electrokinetic Chromatography Using Mixtures of γ-Cyclodextrin and Carnitine-Based Ionic Liquids. Microchem. J. 2020, 157, 105070–105078. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Wang, Q.; He, P. Investigation of Hydroxypropyl-β-Cyclodextrin-Based Synergistic System with Chiral Nematic Mesoporous Silica as Chiral Stationary Phase for Enantiomeric Separation in Microchip Electrophoresis. Talanta 2020, 218, 121121–121128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.-J.; Bang, E.; Na, Y.-C. Chiral Separation of Intact Amino Acids by Capillary Electrophoresis-Mass Spectrometry Employing a Partial Filling Technique with a Crown Ether Carboxylic Acid. J. Chromatogr. A 2019, 1586, 128–138. [Google Scholar] [CrossRef]
- Liu, L.; Bao, P.; Qiao, J.; Zhang, H.; Qi, L. Chiral Ligand Exchange Capillary Electrophoresis with L-Dipeptides as Chiral Ligands for Separation of Dns-D,l-amino Acids. Talanta 2020, 217, 121069–121075. [Google Scholar] [CrossRef]
- Feng, W.; Qiao, J.; Li, D.; Qi, L. Chiral Ligand Exchange Capillary Electrochromatography with Dual Ligands for Enantioseparation of D, l-amino Acids. Talanta 2019, 194, 430–436. [Google Scholar] [CrossRef]
- Xu, Z.; Guan, J.; Shao, H.; Fan, S.; Li, X.; Shi, S.; Yan, F. Combined Use of Cu(II)-L-Histidine Complex and β-Cyclodextrin for the Enantioseparation of Three Amino Acids by CE and a Study of the Synergistic Effect. J. Chromatogr. Sci. 2020, 58, 969–975. [Google Scholar] [CrossRef]
- Evans, K.; Wang, X.; Roper, M.G. Chiral micellar electrokinetic chromatographic separation for determination of l- and D-primary amines released from murine islets of Langerhans. Anal. Methods 2019, 11, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, R.-C.; Bodoki, E.; Servais, A.-C.; Crommen, J.; Oprean, R.; Fillet, M. Selectivity Evaluation of Phenyl Based Stationary Phases for the Analysis of Amino Acid Diastereomers by Liquid Chromatography Coupled with Mass Spectrometry. J. Chromatogr. A 2019, 1590, 80–87. [Google Scholar] [CrossRef]
- Pérez-Míguez, R.; Bruyneel, B.; Castro-Puyana, M.; Marina, M.L.; Somsen, G.W.; Domínguez-Vega, E. Chiral Discrimination of dl-amino Acids by Trapped Ion Mobility Spectrometry after Derivatization with (+)-1-(9-Fluorenyl)Ethyl Chloroformate. Anal. Chem. 2019, 91, 3277–3285. [Google Scholar] [CrossRef] [Green Version]
- Goto, J.; Goto, N.; Nambara, T. New Type of Derivatisation Reagents for Liquid Chromatographic Resolution of Enantiomeric Hydroxyl Compounds. Chem. Pharm. Bull. 1982, 30, 4597–4599. [Google Scholar] [CrossRef] [Green Version]
- Miyano, S.; Okada, S.-I.; Hotta, H.; Takeda, M.; Kabuto, C.; Hashimoto, H. Optical Resolution of 2′-Methoxy-1,1′-Binaphthyl-2-Carboxylic Acid, and Application to Chiral Derivatizing Agent for HPLC Separation of Enantiomeric Alcohols and Amines. Bull. Chem. Soc. Jpn. 1989, 62, 1528–2533. [Google Scholar] [CrossRef] [Green Version]
- Harada, M.; Karakawa, S.; Yamada, N.; Miyano, H.; Shimbo, K. Biaryl Axially Chiral Derivatizing Agent for Simultaneous Separation and Sensitive Detection of Proteinogenic Amino Acid Enantiomers Using Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1593, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Karakawa, S.; Miyano, H.; Shimbo, K. Simultaneous Analysis of d,l-amino Acids in Human Urine Using a Chirality-Switchable Biaryl Axial Tag and Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Symmetry 2020, 12, 913. [Google Scholar] [CrossRef]
- Han, Y.; Jin, M.-N.; Xu, C.-Y.; Qian, Q.; Nan, J.; Jin, T.; Min, J.Z. Evaluation of Chiral Separation Efficiency of a Novel OTPTHE Derivatization Reagent: Applications to Liquid-chromatographic Determination of dl-serine in Human Plasma. Chirality 2019, 31, 1043–1052. [Google Scholar] [CrossRef]
- Russo, M.S.T.; Napylov, A.; Paquet, A.; Vuckovic, D. Comparison of N-Ethyl Maleimide and N-(1-Phenylethyl) Maleimide for Derivatization of Biological Thiols Using Liquid Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2020, 412, 1639–1652. [Google Scholar] [CrossRef]









| d-amino Acids | Proteins/Peptides | Length of Amino Acid Sequence | Position on the Sequence | Ref. |
|---|---|---|---|---|
| d-Asp | Phosphophoryn | 1129 | undetermined | [25] |
| d-Asp | Elastin | 786 | undetermined | [26,27] |
| d-Ala | Ovalbumin | 385 | undetermined | [28] |
| d-Asp | Ovalbumin | 385 | undetermined | [28] |
| d-Glu | Ovalbumin | 385 | undetermined | [28] |
| d-Pro | Ovalbumin | 385 | undetermined | [28] |
| d-Ser | Ovalbumin | 385 | undetermined | [28] |
| d-Asp | Myelin | 304 | 145 | [29] |
| d-isoAsp | Myelin | 304 | 34, 145 | [29] |
| d-Asp, d-isoAsp | βB1-crystallin | 252 | 211 | [23] |
| d-Asp | IgGK light chain | 214 | 151, 170 | [15] |
| d-Asp | βB2-crystallin | 205 | 4 | [12] |
| d-Asp | αB-crystallin | 175 | 36, 62, 140, 143 | [11,30] |
| d-Ser | αB-crystallin | 175 | 59, 66 | [23] |
| d-Asn | αB-crystallin | 173 | undetermined | [21] |
| d-Asp | αB-crystallin | 173 | 58, 84, 151 | [20,23] |
| d-Glu, d-isoGlu | αB-crystallin | 173 | 83 | [23] |
| d-isoAsp | αB-crystallin | 173 | 84 | [23] |
| d-Ser | αB-crystallin | 173 | 59, 62 | [22] |
| d-Thr | αB-crystallin | 173 | undetermined | [21] |
| d-Tyr | Achatin-like neuropeptide | 158 | 56, 86 | [31] |
| d-Asp | Histone H2B | 126 | 25 | [32] |
| d-Asp | Osteocalcin | 100 | undetermined | [33] |
| d-Phe | Phenylseptin | 67 | 50 | [34] |
| d-Trp | ω-agatoxin IV | 48 | 46 | [17] |
| d-Asp | β-amyloid | 42 | 1, 7, 23 | [35] |
| d-Ser | β-amyloid | 42 | 8, 26 | [36] |
| d-Asp | IgG H5 | 27 | 24 | [37] |
| d-Asp | IgG L2 | 24 | 12 | [37] |
| d-allo-Ile | Brombinin H4 | 21 | 2 | [38] |
| d-Phe | Gramicidin S | 10 | cyclopeptide | [39] |
| d-Asp | mAb heavy chain CDR2 peptide (51–59) | 9 | 4 | [40] |
| d-Phe | Hyperglycemic hormone | 8 | 3 | [41] |
| d-Trp | Contryphan | 8 | 4 | [42] |
| d-Ala | Dermorphin | 7 | 2 | [18] |
| d-Ala | Deltorphine | 7 | 2 | [43] |
| d-Met | Dermenkephalin | 7 | 2 | [44] |
| d-Asp | Type 1 collagen C-terminal telopeptide (1209–1214) | 6 | 3 | [45] |
| d-Asn | Fulicin peptide | 5 | 2 | [46] |
| d-Phe | Achatin I peptide | 4 | 2 | [47] |
| d-Amino Acids | Proteins/Peptides/Free AA | Source | Associated Disease/Function | Ref. |
|---|---|---|---|---|
| d-Asp | Elastin | Aorta and skin (H) | Arteriosclerosis | [26,27] |
| Myelin | Brain (H) | [50] | ||
| β-amyloid | Brain (H) | Alzheimer’s disease | [51] | |
| Free AA | Brain (H, R, C) Testis, adrenal and pineal glands (R) | Neuromodulatory effect Inhibit secretion of melatonin Increase testosterone production | [52,53,54] | |
| d-Asp, d-Asn, d-Ser and d-Thr | α-crystallin | Lens (H) | Cataract | [21] |
| d-Ala | Dermorphin Deltorphine | Skin (F) | 1000 times more analgesia than morphine due to presence of d-Ala | [18,43] |
| d-Met | Dermenkephalin | Skin (F) | Analgesia | [44] |
| d-Phe | Achatin I | Ganglia and atrium (S) | Enhances cardiac activity Excitatory action on muscles | [47] |
| Hyperglycemic hormone | Sinus gland (L) | Increase glucose concentration in response to stress | [41,55] | |
| Phenylseptin | Skin (F) | Antimicrobial activity | [34] | |
| d-allo-Ile | Brombinin H4 | Skin (F) | Antimicrobial and antiparasitic activity | [38] |
| d-Asn | Fulicin peptide | Ganglia (S) | Enhance concentration of penis retractor muscle | [46] |
| d-Trp | Contryphan | Venom (CS) | Paralysis of fish prey by snails | [42] |
| ω-agatoxin | Venom (SP) | Calcium channel blocker | [17] | |
| Free AA | Brain (M) | N-methyl d-aspartate (NMDA)/glycine receptor agonist | [52] |
| Types of CSPs | Basic Material | Target Characteristics | Commercial Column |
|---|---|---|---|
| Polysaccharides | Amylose or cellulose | Widely applicable, such as compounds containing amide groups, aromatic ring substituents, carbonyl nitro, sulfonyl, cyano, hydroxyl, amino and other groups, and amino derivatives | Chiralcel®OD, Chiralpak®IB, Lux®Amylose-1, Lux®i-Amylose-1 |
| Cyclodextrins | β-cyclodextin | Widely applicable, such as hydrocarbon compounds, sterols, phenol esters and their derivatives, aromatic amines, polyheterocycles | B-DEXTM225, Astec Cyclobond®, I 2000 RSP, LiChroCART®250-4, ChiraDex® |
| Proteins | Enzymes, plasma proteins, receptor proteins | Water-soluble medicine | Chiralpak®HAS, Resolvosil®BSA, Chiralpak®AGT |
| Crown ethers | Macrocyclic polyester | Amino acids, amino alcohools, primary amines | Crownpak®CR (+)/CR (−), Chirosil®RCA (+)/RAC (−) |
| Pirkle type | Amine, amino alcohol, amino acid derivative compounds, anthrone derivatives | Widely applicable, CSPs designed by analyzing the target | Whelk-O1®, ULMO®, Chirex® |
| Ion exchange type | Cinchona alkaloids, sulfamic acid | N-protected amino acid, N-protected amino group, sulfamic acid, amino phosphoric acid, aromatic carbonyl acid | Chiralpak®QN-AX, Chiralpak®QD-AX, Chiralpak®ZWIX (+), Chiralpak®ZWIX (−) |
| Macrocyclic glygopeptides | Avomycin, Ristomycin A, Vancomycin, Teicoplanon and Teicoplanon aglycone | Widely applicable, such as amino acids, peptides, non-steroidal anti-inflammatory drugs | Astec® CHIROBIOTIC® V, Astec® CHIROBIOTIC® R, Astec® CHIROBIOTIC® TAG |
| Cyclofructans | Cyclic oligosaccharides | Primary amine, acid, secondary amine, tertiary amine, alcohol | Larihc® CF6-RN |
| Porous organic materials | Covalent organic framework, metal organic framework, metal organic cage, mesoporous silica | Halogenated hydrocarbons, ketones, esters, ethers, organic acids, alkylene oxides, alcohols and sulfoxides | / |
| Samples | Column | Ref. | |||||
|---|---|---|---|---|---|---|---|
| ChiroSil RCA (+) | Crownpak (+) | Crownpak (−) | |||||
| Peptides | Rs | α | Rs | α | Rs | α | |
| lll/ddd-(Phe)3 | - | - | - | 5.58 | - | 1.28 | [34] |
| ldl/dld-(Phe)3 | - | - | - | - | - | - | |
| lld/ddl-(Phe)3 | - | - | - | - | - | - | |
| ldd/dll-(Phe)3 | - | - | - | - | - | - | |
| dldl/ldld-Tyr-Arg-Phe-Lys-NH2 | 3.08 | 1.51 | 4.68 | 1.48 | [81] | ||
| ddll/lldd-Tyr-Arg-Phe-Lys-NH2 | 4.76 | 1.92 | 9.46 | 2.39 | |||
| dlll/lddd-Tyr-Arg-Phe-Lys-NH2 | 3.51 | 1.61 | 10.62 | 2.56 | |||
| dldd/ldll-Tyr-Arg-Phe-Lys-NH2 | 3.00 | 1.52 | <0.50 | 1.04 | |||
| ddld/lldl-Tyr-Arg-Phe-Lys-NH2 | 3.14 | 1.51 | 5.75 | 1.65 | |||
| Samples | Column | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ion-Exchange | Zwitterionic | ||||||||
| QN-AX | QD-AX | ZWIX (+) | ZWIX (−) | ||||||
| Peptides | Rs | α | Rs | α | Rs | α | Rs | α | |
| ll-Ala-Ala | 0 | 1.00 | 1.07 | 1.75 | 0.29 | 1.03 | 1.24 | 1.35 | [91] |
| ld-Ala-Ala | 0 | 1.00 | 0 | 1.00 | 0.97 | 1.14 | 0.21 | 1.03 | |
| ll-Ala-Phg | 2.35 | 1.46 | 2.44 | 1.52 | 0.94 | 1.13 | 1.15 | 1.17 | |
| ll-Ala-βPhe | 0 | 1.00 | 0 | 1.00 | 0.90 | 1.09 | 0 | 1.00 | |
| ll-Ala-Phe | 1.82 | 1.25 | 2.43 | 1.40 | 1.03 | 1.13 | 0.80 | 1.12 | |
| ld-Ala-Phe | 0 | 1.00 | 0 | 1.00 | 0.53 | 1.12 | 1.29 | 1.17 | |
| ll-Ala-hPhe | 0 | 1.00 | 1.56 | 1.30 | 0 | 1.00 | 0.90 | 1.20 | |
| βAla-l-Phe | 0 | 1.00 | 1.05 | 1.15 | 4.36 | 1.45 | 2.50 | 1.34 | |
| ll-Ala-Phe-OMe | - | - | - | - | 0 | 1.00 | 0 | 1.00 | |
| ll-Ala-Phe-NH2 | - | - | - | - | 0 | 1.00 | 0.55 | 1.12 | |
| ll-Ala-Tyr | 2.36 | 1.34 | 2.47 | 1.48 | 1.52 | 1.21 | 1.30 | 1.42 | |
| ll-Ala-4-NO2-Phe | 1.88 | 1.25 | 0.82 | 1.09 | 1.96 | 1.28 | 2.00 | 1.46 | |
| ll-Ala-Trp | 2.36 | 1.51 | 2.35 | 1.58 | 7.09 | 2.21 | 3.50 | 1.96 | |
| Gly-l-Phe | 0 | 1.00 | 1.89 | 1.49 | 0 | 1.00 | 0.53 | 1.19 | |
| l-Phe-Gly | 0.63 | 1.14 | 1.52 | 1.28 | 3.21 | 1.33 | 2.00 | 1.26 | |
| ll-Phe-Ala | 4.36 | 1.52 | 4.73 | 2.01 | 2.55 | 1.55 | 2.94 | 1.40 | |
| ld-Phe-Ala | 0 | 1.00 | 0 | 1.00 | 2.71 | 1.44 | 0.97 | 1.17 | |
| ll-Lys-Phe | 0 | 1.00 | 2.14 | 1.71 | 1.95 | 1.28 | 0.94 | 1.12 | |
| ll-Leu-Leu | 3.20 | 2.19 | 4.00 | 2.37 | 1.40 | 1.15 | 0.63 | 1.17 | |
| ld-Leu-Leu | 0 | 1.00 | 0 | 1.00 | 1.77 | 1.24 | 0.53 | 1.16 | |
| Samples | Column | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|
| UHPC-FPP-Titan-Tzwitt | UHPC-SPP-Halo-Tzwitt | Teicoshell | ||||||
| Derivatization | dl-amino acids | Rs | α | Rs | α | Rs | α | |
| Fmoc | Ala | 8.06 | 1.69 | 9.35 | 2.02 | 4.44 | - | [100] |
| Arg | 4.74 | 2.09 | 5.26 | 2.44 | 2.01 | 1.32 | ||
| Arg-(Pbf) | 6.03 | 1.72 | 7.47 | 2.03 | 3.35 | - | ||
| Asn | 2.41 | 1.13 | 2.93 | 1.17 | - | - | ||
| Asn-(Trt) | - | - | 1.15 | 1.07 | - | - | ||
| Asp | - | - | - | 1.02 | - | - | ||
| Asp-(OtBu) | 1.95 | 1.11 | 2.09 | 1.16 | - | - | ||
| Cys | 1.14 | 1.14 | 1.68 | 1.16 | 1.82 | - | ||
| Cys-(Trt) | 1.59 | 1.10 | 2.54 | 1.16 | - | - | ||
| Gln | 7.99 | 1.30 | 4.71 | 1.39 | 1.58 | - | ||
| Gln-(Trt) | 3.23 | 1.22 | 4.46 | 1.32 | 1.96 | - | ||
| Glu | 1.42 | 1.06 | 2.76 | 1.11 | 2.21 | - | ||
| Glu-(OtBu) | 4.28 | 1.49 | 5.99 | 1.64 | 3.34 | - | ||
| His | 3.21 | 1.30 | 3.14 | 1.36 | 1.44 | 1.46 | ||
| His-(Trt) | 4.45 | 1.35 | 5.61 | 1.53 | 3.05 | - | ||
| Ile | 5.33 | 1.43 | 6.43 | 1.70 | 2.18 | - | ||
| Leu | 6.37 | 1.48 | 7.83 | 1.72 | 2.78 | - | ||
| Lys | 3.49 | 1.66 | 4.26 | 2.06 | 2.98 | 1.31 | ||
| Lys-(Boc) | 8.93 | 1.82 | 10.90 | 2.25 | 4.55 | - | ||
| Met | 7.33 | 1.54 | 8.94 | 1.82 | 4.15 | - | ||
| Phe | 4.56 | 1.30 | 5.45 | 1.43 | 2.23 | - | ||
| Pro | - | - | - | - | - | - | ||
| Ser | 2.92 | 1.15 | 3.92 | 1.21 | 2.21 | - | ||
| Ser-(tBu) | 4.30 | 1.27 | 5.54 | 1.41 | 2.35 | - | ||
| Thr | 2.92 | 1.18 | 3.74 | 1.27 | 1.65 | - | ||
| Thr-(tBu) | 1.16 | 1.107 | 1.75 | 1.11 | - | - | ||
| Trp | 3.07 | 1.19 | 3.91 | 1.29 | 1.97 | - | ||
| Trp-(Boc) | - | - | - | - | 2.93 | - | ||
| Tyr | 3.86 | 1.26 | 4.63 | 1.38 | 2.37 | - | ||
| Tyr-(tBu) | 4.78 | 1.60 | 7.56 | 1.82 | 4.16 | - | ||
| Val | 4.89 | 1.33 | 6.38 | 1.52 | 2.36 | - | ||
| Techniques | CSP | Columns | Analytes | Rs and/or α Average | Ref. |
|---|---|---|---|---|---|
| Liquid chromatography | Crown-ether | Cronwpak CR-I (+) | 21 dl-amino acids | Rs: 5.12 (min. 1.49, max. 8.90) | [80] |
| Zwitterrionic | Chiralpak ZWIX (+) | 21 dl-amino acids | α: 2.33 (min. 1.26, max. 5.31) | [93] | |
| Macrocycle antibiotic | UHPC-SPP-Halo-Tzwitt | 28 dl-amino acids | Rs: 5.01 (min. 1.15, max. 10.90) α: 1.52 (min. 1.02, max. 2.44) | [100] | |
| Supercritical fluid chromatography | Crown-ether | Crownpak CR-I (+) | 18 dl-amino acids | Rs: 5.27 (min. 1.99, max. 9.26) | [105] |
| Gas chromatography | Cyclofructan | CF-CSP5 | 7 dl-amino acids | Rs: 1.81 (min. 1.50, max. 2.60) α: 1.04 (min. 1.03, max. 1.06) | [107] |
| Capillary electrophoresis | Crown-ether | BGE containing 18C6H4 | 18 dl-amino acids | Rs: 2.97 (min. 0.70, max. 21.00) α: 1.03 (min. 1.01, max. 1.22) | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morvan, M.; Mikšík, I. Recent Advances in Chiral Analysis of Proteins and Peptides. Separations 2021, 8, 112. https://doi.org/10.3390/separations8080112
Morvan M, Mikšík I. Recent Advances in Chiral Analysis of Proteins and Peptides. Separations. 2021; 8(8):112. https://doi.org/10.3390/separations8080112
Chicago/Turabian StyleMorvan, Marine, and Ivan Mikšík. 2021. "Recent Advances in Chiral Analysis of Proteins and Peptides" Separations 8, no. 8: 112. https://doi.org/10.3390/separations8080112