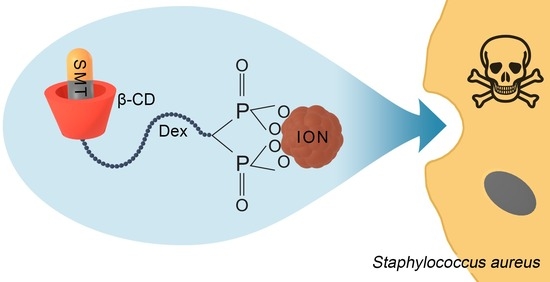
Silver-Sulfamethazine-Conjugated β-Cyclodextrin/Dextran-Coated Magnetic Nanoparticles for Pathogen Inhibition
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Chemicals and Materials
2.2. Synthesis of 4-Toluenesulfonic Anhydride (Ts2O) and 6-Toluenesulfonyl-β-Cyclodextrin (β-CD-Ts)
2.3. Synthesis of 6-Deoxy-6-Hydroxylethylamino-β-Cyclodextrin (β-CD-EA) and 6-Deoxy-6-(2-Hydroxyethyl) (Vinylsulfonyl)Methylamino-β-Cyclodextrin (β-CD-VS)
2.4. Tosylation of Dextran and the Reaction of Dex-Ts with Ethanolamine (EA)
2.5. Functionalization of Dex-EA with β-CD-VS and Modification with VDPA
2.6. Synthesis of Iron Oxide Nanoparticles (IONs)
2.7. Complexation of ION@DPA-Dex-β-CD Nanoparticles with Silver-Sulfamethazine (SMT-Ag)
2.8. Physicochemical Characterization of Particles and Their Coatings
2.9. Disc Diffusion Test (DDT) for Qualitative Antibmicrobial Evaluation
2.10. Minimum Inhibitory and Minimum Bactericidal/Microbicidal Concentrations (MIC and MBC/MMC)
3. Results and Discussion
3.1. Iron Oxide Nanoparticles (IONs)
3.2. Modification of Dex and β-CD
3.3. Preparation of Silver-Sulfamethazine-Conjugated β-Cyclodextrin/Dextran-Coated IONs (ION@DPA-Dex-β-CD-SMT-Ag)
3.4. Antimicrobial Effects
3.4.1. Qualitative DDT Evaluation
3.4.2. Quantitative MIC/MBC/MMC Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Ovung, A.; Bhattacharyya, J. Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 2021, 13, 259–272. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, Y.; Li, X.; Zhu, H.; Zhang, M.; Yan, K.; Chen, H. Colorimetric detection of sulfamethazine based on target resolved calixarene derivative stabilized gold nanoparticles aggregation. Microchim. Acta 2022, 189, 71. [Google Scholar] [CrossRef]
- Tailor, S.M.; Patel, U.H. Synthesis, spectroscopic characterization, antimicrobial activity and crystal structure of silver and copper complexes of sulfamethazine. J. Coord. Chem. 2015, 68, 2192–2207. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Liao, P.-B.; Wu, P.-H.; Chang, W.-J.; Lee, S.-Y.; Huang, W.-M. Cancer cytotoxicity of a hybrid hyaluronan-superparamagnetic iron oxide nanoparticle material: An in-vitro evaluation. Nanomaterials 2022, 12, 496. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-coated magnetite nanoparticles for protein immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 18, 842–852. [Google Scholar] [CrossRef]
- Lemechko, P.; Renard, E.; Guezennec, J.; Guezennec, C.; Langlois, V. Synthesis of dextran-graft-PHBHV amphiphilic copolymer using click chemistry approach. Reac. Funct. Polym. 2012, 72, 487–494. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Wang, H.; Liu, L.; Wang, W.; Wang, C.; Wang, Q.; Liu, W. The biocompatibility of fatty acid modified dextran-agmatine bioconjugate gene delivery vector. Biomaterials 2012, 33, 604–613. [Google Scholar] [CrossRef]
- Heinze, T.; Michealis, N.; Hornig, S. Reactive polymeric nanoparticles based on unconventional dextran derivatives. Eur. Polym. J. 2007, 43, 697–703. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.; Li, Z.; Tan, B.H.; Tang, K.Y.; Tam, K.C. β-Cyclodextrin functionalized magnetic nanoparticles for the removal of pharmaceutical residues in drinking water. J. Ind. Eng. Chem. 2022, 109, 461–474. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; Laíño, R.B.; Calzón, J.A.G.; García, M.E.D. Magnetic nanoparticles grafted with β-cyclodextrin for solid-phase extraction of 5-hydroxy-3-indole acetic acid. Microchim Acta 2014, 181, 941–948. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.-H. Magnetic nanoparticles grafted with cyclodextrin for hydrophobic drug delivery. Chem. Mater. 2007, 19, 6345–6349. [Google Scholar] [CrossRef]
- Tang, X.; Wen, Y.; Zhang, Z.; Zhu, J.; Song, X.; Li, J. Rationally designed multifunctional nanoparticles as GSH-responsive anticancer drug delivery systems based on host-guest polymers derived from dextran and β-cyclodextrin. Carbohydrate Polym. 2023, 320, 121207. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, F.; Huang, D.; Hou, S.; Wang, H.; Wang, M.; Chi, Y.; Zhao, Z. A facile route to magnetic mesoporous core–shell structured silicas containing covalently bound cyclodextrins for the removal of the antibiotic doxycycline from water. RSC Adv. 2018, 8, 31348–31357. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, F.F.; Wu, G.; Qv, X.; Hong, H.; Liu, C. Recovery and separation of erythromycin from industrial wastewater by imprinted magnetic nanoparticles that exploit β-cyclodextrin as the functional monomer. J. Sep. Sci. 2016, 39, 450–459. [Google Scholar] [CrossRef]
- Zoppi, A.; Delrivo, A.; Aiassa, V.; Longhi, M.R. Binding of sulfamethazine to β-cyclodextrin and methyl-β-cyclodextrin. AAPS PharmSciTech 2013, 14, 727–735. [Google Scholar] [CrossRef]
- Abou-El-Sherbini, K.S.; Amer, M.H.A.; Abdel-Aziz, M.S.; Hamzawy, E.M.A.; Sharmoukh, W.; Elnagar, M.M. Encapsulation of biosynthesized nanosilver in silica composites for sustainable antimicrobial functionality. Glob. Chall. 2018, 2, 1800048. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, Q.; Chen, L.; Liu, H.; Ding, M.; Dong, H.; Mou, Y. Therapeutic applications of antimicrobial silver-based biomaterials in dentistry. Int. J. Nanomed. 2022, 17, 443–462. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Li, H.; Zhang, S.; Hong, A.; Jiang, Y.; Hu, N.; Chen, G.; Fu, H.; Yuan, M.; et al. Self-powered electrodeposition system for sub-10-nm silver nanoparticles with high-efficiency antibacterial activity. J. Phys. Chem. Lett. 2022, 13, 6721–6730. [Google Scholar] [CrossRef]
- Degenhardt, C.R.; Burdsall, D.C. Synthesis of ethenylidenebis(phosphonic acid) and its tetraalkyl esters. J. Org. Chem. 1986, 51, 3488–3490. [Google Scholar] [CrossRef]
- Zhong, N.; Byun, H.-S.; Bittrnan, R. An improved synthesis of 6-O-monotosyl-6-deoxy-β-cyclodextrin. Tetrahedron Lett. 1998, 39, 2919–2920. [Google Scholar] [CrossRef]
- del Castillo, T.; Marales-Sanfrutos, J.; Santoyo-González, F.; Magez, S.; Lopez-Jaramillo, F.J.; Garcia-Salcedo, J.A. Monovinyl sulfone β-cyclodextrin. A flexible drug carrier systém. ChemMedChem 2014, 9, 383–389. [Google Scholar] [CrossRef]
- Zasońska, B.A.; Boiko, N.; Klyuchivska, O.; Trchová, M.; Petrovský, E.; Stoika, R.; Horák, D. Silica-coated γ-Fe2O3 nanoparticles: Preparation and engulfment by mammalian macrophages. J. Nanopharm. Drug Deliv. 2013, 1, 182–192. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing, Version 11.0. Available online: www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2023_manuals/Manual_v_11.0_EUCAST_Disk_Test_2023.pdf (accessed on 1 January 2023).
- Nweze, E.I.; Mukherjee, P.K.; Ghannoum, M.A. Agar-based disk diffusion assay for susceptibility testing of dermatophytes. J. Clin. Microbiol. 2010, 48, 3750–3752. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Zasońska, B.A.; Bober, P.; Jošt, P.; Petrovský, E.; Boštík, P.; Horák, D. Magnetoconductive maghemite core/polyaniline shell nanoparticles as promising tools for biomedical applications. Colloids Surf. B 2016, 141, 382–389. [Google Scholar] [CrossRef]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A review of the current state of magnetic force microscopy to unravel the magnetic properties of nanomaterials applied in biological systems and future directions for quantum technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Babič, M.; Horák, D.; Jendelová, P.; Glogarová, K.; Herynek, V.; Trchová, M.; Likavčanová, K.; Hájek, M.; Syková, E. Poly(N,N-dimethylacrylamide)-coated maghemite nanoparticles for stem cell labeling. Bioconjugate Chem. 2009, 20, 283–294. [Google Scholar] [CrossRef]
- Carone, A.; Emilsson, S.; Mariani, P.; Désert, A.; Parola, S. Gold nanoparticle shape dependence of colloidal stability domains. Nanoscale Adv. 2023, 5, 2017–2026. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef]
- Schneider, H.J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1785. [Google Scholar] [CrossRef]
- Purama, R.K.; Goswami, P.; Khan, A.T.; Goyal, A. Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr. Polym. 2009, 76, 30–35. [Google Scholar] [CrossRef]
- Kuniaki, F. The infrared spectrum of dimethyl sulfone. Bull. Chem. Soc. Jpn. 1959, 32, 1374–1376. [Google Scholar] [CrossRef]
- Gong, W. A real-time in-situ ATR-FITIR spectroscopic study of linear phosphate on titania surfaces. Int. J. Miner. Process. 2001, 63, 147–165. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Gatrone, R.C.; Nash, K.L. Membrane Extraction with Thermodynamically Unstable Diphosphonic Acid Derivatives. US Patent 5,678,242, 1997. Available online: https://www.osti.gov/biblio/541723 (accessed on 14 October 1997).
- Sandmann, B.J.; Nesbitt, R.U.; Sandmann, R.A. Characterization of silver sulfadiazine and related compounds. J. Pharm. Sci. 1974, 63, 948–951. [Google Scholar] [CrossRef]
- Guo, L.; Ping, J.; Qin, J.; Yang, M.; Wu, X.; You, M.; You, F.; Peng, H.A. Comprehensive study of drug loading in hollow mesoporous silica nanoparticles: Impacting factors and loading efficiency. Nanomaterials 2021, 11, 1293. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Gong, M.; Lin, Y.; Xu, Y.; Ye, L.; Yu, X.; Liu, J.; Liu, J.; He, S.; et al. Synergistic effects of nanoparticle heating and amoxicillin on H. pylori inhibition. J. Magn. Magn. Mater. 2019, 485, 95–104. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N. (Eds.) Hybrid Nanostructures for Cancer Theranostics; Elsevier: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Mutlu-Ağardan, N.B.; Tort, S.; Aydoğduoğlu, Ş.; Kıymacı, M.E. A new insight to silver sulfadiazine antibacterial dressings: Nanoparticle-loaded nanofibers for controlled drug delivery. AAPS PharmSciTech 2023, 24, 8. [Google Scholar] [CrossRef]
- Parzymies, M.; Pudelska, K.; Poniewozik, M. The use of nano-silver for disinfection of Pennisetum alopecuroides plant material for tissue culture. Acta Sci. Pol. Hortorum Cultus 2019, 18, 127–135. [Google Scholar] [CrossRef]
- Sarmast, M.K.; Salehi, H.; Khosh-Khui, M. Nano silver treatment is effective in reducing bacterial contaminations of Araucaria excelsa R. Br. var. glauca explants. Acta Biol. Hung. 2011, 62, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed]





| Dn (nm) | Ð | Dh (nm) | PD | ζ-Potential (mV) | Coating (wt.%) | |
|---|---|---|---|---|---|---|
| IONs | 8 ± 2 | 1.29 | 108 ± 1 | 0.13 | −26 ± 5 | - |
| ION@DPA-Dex-β-CD | 9 ± 2 | 1.28 | 155 ± 2 | 0.07 | −18 ± 4 | 10.8 |
| ION@DPA-Dex-β-CD-SMT-Ag | 8 ± 2 | 1.26 | 244 ± 3 | 0.29 | −36 ± 2 | 13.1 |
| SMT-Ag Content (µg/mg of IONs) | LE (%) | |
|---|---|---|
| IONs | 3 ± 0.2 | 0.3 ± 0.02 |
| ION@DPA-Dex-β-CD | 24 ± 0.6 | 2.1 ± 0.03 |
| Microorganism | Staphylococcus aureus CCM 2022 | Escherichia coli CCM 3954 | Candida albicans CCM 8186 | Aspergillus niger | |||||
|---|---|---|---|---|---|---|---|---|---|
| Test | DDT | MIC/MBC (μg/mL) | DDT | MIC/MBC (μg/mL) | DDT | MIC/MMC (μg/mL) | DDT | MIC/MMC (μg/mL) | |
| Tested Particles | |||||||||
| IONs | Growth around the disc/NT | NE/NE | Growth around the disc/NT | NT | Growth around the disc/growth within the drop | NT | Growth around the disc/NT | NT | |
| ION@DPA-Dex-β-CD | Growth around the disc/NT | NE/NE | Growth around the disc/NT | NT | Growth around the disc/growth within the drop | NT | Growth around the disc/NT | NT | |
| ION@DPA-Dex-β-CD-SMT-Ag | 2 mm inhibition zone/no growth within the drop | 500/NE | 1 mm inhibition zone/no growth within the drop | 500/1000 | Growth around the disc/growth within the drop | 250/NE | 2 mm inhibition zone/no growth within the drop | 250/1000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatan, A.B.; Patsula, V.; Macková, H.; Mahun, A.; Lehotská, R.; Piecková, E.; Horák, D. Silver-Sulfamethazine-Conjugated β-Cyclodextrin/Dextran-Coated Magnetic Nanoparticles for Pathogen Inhibition. Nanomaterials 2024, 14, 371. https://doi.org/10.3390/nano14040371
Shatan AB, Patsula V, Macková H, Mahun A, Lehotská R, Piecková E, Horák D. Silver-Sulfamethazine-Conjugated β-Cyclodextrin/Dextran-Coated Magnetic Nanoparticles for Pathogen Inhibition. Nanomaterials. 2024; 14(4):371. https://doi.org/10.3390/nano14040371
Chicago/Turabian StyleShatan, Anastasiia B., Vitalii Patsula, Hana Macková, Andrii Mahun, Renáta Lehotská, Elena Piecková, and Daniel Horák. 2024. "Silver-Sulfamethazine-Conjugated β-Cyclodextrin/Dextran-Coated Magnetic Nanoparticles for Pathogen Inhibition" Nanomaterials 14, no. 4: 371. https://doi.org/10.3390/nano14040371