Metallic Effects on p-Hydroxyphenyl Porphyrin Thin-Film-Based Planar Optical Waveguide Gas Sensor: Experimental and Computational Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of THPP Metalloporphyrin Thin Film
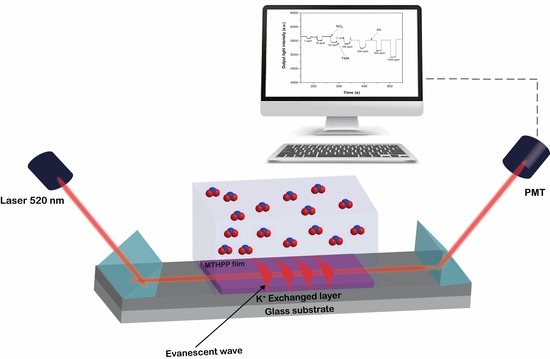
2.2. Characterization of Metalloporphyrin Film-Gas Interaction
2.3. Molecular Dynamic Simulations and Quantum Mechanical Calculations
3. Results and Discussion
3.1. Characterization of THPP Metalloporphyrin Complex
3.2. Interaction Analysis of Metalloporphyrin Film and NO2 Gas
3.3. Molecular Dynamic Simulations and Quantum Mechanical Calculations Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doğan, N.; Dumanoğulları, F.M.; Hayvalı, M.; Yılmaz, H.; Kürüm, U.; Yaglioglu, H.G.; Elmali, A. Nonlinear absorption behaviors of filled and unfilled d shell metal complexes of 5, 10, 15, 20-tetrakis(4-hydroxyphenyl)porphyrin. Chem. Phys. Lett. 2011, 508, 265–269. [Google Scholar] [CrossRef]
- Rosenblatt, M.M.; Wang, J.; Suslick, K.S. De novo designed cyclic-peptide heme complexes. Proc. Natl. Acad. Sci. USA 2003, 100, 13140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suslick, K.S.; Rakow, N.A.; Sen, A. Colorimetric sensor arrays for molecular recognition. Tetrahedron 2004, 60, 11133–11138. [Google Scholar] [CrossRef]
- Li, Z.; Jang, M.; Askim, J.R.; Suslick, K.S. Identification of accelerants, fuels and post-combustion residues using a colorimetric sensor array. Analyst 2015, 140, 5929–5935. [Google Scholar] [CrossRef] [PubMed]
- Askim, J.R.; Li, Z.; LaGasse, M.K.; Rankin, J.M.; Suslick, K.S. An optoelectronic nose for identification of explosives. Chem. Sci. 2016, 7, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Askim, J.R.; Zhong, W.; Orlean, P.; Suslick, K.S. Identification of pathogenic fungi with an optoelectronic nose. Analyst 2014, 139, 1922–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Suslick, K.S. Portable Optoelectronic Nose for Monitoring Meat Freshness. ACS Sens. 2016, 1, 1330–1335. [Google Scholar] [CrossRef]
- Suslick, B.A.; Feng, L.; Suslick, K.S. Discrimination of Complex Mixtures by a Colorimetric Sensor Array: Coffee Aromas. Anal. Chem. 2010, 82, 2067–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Bailey, D.P.; Suslick, K.S. Colorimetric Sensor Arrays for the Analysis of Beers: A Feasibility Study. J. Agric. Food Chem. 2006, 54, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Suslick, K.S. Colorimetric Sensor Array for Soft Drink Analysis. J. Agric. Food Chem. 2007, 55, 237–242. [Google Scholar] [CrossRef]
- Musto, C.J.; Lim, S.H.; Suslick, K.S. Colorimetric Detection and Identification of Natural and Artificial Sweeteners. Anal. Chem. 2009, 81, 6526–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suslick, K.S. An Optoelectronic Nose:“Seeing” Smells by Means of Colorimetric Sensor Arrays. MRS Bull. 2004, 29, 720–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yimit, A.; Rossberg, A.G.; Amemiya, T.; Itoh, K. Thin film composite optical waveguides for sensor applications: A review. Talanta 2005, 65, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sergey, S.S.; Michael, J.C.; Courtney, B.; Darnell, E.D.; James, G.G.; Frank Kenneth, H. Planar optical waveguide sensor of ammonia. Proc. SPIE 2004, 5586, 33–44. [Google Scholar]
- Dilbar, A.; Erkin, T.; Xawkat, A.; Abliz, Y. Fabrication of Composite Optical Waveguides Based on Thin Films Consisted of Iron Phosphate Nanoparticles and Their Applications as Ammonia Gas Sensor. Chin. J. Appl. Chem. 2010, 27, 965–969. [Google Scholar] [CrossRef]
- Kadir, R.; Yimit, A.; Ablat, H.; Mahmut, M.; Itoh, K. Optical Waveguide BTX Gas Sensor Based on Polyacrylate Resin Thin Film. Environ. Sci. Technol. 2009, 43, 5113–5116. [Google Scholar] [CrossRef] [PubMed]
- Yasin, P.; Yimit, A.; Rahman, E.; Nizamidin, P. Cyclodextrin Polymer Films Optical Waveguide Sensor for Volatile Organic Gas Detection. Chem. Res. Chin. Univ. 2012, 4, 682–685. [Google Scholar]
- Mohemati, M.; Yimit, A.; Turahun, Y. Zinc Oxide Thin Film/Tin-diffused Optical Waveguide Sensor for Chlorobenzene Gas Detection. Chin. J. Anal. Chem. 2011, 39, 120–124. [Google Scholar] [CrossRef]
- Mamtmin, G.; Kari, N.; Abdurahman, R.; Nizamidin, P.; Yimit, A. 5, 10, 15, 20-tetrakis-(4-methoxyphenyl) porphyrin film/K+ ion-exchanged optical waveguide gas sensor. Opt. Laser Technol. 2020, 128, 106260. [Google Scholar] [CrossRef]
- Kari, N.; Zannotti, M.; Mamtmin, G.; Giovannetti, R.; Minofar, B.; Řeha, D.; Maimaiti, P.; Kutilike, B.; Yimit, A. Substituent Effect on Porphyrin Film-Gas Interaction by Optical Waveguide: Spectrum Analysis and Molecular Dynamic Simulation. Materials 2020, 13, 5613. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the preparation of metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.A.; Cheatham, T.E.I.; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Merz, K.M.; Wang, B.; Pearlman, D.A.; et al. Amber 8; University of California: San Francisco, CA, USA, 2004. [Google Scholar]
- Ghahremanpour, M.M.; Van Maaren, P.; Van der Spoel, D. Alexandria Library; Zenodo, Ed.; 2017; Available online: https://zenodo.org/record/1004711#.Yi1nVjURVPZ (accessed on 22 December 2021). [CrossRef]
- Ghahremanpour, M.M.; van Maaren, P.J.; Ditz, J.C.; Lindh, R.; van der Spoel, D. Large-scale calculations of gas phase thermochemistry: Enthalpy of formation, standard entropy, and heat capacity. J. Chem. Phys. 2016, 145, 114305. [Google Scholar] [CrossRef] [Green Version]
- Ghahremanpour, M.M.; van Maaren, P.J.; van der Spoel, D. The Alexandria library, a quantum-chemical database of molecular properties for force field development. Sci. Data 2018, 5, 180062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghahremanpour, M.M.; van Maaren, P.J.; Caleman, C.; Hutchison, G.R.; van der Spoel, D. Polarizable Drude Model with s-Type Gaussian or Slater Charge Density for General Molecular Mechanics Force Fields. J. Chem. Theory Comput. 2018, 14, 5553–5566. [Google Scholar] [CrossRef] [PubMed]
- Walz, M.-M.; Ghahremanpour, M.M.; van Maaren, P.J.; van der Spoel, D. Phase-Transferable Force Field for Alkali Halides. J. Chem. Theory Comput. 2018, 14, 5933–5948. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.01; Gaussian Inc: Wallingford, CT, USA, 2004. [Google Scholar]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Martínez, L. Packing optimization for automated generation of complex system’s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. Mol. Model. Annu. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Zwaans, R.; van Lenthe, J.H.; den Boer, D.H.W. Ab initio calculations on first-row transition metal porphyrins Part 2. Ground state spin multiplicities, calculated ionisation potentials and electron affinities and their relation to catalytic activity. J. Mol. Struct. THEOCHEM 1996, 367, 15–24. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Maximiano, R.V.; Piovesan, E.; Zílio, S.C.; Machado, A.E.H.; de Paula, R.; Cavaleiro, J.A.S.; Borissevitch, I.E.; Ito, A.S.; Gonçalves, P.J.; Barbosa Neto, N.M. Excited-state absorption investigation of a cationic porphyrin derivative. J. Photochem. Photobiol. A Chem. 2010, 214, 115–120. [Google Scholar] [CrossRef]
- Zannotti, M.; Giovannetti, R.; Minofar, B.; Řeha, D.; Plačková, L.; D’Amato, C.A.; Rommozzi, E.; Dudko, H.V.; Kari, N.; Minicucci, M. Aggregation and metal-complexation behaviour of THPP porphyrin in ethanol/water solutions as function of pH. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, W.; Wang, Z. Porphin-Based Carbon Dots for “Turn Off–On” Phosphate Sensing and Cell Imaging. Nanomaterials 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, K.A.D.F.; Silva, S.; Pereira, P.M.R.; Simões, M.M.Q.; Neves, M.d.G.P.M.S.; Cavaleiro, J.A.S.; Wypych, F.; Tomé, J.P.C.; Nakagaki, S. Galactodendritic Porphyrinic Conjugates as New Biomimetic Catalysts for Oxidation Reactions. Inorg. Chem. 2015, 54, 4382–4393. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, P.B.; Gujarathi, B.P. Synthesis and characterization of new meso-substituted unsymmetrical metalloporphyrins. J. Chem. Sci. 2008, 120, 259–266. Available online: https://www.ias.ac.in/article/fulltext/jcsc/120/02/0259-0266 (accessed on 22 December 2021). [CrossRef]
- Lu, G.; Zhang, X.; Cai, X.; Jiang, J. Tuning the morphology of self-assembled nanostructures of amphiphilic tetra(p-hydroxyphenyl)porphyrins with hydrogen bonding and metal–ligand coordination bonding. J. Mater. Chem. 2009, 19, 2417–2424. [Google Scholar] [CrossRef]
- Diller, K.; Papageorgiou, A.C.; Klappenberger, F.; Allegretti, F.; Barth, J.V.; Auwärter, W. In vacuo interfacial tetrapyrrole metallation. Chem. Soc. Rev. 2016, 45, 1629–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.A.; Gaddam, R.R.; Suresh, M.; Varanasi, S.R.; Yang, D.; Bhatia, S.K.; Zhao, X.S. Porphyrin–graphene oxide frameworks for long life sodium ion batteries. J. Mater. Chem. A 2017, 5, 13204–13211. [Google Scholar] [CrossRef]
- Bai, Y.; Sekita, M.; Schmid, M.; Bischof, T.; Steinrück, H.-P.; Gottfried, J.M. Interfacial coordination interactions studied on cobalt octaethylporphyrin and cobalt tetraphenylporphyrin monolayers on Au(111). Phys. Chem. Chem. Phys. 2010, 12, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Lukasczyk, T.; Flechtner, K.; Merte, L.R.; Jux, N.; Maier, F.; Gottfried, J.M.; Steinrück, H.-P. Interaction of Cobalt(II) Tetraarylporphyrins with a Ag(111) Surface Studied with Photoelectron Spectroscopy. J. Phys. Chem. Chem. 2007, 111, 3090–3098. [Google Scholar] [CrossRef]
- Mangione, G.; Carlotto, S.; Sambi, M.; Ligorio, G.; Timpel, M.; Vittadini, A.; Nardi, M.V.; Casarin, M. Electronic structures of CuTPP and CuTPP(F) complexes. A combined experimental and theoretical study I. Phys. Chem. Chem. Phys. 2016, 18, 18727–18738. [Google Scholar] [CrossRef] [PubMed]
- Fatayer, S.; Veiga, R.G.A.; Prieto, M.J.; Perim, E.; Landers, R.; Miwa, R.H.; de Siervo, A. Self-assembly of NiTPP on Cu(111): A transition from disordered 1D wires to 2D chiral domains. Phys. Chem. Chem. Phys. 2015, 17, 18344–18352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, M.; Naik, T.R.; Pathak, C.S.; Nagarajan, S.; Rao, V.R.; Singh, R. Significant improvement in the electrical characteristics of Schottky barrier diodes on molecularly modified Gallium Nitride surfaces. Appl. Phys. Lett. 2018, 112, 163502. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, S.; Yoon, H.; Jang, W.-D. Strong Binding Affinity of a Zinc–Porphyrin-Based Receptor for Halides through the Cooperative Effects of Quadruple C—H Hydrogen Bonds and Axial Ligation. Chem.—A Eur. J. 2011, 17, 13898–13903. [Google Scholar] [CrossRef] [PubMed]













| Soret Band | Q Band | |||||
|---|---|---|---|---|---|---|
| Complexes | λ (nm) | ε (M−1 cm−1) | Λ (nm) | ε (M−1 cm−1) | Λ (nm) | ε (M−1 cm−1) |
| Co–THPP | 420 | 2.87 × 105 | 534 | 1.09 × 104 | 590 | 3.19 × 103 |
| Ni–THPP | 421 | 1.38 × 105 | 531 | 9.15 × 104 | 570 | 2.01 × 103 |
| Cu–THPP | 421 | 9.38 × 105 | 542 | 4.42 × 104 | 582 | 1.30 × 104 |
| Zn–THPP | 429 | 5.00 × 108 | 562 | 1.46 × 107 | 604 | 1.14 × 107 |
| Complexes | λSoret Solution (nm) | λSoret Film (nm) | Shift Δλ (nm) |
|---|---|---|---|
| Co–THPP | 420 | 446 | 26 |
| Ni–THPP | 421 | 431 | 10 |
| Cu–THPP | 421 | 429 | 8 |
| Zn–THPP | 429 | 440 | 11 |
| Soret Band | Q Band | |||
|---|---|---|---|---|
| Complexes | Before | After NO2 | Before | After NO2 |
| Co–THPP | 445 | 455 | 553, 596 | - |
| Cu–THPP | 427 | 448 | 550 | - |
| Ni–THPP | 431 | 474 | 536 | - |
| Zn–THPP | 440 | 474 | 563, 607 | 700 |
| System | Interaction Energy [kcal/mole] | Interaction Energy [kcal/mole] |
|---|---|---|
| Conformation 1 | Conformation 2 | |
| NO2—Zn–THPP | −11.0 | −29.7 |
| NO2—Cu–THPP | +0.33 | −2.98 |
| NO2—Co–THPP | −11.8 | −30.6 |
| NO2—Ni–THPP | −14.9 | −19.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kari, N.; Zannotti, M.; Giovannetti, R.; Řeha, D.; Minofar, B.; Abliz, S.; Yimit, A. Metallic Effects on p-Hydroxyphenyl Porphyrin Thin-Film-Based Planar Optical Waveguide Gas Sensor: Experimental and Computational Studies. Nanomaterials 2022, 12, 944. https://doi.org/10.3390/nano12060944
Kari N, Zannotti M, Giovannetti R, Řeha D, Minofar B, Abliz S, Yimit A. Metallic Effects on p-Hydroxyphenyl Porphyrin Thin-Film-Based Planar Optical Waveguide Gas Sensor: Experimental and Computational Studies. Nanomaterials. 2022; 12(6):944. https://doi.org/10.3390/nano12060944
Chicago/Turabian StyleKari, Nuerguli, Marco Zannotti, Rita Giovannetti, David Řeha, Babak Minofar, Shawket Abliz, and Abliz Yimit. 2022. "Metallic Effects on p-Hydroxyphenyl Porphyrin Thin-Film-Based Planar Optical Waveguide Gas Sensor: Experimental and Computational Studies" Nanomaterials 12, no. 6: 944. https://doi.org/10.3390/nano12060944