Novel Insights into the Effect of Pythium Strains on Rapeseed Metabolism
Abstract
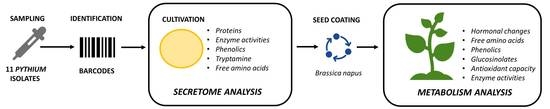
:1. Introduction
2. Materials and Methods
2.1. Isolation and Selection of Pythium Isolates
2.2. Cultivation of Pythium Isolates
2.3. Identification of Pythium Isolates
2.4. Plant Material
2.5. Protein Content
2.6. Electrophoretic Separation
2.7. Free Amino Acids and Tryptamine Content
2.8. Phenolics and Antioxidant Capacity
2.9. Phytohormone Analysis
2.10. Extraction and Separation of Glucosinolates
2.11. Enzyme Activities
2.12. Carbon, Nitrogen, and Sulfur Content Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Identification of Tested Pythium spp. Revealed not yet Described Pythium Species
3.2. Secretomes Contained Proteins, Amino Acids, and Phenolic Compounds
3.3. Pythium Secretes a Cocktail of Host Cell Wall-Degrading Enzymes
3.4. How Do Pythium spp. Affect Metabolism in Inoculated Rapeseed Plants?
3.5. The Concentration of Phytohormones is Affected by Pythium spp. in the Rapeseed Plants
3.6. Pythium Alters the Glucosinolate Composition of Inoculated Rapeseed Plants
3.7. Could Pythium spp. Support the Activity of Antioxidant Enzymes in Rapeseed Plants?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerbore, J.; Benhamou, N.; Vallance, J.; Le Floch, G.; Grizard, D.; Regnault-Roger, C.; Rey, P. Biological control of plant pathogens: Advantages and limitations seen through the case study of Pythium oligandrum. Environ. Sci. Pollut. Res. Int. 2014, 21, 4847–4860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Elad, Y.; Chet, I. Biological control effects of a new isolate of Trichoderma harzianum on Pythium aphanidermatum. Phytopathology 1984, 74, 498–501. [Google Scholar] [CrossRef]
- Benhamou, N.; le Floch, G.; Vallance, J.; Gerbore, J.; Grizard, D.; Rey, P. Pythium oligandrum: An example of opportunistic success. Microbiology 2012, 158, 2679–2694. [Google Scholar] [CrossRef] [PubMed]
- Gabrielova, A.; Mencl, K.; Suchanek, M.; Klimes, R.; Hubka, V.; Kolarik, M. The oomycete Pythium oligandrum can suppress and kill the causative agents of dermatophytoses. Mycopathologia 2018, 183, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, S.K.; Vetukuri, R.R.; Grenville-Briggs, L.J. Draft genome sequence of the mycoparasitic oomycete Pythium periplocum strain CBS 532.74. Genome Announc. 2017, 5, e00057–17. [Google Scholar] [CrossRef] [Green Version]
- Ponchet, M.; Panabieres, F.; Milat, M.L.; Mikes, V.; Montillet, J.L.; Suty, L.; Triantaphylides, C.; Tirilly, Y.; Blein, J.P. Are elicitins cryptograms in plant-Oomycete communications? Cell Mol. Life Sci. 1999, 56, 1020–1047. [Google Scholar] [CrossRef]
- Masunaka, A.; Sekiguchi, H.; Takahashi, H.; Takenaka, S. Distribution and expression of elicitin-like protein genes of the biocontrol agent Pythium oligandrum. J. Phytopathol. 2010, 158, 417–426. [Google Scholar] [CrossRef]
- Le Floch, G.; Rey, P.; Benizri, E.; Benhamou, N.; Tirilly, Y. Impact of auxin-compounds produced by the antagonistic fungus Pythium oligandrum or the minor pathogen Pythium group F on plant growth. Plant Soil 2003, 257, 459–470. [Google Scholar] [CrossRef]
- Whipps, J.M. Effect of media on growth and interactions between a range of soil-born glasshouse pathogens and antagonistic fungi. New Phytol. 1987, 107, 127–142. [Google Scholar] [CrossRef]
- Vesely, D. Studies of the mycoparasitism in rhizosphere of emerging sugar-beet. Zentralbl. Bakteriol. Naturwiss. 1978, 133, 195–200. [Google Scholar] [CrossRef]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D. One stop shop: Backbones trees for important phytopathogenic genera: I. Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- Robideau, G.P.; De Cock, A.W.; Coffey, M.D.; Voglmayr, H.; Brouwer, H.; Bala, K.; Chitty, D.W.; Desaulniers, N.; Eggertson, Q.A.; Gachon, C.M.; et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol. Ecol. Resour. 2011, 11, 1002–1011. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Chen, J.; Lü, L.; Ye, W.; Wang, Y.-C.; Zheng, X.-B. Pythium cedri sp. nov. (Pythiaceae, Pythiales) from southern China based on morphological and molecular characters. Phytotaxa 2017, 309, 135–142. [Google Scholar] [CrossRef]
- Faure, C.; Veyssiere, M.; Boelle, B.; San Clemente, H.; Bouchez, O.; Lopez-Roques, C.; Chaubet, A.; Martinez, Y.; Bezouska, K.; Suchanek, M.; et al. Long-read genome sequence of the sugar beet rhizosphere mycoparasite Pythium oligandrum. G3 Genes Genom. Genet. 2020, 10, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Berger, H.; Yacoub, A.; Gerbore, J.; Grizard, D.; Rey, P.; Sessitsch, A.; Compant, S. Draft genome sequence of biocontrol agent Pythium oligandrum strain Po37, an Oomycota. Genome Announc. 2016, 4, e00215–00216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schagger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hodek, O.; Krizek, T.; Coufal, P.; Ryslava, H. Design of experiments for amino acid extraction from tobacco leaves and their subsequent determination by capillary zone electrophoresis. Anal. Bional. Chem. 2017, 409, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Tupec, M.; Hyskova, V.; Belonoznikova, K.; Hranicek, J.; Cerveny, V.; Ryslava, H. Characterization of some potential medicinal plants from Central Europe by their antioxidant capacity and the presence of metal elements. Food Biosci. 2017, 20, 43–50. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Kaminek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. Methods Mol. Biol. 2012, 913, 251–261. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Konradyova, V.; Knirsch, V.; Gaudinova, A.; Kramna, B.; Kazda, J.; Ludwig-Muller, J.; Vankova, R. Hormonal responses to Plasmodiophora brassicae infection in Brassica napus cultivars differing in their pathogen resistance. Int. J. Mol. Sci. 2018, 19, 4024. [Google Scholar] [CrossRef] [Green Version]
- Lechtenberg, M.; Hensel, A. Determination of glucosinolates in broccoli-based dietary supplements by cyclodextrin-mediated capillary zone electrophoresis. J. Food Compos. Anal. 2019, 78, 138–149. [Google Scholar] [CrossRef]
- Hyskova, V.; Pliskova, V.; Cerveny, V.; Ryslava, H. NADP-dependent enzymes are involved in response to salt and hypoosmotic stress in cucumber plants. Gen. Physiol. Biophys. 2017, 36, 247–258. [Google Scholar] [CrossRef]
- Yannarelli, G.G.; Fernandez-Alvarez, A.J.; Santa-Cruz, D.M.; Tomaro, M.L. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochemistry 2007, 68, 505–512. [Google Scholar] [CrossRef]
- Coelho, D.F.; Saturnino, T.P.; Fernandes, F.F.; Mazzola, P.G.; Silveira, E.; Tambourgi, E.B. Azocasein substrate for determination of proteolytic activity: Reexamining a traditional method using bromelain samples. BioMed Res. Int. 2016, 2016, ID8409183. [Google Scholar] [CrossRef] [Green Version]
- Nakata, H.; Ishii, S. Substrate activation of trypsin and acetyltrypsin caused by -N-benzoyl-L-arginine p-nitroanilide. J. Biochem. 1972, 72, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barrett, D.M. Determination of reducing sugars with 3-methyl-2-benzothiazolinonehydrazone. Anal. Biochem. 2002, 305, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Maseko, S.T.; Dakora, F.D. Rhizosphere acid and alkaline phosphatase activity as a marker of P nutrition in nodulated Cyclopia and Aspalathus species in the Cape fynbos of South Africa. S. Afr. J. Bot. 2013, 89, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Synkova, H.; Semoradova, S.; Schnablova, R.; Muller, K.; Pospisilova, J.; Ryslava, H.; Malbeck, J.; Cerovska, N. Effects of biotic stress caused by Potato virus Y on photosynthesis in ipt transgenic and control Nicotiana tabacum L. Plant Sci. 2006, 171, 607–616. [Google Scholar] [CrossRef]
- Spoustova, P.; Hyskova, V.; Muller, K.; Schnablova, R.; Ryslava, H.; Cerovska, N.; Malbeck, J.; Cvikrova, M.; Synkova, H. Tobacco susceptibility to Potato virus Y-NTN infection is affected by grafting and endogenous cytokinin content. Plant Sci. 2015, 235, 25–36. [Google Scholar] [CrossRef]
- Plaats-Niterink, A.J.v.d. Monograph of the genus Pythium. Stud. Mycol. 1981, 21, 1–244. [Google Scholar]
- McGowan, J.; Fitzpatrick, D.A. Genomic, network, and phylogenetic analysis of the oomycete effector arsenal. MSphere 2017, 2, e00408–17. [Google Scholar] [CrossRef] [Green Version]
- Judelson, H.S. Metabolic diversity and novelties in the oomycetes. Annu. Rev. Microbiol. 2017, 71, 21–39. [Google Scholar] [CrossRef]
- Brunner, F.; Wirtz, W.; Rose, J.K.; Darvill, A.G.; Govers, F.; Scheel, D.; Nurnberger, T. A beta-glucosidase/xylosidase from the phytopathogenic oomycete, Phytophthora infestans. Phytochemistry 2002, 59, 689–696. [Google Scholar] [CrossRef]
- Bowyer, P.; Clarke, B.R.; Lunness, P.; Daniels, M.J.; Osbourn, A.E. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science 1995, 267, 371–374. [Google Scholar] [CrossRef]
- Crombie, W.M.L.; Crombie, L.; Green, J.B.; Lucas, J.A. Pathogenicity of ‘take-all’ fungus to oats: Its relationship to the concentration and detoxification of the four avenacins. Phytochemistry 1986, 25, 2075–2083. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Bernards, M.A. Ginsenosidases and the pathogenicity of Pythium irregulare. Phytochemistry 2012, 78, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Brzobohaty, B.; Moore, I.; Kristoffersen, P.; Bako, L.; Campos, N.; Schell, J.; Palme, K. Release of active cytokinin by a beta-glucosidase localized to the maize root meristem. Science 1993, 262, 1051–1054. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 1–11. [Google Scholar] [CrossRef]
- Tsuda, K. Division of Tasks: Defense by the Spatial Separation of Antagonistic Hormone Activities. Plant Cell Physiol. 2017, 54, 3–4. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, Y.; Takenaka, S.; Hase, S.; Kubota, M.; Ichinose, Y.; Kanayama, Y.; Nakaho, K.; Klessig, D.F.; Takahashi, H. Enhanced defense responses in Arabidopsis induced by the cell wall protein fractions from Pythium oligandrum require SGT1, RAR1, NPR1 and JAR1. Plant Cell Physiol. 2009, 50, 924–934. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S.; Yamaguchi, K.; Masunaka, A.; Hase, S.; Inoue, T.; Takahashi, H. Implications of oligomeric forms of POD-1 and POD-2 proteins isolated from cell walls of the biocontrol agent Pythium oligandrum in relation to their ability to induce defense reactions in tomato. J. Plant Physiol. 2011, 168, 1972–1979. [Google Scholar] [CrossRef]
- Bjorkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health-influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Maruyama, A.; Yamamoto, Y.; Hara, S. Extraction and characterization of glucosinolates and isothiocyanates from rape seed meal. J. Oleo Sci. 2014, 63, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, H.L.; Brandes, H.; Ulber, B.; Becker, H.C.; Vidal, S. Evaluation of nine genotypes of oilseed rape (Brassica napus L.) for larval infestation and performance of rape stem weevil (Ceutorhynchus napi Gyll.). PLoS ONE 2017, 12, e0180807. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Dosz, E.B.; Sun, J.; Hoeflinger, J.L.; Van Tassell, M.L.; Chen, P.; Harnly, J.M.; Miller, M.J.; Jeffery, E.H. Myrosinase-dependent and -independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front. Plant Sci. 2015, 6, 831. [Google Scholar] [CrossRef] [Green Version]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Wittstock, U.; Kliebenstein, D.J.; Lambrix, V.; Reichelt, M.; Gershenzon, J. Chapter five Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores. Recent Adv. Phytochem. 2003, 37, 101–125. [Google Scholar] [CrossRef]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef]
- Giamoustaris, A.; Mithen, R. Glucosinolates and disease resistance in oilseed rape (Brassica napus ssp. oleifera). Plant Pathol. 1997, 46, 271–275. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Abou-Mansour, E.; Buchala, A.; Mauch, F. Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J. 2010, 62, 840–851. [Google Scholar] [CrossRef] [Green Version]









| Identity | Strain | Accession No. | |
|---|---|---|---|
| ITS rDNA | COI | ||
| P. oligandrum | 00X5 | LR760206 | LR760205 |
| P. oligandrum | 00X11 | MT249384 | LR760204 |
| P. oligandrum | 00X23 | LR760202 | |
| P. oligandrum | 00X30 | LR760200 | |
| P. oligandrum | 00X34 | LR760199 | |
| P. oligandrum | 00X40 | LR760209 | LR760201 |
| P. oligandrum | 00X42 | LR760210 | LR760197 |
| P. oligandrum | 00X48 | LR760208 | LR760198 |
| P. oligandrum | X40 | LR760207 | LR760196 |
| Pythium sp. | X42 | LR760211 | LR760203 |
| C [µmol.L-1] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | M1 | 00X5 | 00X11 | 00X23 | 00X30 | 00X34 | 00X40 | 00X42 | 00X48 | X40 | X42 | |
| Ala | 993 ± 70 | 2578 ± 134 | 1523 ± 120 | 3055 ± 199 | 7391 ± 198 | 1951 ± 52 | 2984 ± 61 | 1625 ± 32 | 9734 ± 6 | 1832 ± 37 | 1130 ± 45 | 8088 ± 1769 |
| Arg | 32 ± 8 | 975 ± 50 | 1274 ± 42 | 1507 ± 66 | 457 ± 25 | 1272 ± 21 | 1036 ± 41 | 820 ± 8 | 908 ± 59 | 2039 ± 89 | 1057 ± 83 | 1088 ± 89 |
| Asn | 345 ± 39 | 685 ± 97 | 482 ± 109 | 1167 ± 80 | 784 ± 14 | 812 ± 43 | 1056 ± 65 | 794 ± 19 | 1171 ± 54 | 623 ± 92 | 442 ± 28 | 1462 ± 183 |
| Asp | <LOD | 426 ± 33 | 181 ± 50 | 1077 ± 219 | 709 ± 157 | 353 ± 130 | 854 ± 85 | 455 ± 98 | 2027 ± 67 | 295 ± 113 | 225 ± 131 | 2552 ± 120 |
| Cys | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Gln | <LOD | 1207 ± 24 | 771 ± 12 | 2555 ± 241 | 2486 ± 65 | 1488 ± 116 | 2410 ± 102 | 2169 ± 115 | 3609 ± 94 | 777 ± 36 | 797 ± 47 | 4169 ± 630 |
| Glu | 63 ± 31 | 1731 ± 59 | 1208 ± 112 | 2737 ± 132 | 3332 ± 90 | 1934 ± 7 | 2806 ± 87 | 1415 ± 79 | 7537 ± 106 | 1106 ± 30 | 991 ± 30 | 8597 ± 937 |
| Gly | 276 ± 31 | 1223 ± 60 | 476 ± 69 | 1638 ± 137 | 2445 ± 107 | 861 ± 69 | 1726 ± 28 | 806 ± 17 | 4364 ± 181 | 778 ± 43 | 417 ± 83 | 4338 ± 927 |
| His | 1 ± 0 | 251 ± 13 | 204 ± 35 | 383 ± 37 | 128 ± 8 | 249 ± 1 | 337 ± 23 | 199 ± 27 | 351 ± 8 | 250 ± 46 | 163 ± 14 | 360 ± 111 |
| Ile | 106 ± 64 | 650 ± 71 | 471 ± 81 | 1038 ± 48 | 705 ± 45 | 645 ± 15 | 901 ± 87 | 517 ± 19 | 1365 ± 70 | 533 ± 47 | 371 ± 12 | 1662 ± 147 |
| Leu | 659 ± 43 | 1237 ± 12 | 1068 ± 161 | 1895 ± 67 | 1367 ± 109 | 1311 ± 52 | 1602 ± 87 | 1014 ± 132 | 2513 ± 66 | 1193 ± 92 | 811 ± 121 | 3099 ± 514 |
| Lys | 26 ± 7 | 900 ± 113 | 750 ± 26 | 1327 ± 32 | 779 ± 53 | 935 ± 20 | 1096 ± 17 | 679 ± 11 | 1408 ± 55 | 896 ± 27 | 546 ± 19 | 1669 ± 100 |
| Met | 134 ± 28 | 352 ± 10 | 323 ± 45 | 498 ± 147 | 313 ± 47 | 386 ± 31 | 331 ± 84 | 256 ± 27 | 640 ± 55 | 316 ± 29 | 251 ± 14 | 737 ± 129 |
| Phe | 138 ± 67 | 521 ± 47 | 374 ± 84 | 904 ± 182 | 476 ± 127 | 534 ± 43 | 748 ± 69 | 460 ± 116 | 910 ± 82 | 510 ± 38 | 354 ± 19 | 1441 ± 240 |
| Pro | 573 ± 146 | 2030 ± 155 | 2080 ± 292 | 1970 ± 163 | 4881 ± 89 | 2866 ± 115 | 1494 ± 105 | 1199 ± 102 | 3888 ± 108 | 3829 ± 138 | 1677 ± 89 | 6118 ± 983 |
| Ser | 228 ± 45 | 879 ± 22 | 558 ± 99 | 1331 ± 67 | 1865 ± 102 | 809 ± 44 | 1249 ± 78 | 734 ± 79 | 2859 ± 77 | 715 ± 133 | 470 ± 12 | 3113 ± 393 |
| Thr | 170 ± 42 | 846 ± 0 | 520 ± 63 | 1211 ± 102 | 1358 ± 47 | 793 ± 35 | 1116 ± 125 | 633 ± 28 | 2068 ± 42 | 712 ± 43 | 420 ± 22 | 2417 ± 452 |
| Trp | <LOD | 122 ± 12 | 50 ± 15 | 141 ± 56 | 87 ± 56 | 89 ± 17 | 127 ± 24 | 81 ± 14 | 266 ± 46 | 82 ± 21 | 60 ± 20 | 250 ± 61 |
| Tyr | <LOD | 419 ± 65 | 378 ± 78 | 676 ± 26 | 339 ± 26 | 440 ± 34 | 555 ± 58 | 351 ± 55 | 842 ± 32 | 376 ± 31 | 277 ± 32 | 1118 ± 238 |
| Val | 302 ± 31 | 1058 ± 158 | 821 ± 35 | 1623 ± 47 | 1353 ± 88 | 1095 ± 11 | 1443 ± 102 | 784 ± 113 | 2461 ± 71 | 884 ± 41 | 656 ± 41 | 2806 ± 347 |
| Glucobrassicin | Glucoraphanin | Progoitrin | |
|---|---|---|---|
| [mg g(DW)−1] | [mg g(DW)−1] | [mg g(DW)−1] | |
| C | 0.142 ± 0.002 a | 0 ± 0 i | 0.196 ± 0.004 l,n |
| M1 | 0.214 ± 0.004 b,c | 0.001 ± 0.000 i | 0.133 ± 0.008 m,p |
| 00X5 | 0.200 ± 0.004 b | 0.002 ± 0.002 i,j | 0.166 ± 0.005 l,m |
| 00X11 | 0.230 ± 0.008 c | 0.003 ± 0.000 i,j | 0.139 ± 0.004 m,p |
| 00X23 | 0.180 ± 0.003 d | 0.009 ± 0.004 j,k | 0.210 ± 0.007 n,q |
| 00X30 | 0.418 ± 0.013 e | 0.005 ± 0.004 i,j | 0.142 ± 0.026 m,p |
| 00X34 | 0.049 ± 0.005 f | 0.014 ± 0.004 k | 0.086 ± 0.022 o |
| 00X40 | 0.097 ± 0.002 g | 0.005 ± 0.001 i,j | 0.120 ± 0.005 o,p |
| 00X42 | 0.318 ± 0.003 h | 0.003 ± 0.001 i,j | 0.226 ± 0.018 n |
| 00X48 | 0.179 ± 0.002 d | 0.005 ± 0.002 i,j | 0.175 ± 0.008 l,m,q |
| X42 | 0.148 ± 0.003 a | 0 ± 0 i | 0.078 ± 0.017 o |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bělonožníková, K.; Vaverová, K.; Vaněk, T.; Kolařík, M.; Hýsková, V.; Vaňková, R.; Dobrev, P.; Křížek, T.; Hodek, O.; Čokrtová, K.; et al. Novel Insights into the Effect of Pythium Strains on Rapeseed Metabolism. Microorganisms 2020, 8, 1472. https://doi.org/10.3390/microorganisms8101472
Bělonožníková K, Vaverová K, Vaněk T, Kolařík M, Hýsková V, Vaňková R, Dobrev P, Křížek T, Hodek O, Čokrtová K, et al. Novel Insights into the Effect of Pythium Strains on Rapeseed Metabolism. Microorganisms. 2020; 8(10):1472. https://doi.org/10.3390/microorganisms8101472
Chicago/Turabian StyleBělonožníková, Kateřina, Kateřina Vaverová, Tomáš Vaněk, Miroslav Kolařík, Veronika Hýsková, Radomíra Vaňková, Petre Dobrev, Tomáš Křížek, Ondřej Hodek, Kateřina Čokrtová, and et al. 2020. "Novel Insights into the Effect of Pythium Strains on Rapeseed Metabolism" Microorganisms 8, no. 10: 1472. https://doi.org/10.3390/microorganisms8101472