Production and Characterization of Rhamnolipids Produced by Pseudomonas aeruginosa DBM 3774: Response Surface Methodology Approach
Abstract
:1. Introduction
2. Material and Methods
2.1. Microorganism
2.2. Cultivation in Erlenmeyer Flasks
2.3. Response Surface Methodology (RSM) Optimization of Culture Medium
2.4. Rhamnolipid Determination and Isolation
2.5. Mass Spectrometry
2.6. Critical Micelle Concentration
2.7. Determination of Emulsification Activity
2.8. Oil Displacement Test
2.9. Solubilization of Phenanthrene
3. Results and Discussion
3.1. Effect of Carbon and Nitrogen Source on Rhamnolipid Production
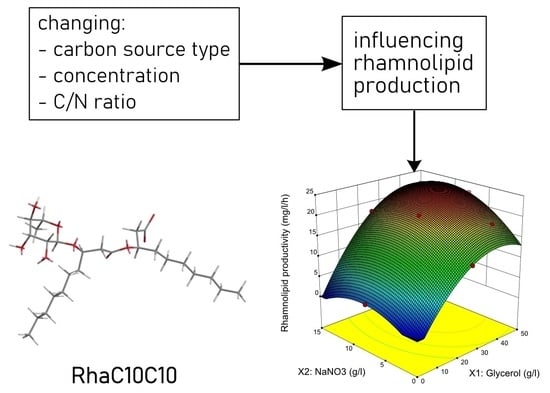
3.2. Optimization of Culture Medium for Rhamnolipid Production by RSM
3.3. Experimental Verification of the RSM Model
3.4. Rhamnolipid Characterization
3.4.1. Structural Characterization of Rhamnolipids
3.4.2. Critical Micelle Concentration
3.4.3. Emulsification Index
3.4.4. Oil Displacement Test
3.4.5. Phenanthrene Solubilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Savla, N.; Pandit, S.; Khanna, N.; Mathuriya, A.S.; Jung, S.P. Microbially powered electrochemical systems coupled with membrane-based technology for sustainable desalination and efficient wastewater treatment. J. Korean Soc. Environ. Eng. 2020, 42, 360–380. [Google Scholar] [CrossRef]
- Koo, B.; Jung, S.P. Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem. Eng. J. 2021, 424, 130388. [Google Scholar] [CrossRef]
- Lepine, F.; Deziel, E.; Milot, S.; Villemur, R. Liquid chromatographic/mass spectrometric detection of the 3-(3-hydroxyalkanoyloxy)alkanoic acid precursors of rhamnolipids in Pseudomonas aeruginosa cultures. J. Mass Spectrom. 2002, 37, 41–46. [Google Scholar] [CrossRef]
- Maier, R.M.; Soberon-Chavez, G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biot. 2000, 54, 625–633. [Google Scholar] [CrossRef]
- Wittgens, A.; Kovacic, F.; Müller, M.M.; Gerlitzki, M.; Santiago-Schübel, B.; Hofmann, D.; Tiso, T.; Blank, L.M.; Henkel, M.; Hausmann, R. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Appl. Microbiol. Biot. 2017, 101, 2865–2878. [Google Scholar] [CrossRef] [Green Version]
- Jirku, V.; Cejkova, A.; Schreiberova, O.; Jezdik, R.; Masak, J. Multicomponent biosurfactants-A “Green Toolbox” extension. Biotechnol. Adv. 2015, 33, 1272–1276. [Google Scholar] [CrossRef]
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; Garcia, F.; Manresa, A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir 2001, 17, 1367–1371. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Hruzova, K.; Patel, A.; Masak, J.; Matatkova, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. A novel approach for the production of green biosurfactant from Pseudomonas aeruginosa using renewable forest biomass. Sci. Total Environ. 2020, 711, 135099. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Muller, M.M.; Kugler, J.H.; Henkel, M.; Gerlitzki, M.; Hormann, B.; Pohnlein, M.; Syldatk, C.; Hausmann, R. Rhamnolipids-Next generation surfactants? J. Biotechnol. 2012, 162, 366–380. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lu, W.B.; Wei, Y.H.; Chen, W.M.; Chang, J.S. Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol. Prog. 2007, 23, 661–666. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Aboulwafa, M.M.; Aboshanab, K.A.; Hassouna, N.A.H. Optimization of rhamnolipid production by P. aeruginosa isolate p6. J. Surfactants Deterg. 2016, 19, 943–955. [Google Scholar] [CrossRef]
- Al-Araji, L.I.Y.; Rahman, R.N.Z.R.A.; Basri, M.; Salleh, A.B. Optimisation of rhamnolipids produced by Pseudomonas aeruginosa 181 using Response Surface Modeling. Ann. Microbiol. 2007, 57, 571–575. [Google Scholar] [CrossRef]
- Jamal, A.; Qureshi, M.Z.; Ali, N.; Ali, M.I.; Hameed, A. Enhanced production of rhamnolipids by Pseudomonas aeruginosa JQ927360 using response surface methodology. Asian J. Chem. 2014, 26, 1044–1048. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dobler, L.; de Carvalho, B.R.; Alves, W.D.; Neves, B.C.; Freire, D.M.G.; Almeida, R.V. Enhanced rhamnolipid production by Pseudomonas aeruginosa overexpressing estA in a simple medium. PLoS ONE 2017, 12, e0183857. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mawgoud, A.M.; Lepine, F.; Deziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [Green Version]
- Hoskova, M.; Jezdik, R.; Schreiberova, O.; Chudoba, J.; Sir, M.; Cejkova, A.; Masak, J.; Jirku, V.; Rezanka, T. Structural and physiochemical characterization of rhamnolipids produced by Acinetobacter calcoaceticus, Enterobacter asburiae and Pseudomonas aeruginosa in single strain and mixed cultures. J. Biotechnol. 2015, 193, 45–51. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle interpretation in terms of solid surface tension. Colloids Surf. A 2000, 161, 31–48. [Google Scholar] [CrossRef]
- Maťátková, O.; Kolouchová, I.; Lokočová, K.; Michailidu, J.; Jaroš, P.; Kulišová, M.; Řezanka, T.; Masák, J. Rhamnolipids as a tool for eradication of Trichosporon cutaneum biofilm. Biomolecules 2021, 11, 1727. [Google Scholar] [CrossRef] [PubMed]
- Pornsunthorntawee, O.; Wongpanit, P.; Chavadej, S.; Abe, M.; Rujiravanit, R. Structural and physicochemical characterization of crude biosurfactant produced by Pseudomonas aeruginosa SP4 isolated from petroleum-contaminated soil. Bioresour. Technol. 2008, 99, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; Kim, K.W.; Seagren, E.A. Combined effects of pH and biosurfactant addition on solubilization and biodegradation of phenanthrene. Appl. Microbiol. Biotechnol. 2004, 65, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Yeh, K.L.; Lu, W.B.; Lin, C.L.; Chang, J.S. Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour. Technol. 2008, 99, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Sampaio, A.P.W.; Vasquez, G.S.; Santa Anna, L.M.; Pereira, N.; Freire, D.M.G. Evaluation of different carbon and nitrogen sources in production of rhamnolipids by a strain of Pseudomonas aeruginosa. Appl. Biochem. Biotechnol. 2002, 98, 1025–1035. [Google Scholar] [CrossRef]
- Sim, L.; Ward, O.P.; Li, Z.Y. Production and characterisation of a biosurfactant isolated from Pseudomonas aeruginosa UW-1. J. Ind. Microbiol. Biotechnol. 1997, 19, 232–238. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipid surfactants: An update on the general aspects of these remarkable biomolecules. Biotechnol. Prog. 2005, 21, 1593–1600. [Google Scholar] [CrossRef]
- Deziel, E.; Lepine, F.; Milot, S.; Villemur, R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiol-Sgm 2003, 149, 2005–2013. [Google Scholar] [CrossRef] [Green Version]
- Rezanka, T.; Siristova, L.; Sigler, K. Rhamnolipid-producing thermophilic bacteria of species Thermus and Meiothermus. Extremophiles 2011, 15, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.S.; Pereira, A.G.; Neves, B.C.; Freire, D.M. Gene regulation of rhamnolipid production in Pseudomonas aeruginosa-a review. Bioresour. Technol. 2011, 102, 6377–6384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata-Sandoval, J.C.; Karns, J.; Torrents, A. High-performance liquid chromatography method for the characterization of rhamnolipid mixtures produced by Pseudomonas aeruginosa UG2 on corn oil. J. Chromatogr. A 1999, 864, 211–220. [Google Scholar] [CrossRef]
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Anton. Leeuw. Int. J. G 2004, 85, 1–8. [Google Scholar] [CrossRef]
- Haba, E.; Abalos, A.; Jauregui, O.; Espuny, M.J.; Manresa, A. Use of liquid chromatography-mass spectroscopy for studying the composition and properties of rhamnolipids produced by different strains of Pseudomonas aeruginosa. J. Surfactants Deterg. 2003, 6, 155–161. [Google Scholar] [CrossRef]
- Lovaglio, R.B.; dos Santos, F.J.; Jafelicci, M.; Contiero, J. Rhamnolipid emulsifying activity and emulsion stability: pH rules. Colloids Surf. B Biointerfaces 2011, 85, 301–305. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Mohamed, M.S.; Samak, N. Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Braz. J. Chem. Eng. 2014, 31, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.R.; Teixeira, J.A.; van der Mei, H.C.; Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloids Surf. B Biointerfaces 2006, 49, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Bognolo, G. Biosurfactants as emulsifying agents for hydrocarbons. Colloid Surface A 1999, 152, 41–52. [Google Scholar] [CrossRef]
- Shin, K.H.; Kim, K.W.; Ahn, Y. Use of biosurfactant to remediate phenanthrene-contaminated soil by the combined solubilization-biodegradation process. J. Hazard. Mater. 2006, 137, 1831–1837. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.M.; Zhong, H.; Wang, Z.Q.; Liu, Z.F.; Cheng, M.; Liu, G.S.; Yang, X.; Liu, S.H. Effect of rhamnolipid solubilization on hexadecane bioavailability: Enhancement or reduction? J. Hazard. Mater. 2017, 322, 394–401. [Google Scholar] [CrossRef]
- Yin, H.; Qiang, J.; Jia, Y.; Ye, J.S.; Peng, H.; Qin, H.M.; Zhang, N.; He, B.Y. Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil-containing wastewater. Process Biochem. 2009, 44, 302–308. [Google Scholar] [CrossRef]



| Coded Levels of Variables | |||||
|---|---|---|---|---|---|
| Variable | −1.41 | −1 | 0 | 1 | 1.41 |
| Glycerol (g/L) | 0 | 7.3 | 25 | 42.7 | 50 |
| NaNO3 (g/L) | 0 | 2.2 | 7.5 | 12.8 | 15 |
| Carbon Source Concentration | Biomass | Max. Production Time | Yield | Productivity | |
|---|---|---|---|---|---|
| Carbon Source | (g/L) | (g/L) | (h) | (grhamnose/L) | (mgrhamnose/L/h) |
| sodium citrate | 5 | 0.39 | 193 | 0.43 | 2.20 |
| 10 | 0.73 | 241 | 0.47 | 1.95 | |
| 20 | 1.43 | 193 | 0.93 | 4.82 | |
| 40 | 0.87 | 143 | 0.70 | 4.91 | |
| glycerol | 5 | 0.86 | 193 | 0.83 | 4.30 |
| 10 | 1.61 | 168 | 1.64 | 9.76 | |
| 20 | 1.68 | 221 | 3.16 | 14.30 | |
| 40 | 1.88 | 193 | 4.37 | 22.66 | |
| succinic acid | 5 | 0.40 | 221 | 0.33 | 1.48 |
| 10 | 0.97 | 241 | 0.63 | 2.61 | |
| 20 | 1.00 | 193 | 0.45 | 2.33 | |
| 40 | 0.60 | 193 | 0.43 | 2.23 | |
| FAME | 5 | nd | 145 | 0.44 | 3.03 |
| 10 | nd | 168 | 0.89 | 5.32 | |
| 20 | nd | 234 | 2.23 | 9.53 | |
| 40 | nd | 192 | 2.31 | 12.01 | |
| sunflower oil | 5 | nd | 192 | 1.17 | 6.08 |
| 10 | nd | 192 | 1.51 | 7.86 | |
| 20 | nd | 234 | 2.16 | 9.24 | |
| 40 | nd | 192 | 2.61 | 13.57 | |
| hexadecane | 5 | nd | 168 | 0.24 | 1.42 |
| 10 | nd | 168 | 0.26 | 1.53 | |
| 20 | nd | 168 | 0.26 | 1.55 | |
| 40 | nd | 145 | 0.31 | 2.11 |
| Glycerol | NaNO3 | Max. Production Time | Rhamnolipid Yield | Rhamnolipid Productivity | |||
|---|---|---|---|---|---|---|---|
| Run | Coded Variable | c (g/L) | Coded Variable | c (g/L) | (h) | (grhamnose/L) | (mgrhamnose/L/h) |
| 1 | 0 | 25 | 0 | 7.5 | 194 | 3.90 | 20.10 |
| 2 | 1 | 42.7 | −1 | 2.2 | 169 | 3.07 | 18.13 |
| 3 | −1 | 7.3 | 1 | 12.8 | 237 | 1.89 | 7.99 |
| 4 | −1.41 | 0 | 0 | 7.5 | 357 | 1.12 | 3.14 |
| 5 | 1 | 42.7 | 1 | 12.8 | 264 | 6.20 | 23.45 |
| 6 | 0 | 25 | −1.41 | 0 | 132 | 1.66 | 12.58 |
| 7 | 0 | 25 | 1.41 | 15 | 244 | 4.29 | 17.59 |
| 8 | 1.41 | 50 | 0 | 7.5 | 241 | 5.41 | 22.49 |
| 9 | −1 | 7.3 | −1 | 0 | 210 | 1.31 | 6.23 |
| 10 | 0 | 25 | 0 | 7.5 | 195 | 3.92 | 20.15 |
| 11 | 0 | 25 | 0 | 7.5 | 193 | 3.87 | 20.08 |
| 12 | 0 | 25 | 0 | 7.5 | 194 | 3.91 | 20.11 |
| 13 | 0 | 25 | 0 | 7.5 | 190 | 3.82 | 20.08 |
| RSM Medium | Control Medium | RSM Medium | Control Medium | ||
|---|---|---|---|---|---|
| RhaRhaFAFA | RhaFAFA | ||||
| RhaRhaC8C8 | 3.0 | 0.0 | RhaC8C8 | 8.0 | 0.02 |
| RhaRhaC8C10 | 2.2 | 2.0 | RhaC8C10 | 5.4 | 2.9 |
| RhaRhaC10C8 | 4.6 | 1.5 | RhaC10C8 | 4.5 | 2.0 |
| RhaRhaC10C10 | 8.7 | 26.2 | RhaC10C10 | 23.5 | 25.9 |
| RhaRhaC10C10:1 | 0.0 | 0.2 | RhaC10C10:1 | 0.0 | 0.3 |
| RhaRhaC10:1C10 | 0.0 | 0.2 | RhaC10:1C10 | 0.0 | 0.2 |
| RhaRhaC10C12:1 | 0.0 | 4.1 | RhaC10C12:1 | 0.0 | 4.0 |
| RhaRhaC12:1C10 | 0.0 | 5.7 | RhaC12:1C10 | 0.0 | 3.4 |
| RhaRhaC10:1C12:1 | 0.0 | 0.0 | RhaC10:1C12:1 | 0.0 | 0.01 |
| RhaRhaC10C12 | 1.0 | 5.6 | RhaC10C12 | 4.0 | 3.7 |
| RhaRhaC12C10 | 0.4 | 4.0 | RhaC12C10 | 3.5 | 4.5 |
| RhaRhaC12C12 | 0.0 | 0.3 | RhaC12C12 | 1.0 | 0.1 |
| RhaRhaC12:1C12:1 | 0.0 | 0.02 | RhaC12:1C12:1 | 0.0 | 0.02 |
| RhaRhaC12C12:1 | 0.0 | 0.4 | RhaC12C12:1 | 0.0 | 0.2 |
| total RhaRhaFAFA | 19.9 | 50.2 | total RhaFAFA | 49.9 | 47.3 |
| RhaFA | RhaRhaFA | ||||
| RhaC8 | 3.2 | 0.03 | RhaRhaC8 | 4.1 | 0.1 |
| RhaC10:1 | 0.0 | 0.03 | RhaRhaC10:1 | 0.0 | 0.1 |
| RhaC10 | 9.6 | 0.4 | RhaRhaC10 | 9.2 | 1.7 |
| RhaC12 | 2.3 | 0.02 | RhaRhaC12:1 | 0.0 | 0 |
| RhaC12:1 | 0.0 | 0 | RhaRhaC12 | 1.8 | 0.06 |
| total RhaFA | 15.1 | 0.5 | total RhaRhaFA | 15.1 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maťátková, O.; Michailidu, J.; Ježdík, R.; Jarošová Kolouchová, I.; Řezanka, T.; Jirků, V.; Masák, J. Production and Characterization of Rhamnolipids Produced by Pseudomonas aeruginosa DBM 3774: Response Surface Methodology Approach. Microorganisms 2022, 10, 1272. https://doi.org/10.3390/microorganisms10071272
Maťátková O, Michailidu J, Ježdík R, Jarošová Kolouchová I, Řezanka T, Jirků V, Masák J. Production and Characterization of Rhamnolipids Produced by Pseudomonas aeruginosa DBM 3774: Response Surface Methodology Approach. Microorganisms. 2022; 10(7):1272. https://doi.org/10.3390/microorganisms10071272
Chicago/Turabian StyleMaťátková, Olga, Jana Michailidu, Richard Ježdík, Irena Jarošová Kolouchová, Tomáš Řezanka, Vladimír Jirků, and Jan Masák. 2022. "Production and Characterization of Rhamnolipids Produced by Pseudomonas aeruginosa DBM 3774: Response Surface Methodology Approach" Microorganisms 10, no. 7: 1272. https://doi.org/10.3390/microorganisms10071272