Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications
Abstract
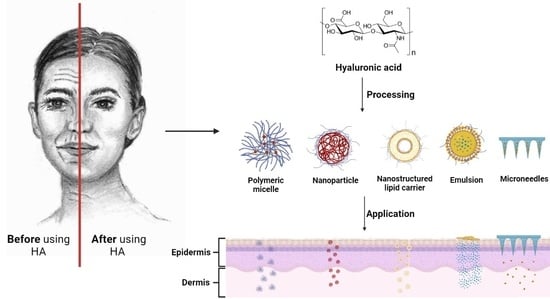
:1. Introduction
2. The Use of Native Versus Modified Hyaluronan in Products
3. The Mechanism of Skin Permeation and Retention of Hyaluronan
3.1. In Vitro and Ex Vivo Studies
3.2. In Vivo Therapeutic Effect of HA
3.3. Clinical Studies
4. Formulations of HA Used for Skin
4.1. Nano and Microemulsions
4.2. Nanostructured Lipid Carriers
4.3. Liposomes
4.4. Ethosomes
4.5. Niosomes
4.6. Nanoparticles
4.7. Polymeric Micelles
4.8. Microneedles
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarango-Granda, P.; Espinoza, L.C.; Díaz-Garrido, N.; Alvarado, H.; Rodríguez-Lagunas, M.J.; Baldomá, L.; Calpena, A. Calpena, Effect of Penetration Enhancers and Safety on the Transdermal Delivery of Apremilast in Skin. Pharmaceutics 2022, 14, 1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Guo, X.; Zhao, Y.; Ren, S.; Zhang, Z.; Lv, H. Hyaluronic acid-cyclodextrin encapsulating paeonol for treatment of atopic dermatitis. Int. J. Pharm. 2022, 623, 121916. [Google Scholar] [CrossRef] [PubMed]
- De Vita, F.; Ferravante, A.; Vecchi, G.; Nobile, V.; Giori, A.M. Evaluation of the Efficacy of IALUSET VITAL® Cream in Helping the Improvement of the Atopic Dermatitis Symptoms in Adults: A Randomized, Double Blind, Vehicle-Controlled Clinical Trial. Allergies 2021, 1, 195–205. [Google Scholar] [CrossRef]
- Du, H.Y.; Liu, P.; Zhu, J.J.; Lan, J.J.; Li, Y.; Zhang, L.B.; Zhu, J.T.; Tao, J. Hyaluronic Acid-Based Dissolving Microneedle Patch Loaded with Methotrexate for Improved Treatment of Psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef] [PubMed]
- Jacobus, B.S.; De Villa, D.; Maschmann Inácio, L.A.; Davies, S.; Zatta, K.C.; Guterres, S.S.; Külkamp-Guerreiro, I.C. Azelaic acid-loaded nanoemulsion with hyaluronic acid—A new strategy to treat hyperpigmentary skin disorders. Drug Dev. Ind. Pharm. 2019, 45, 642–650. [Google Scholar] [CrossRef]
- Huerta-Angeles, G.; Brandejsova, M.; Stepan, P.; Pavlik, V.; Starigazdova, J.; Orzol, P.; Kopecka, K.; Halamkova, P.; Kulhanek, J.; Velebny, V. Retinoic acid grafted to hyaluronan for skin delivery: Synthesis, stability studies, and biological evaluation. Carbohydr. Polym. 2020, 231, 115733. [Google Scholar] [CrossRef]
- Ulrich, M.; Pellacani, G.; Ferrandiz, C.; Lear, J.T. Evidence for field cancerisation treatment of actinic keratoses with topical diclofenac in hyaluronic acid. Eur. J. Dermatol. 2014, 24, 158–167. [Google Scholar] [CrossRef]
- Zhu, J.J.; Dong, L.Y.; Du, H.Y.; Mao, J.Z.; Xie, Y.; Wang, H.; Lan, J.J.; Lou, Y.C.; Fu, Y.X.; Wen, J.J.; et al. 5-Aminolevulinic Acid-Loaded Hyaluronic Acid Dissolving Microneedles for Effective Photodynamic Therapy of Superficial Tumors with Enhanced Long-Term Stability. Adv. Healthc. Mater. 2019, 8, 1900896. [Google Scholar] [CrossRef]
- Evrard, C.; Lambert de Rouvroit, C.; Poumay, Y. Epidermal Hyaluronan in Barrier Alteration-Related Disease. Cells 2021, 10, 3096. [Google Scholar] [CrossRef]
- Huerta-Angeles, G.; Nesporova, K.; Ambrozova, G.; Kubala, L.; Velebny, V. An Effective Translation: The Development of Hyaluronan-Based Medical Products From the Physicochemical, and Preclinical Aspects. Front. Bioeng. Biotechnol. 2018, 6, 62. [Google Scholar] [CrossRef]
- Reed, R.K.; Lilja, K.; Laurent, T.C. Hyaluronan in the rat with special reference to the skin. Acta Physiol. Scand. 1988, 134, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ghadi, Z.; Ebrahimnejad, P. Curcumin entrapped hyaluronan containing niosomes: Preparation, characterisation and in vitro/in vivo evaluation. J. Microencapsul. 2019, 36, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Xie, J.; Ji, Y.; Xue, W.; Ma, D.; Hu, Y. Hyaluronic acid-containing ethosomes as a potential carrier for transdermal drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 323–329. [Google Scholar] [CrossRef]
- Yuan, M.; Niu, J.; Xiao, Q.; Ya, H.; Zhang, Y.; Fan, Y.; Li, L.; Li, X. Hyaluronan-modified transfersomes based hydrogel for enhanced transdermal delivery of indomethacin. Drug Deliv. 2022, 29, 1232–1242. [Google Scholar] [CrossRef]
- Kong, M.; Hou, L.; Wang, J.; Feng, C.; Liu, Y.; Cheng, X.; Chen, X. Enhanced transdermal lymphatic drug delivery of hyaluronic acid modified transfersomes for tumor metastasis therapy. Chem. Commun. 2015, 51, 1453–1456. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Čepa, M.; Machalová, V.; Luptáková, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, H.; Wang, Z.; Yang, X.; Zhang, M.; Liu, X.; Wang, B.; Wu, Z.; Chen, D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv Transl. Res. 2020, 10, 1520–1530. [Google Scholar] [CrossRef]
- Kang, H.; Zuo, Z.; Lin, R.; Yao, M.; Han, Y.; Han, J. The most promising microneedle device: Present and future of hyaluronic acid microneedle patch. Drug Deliv. 2022, 29, 3087–3110. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vávrová, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 2015, 12, 1391–1401. [Google Scholar] [CrossRef]
- Martins, M.; Azoia, N.G.; Shimanovich, U.; Matamá, T.; Gomes, A.C.; Silva, C.; Cavaco-Paulo, A. Design of Novel BSA/Hyaluronic Acid Nanodispersions for Transdermal Pharma Purposes. Mol. Pharm. 2014, 11, 1479–1488. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.Y.W. Matrix Hyaluronan-Activated CD44 Signaling Promotes Keratinocyte Activities and Improves Abnormal Epidermal Functions. Am. J. Pathol. 2014, 184, 1912–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.Y.; Chang, C.H.; Yu, C.H.; Huang, L.L.H. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell 2017, 16, 451–460. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Diaz, I.; Namkoong, J.; Wu, J.; Boyd, T. Efficacy Evaluation of a Topical Hyaluronic Acid Serum in Facial Photoaging. Dermatol. Ther. 2021, 11, 1385–1394. [Google Scholar] [CrossRef]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.-H. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef]
- Radrezza, S.; Baron, G.; Nukala, S.B.; Depta, G.; Aldini, G.; Carini, M.; D’Amato, A. Advanced quantitative proteomics to evaluate molecular effects of low-molecular-weight hyaluronic acid in human dermal fibroblasts. J. Pharm. Biomed. Anal. 2020, 185, 113199. [Google Scholar] [CrossRef]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The Degradation of Hyaluronan in the Skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Funt, D.K. Treatment of Delayed-onset Inflammatory Reactions to Hyaluronic Acid Filler: An Algorithmic Approach. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4362. [Google Scholar] [CrossRef] [PubMed]
- Jouy, F.; Lohmann, N.; Wandel, E.; Ruiz-Gómez, G.; Pisabarro, M.T.; Beck-Sickinger, A.G.; Schnabelrauch, M.; Möller, S.; Simon, J.C.; Kalkhof, S.; et al. Sulfated hyaluronan attenuates inflammatory signaling pathways in macrophages involving induction of antioxidants. Proteomics 2017, 17, 1700082. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Yang, H.; Qiu, P.; Cong, Z.; Zou, Y.; Song, L.; Guo, J.; Anastassiades, T.P. A Low Molecular Weight Hyaluronic Acid Derivative Accelerates Excisional Wound Healing by Modulating Pro-Inflammation, Promoting Epithelialization and Neovascularization, and Remodeling Collagen. Int. J. Mol. Sci. 2019, 20, 3722. [Google Scholar] [CrossRef] [Green Version]
- Hauck, S.; Zager, P.; Halfter, N.; Wandel, E.; Torregrossa, M.; Kakpenova, A.; Rother, S.; Ordieres, M.; Räthel, S.; Berg, A.; et al. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact. Mater. 2021, 6, 4342–4359. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M.H.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Brown, T.J.; Alcorn, D.; Fraser, J.R.E. Absorption of Hyaluronan Applied to the Surface of Intact Skin. J. Investig. Dermatol. 1999, 113, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Tokudome, Y.; Komi, T.; Omata, A.; Sekita, M. A new strategy for the passive skin delivery of nanoparticulate, high molecular weight hyaluronic acid prepared by a polyion complex method. Sci. Rep. 2018, 8, 2336. [Google Scholar] [CrossRef] [Green Version]
- Shigefuji, M.; Tokudome, Y. Nanoparticulation of hyaluronic acid: A new skin penetration enhancing polyion complex formulation: Mechanism and future potential. Materialia 2020, 14, 100879. [Google Scholar] [CrossRef]
- Starigazdová, J.; Nešporová, K.; Čepa, M.; Šínová, R.; Šmejkalová, D.; Huerta-Angeles, G.; Velebný, V. In vitro investigation of hyaluronan-based polymeric micelles for drug delivery into the skin: The internalization pathway. Eur. J. Pharm. Sci. 2020, 143, 105168. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Skin Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development. Guidance document for the conduct of skin absorption studies. Env/Jm/Mono 2004, 2, 1–31. [Google Scholar]
- Organization for Economic Cooperation and Development. Test No. 428: Skin Absorption: In Vitro Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic Skin Penetration Enhancer and Nanoemulsion Formulations Promote the Human Epidermal Permeation of Caffeine and Naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kozaka, S.; Kashima, A.; Wakabayashi, R.; Nakata, T.; Ueda, T.; Goto, M. Effective Transcutaneous Delivery of Hyaluronic Acid Using an Easy-to-Prepare Reverse Micelle Formulation. Cosmetics 2020, 7, 52. [Google Scholar] [CrossRef]
- de Oliveira, J.K.; Ueda-Nakamura, T.; Corrêa, A.G.; Petrilli, R.; Lopez, R.F.V.; Nakamura, C.V.; Auzely-Velty, R. Liposome-based nanocarrier loaded with a new quinoxaline derivative for the treatment of cutaneous leishmaniasis. Mater. Sci. Eng. C 2020, 110, 110720. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr. Polym. 2021, 253, 117295. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Gunasegaran, T.A.P.; Nathan, S.S.; Md, S.; Gorain, B.; Tripathy, M.; Hussain, Z. Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: Fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 2019, 9, 520–533. [Google Scholar] [CrossRef]
- Yue, Y.; Zhao, D.; Yin, Q. Hyaluronic acid modified nanostructured lipid carriers for transdermal bupivacaine delivery: In vitro and in vivo anesthesia evaluation. Biomed. Pharmacother. 2018, 98, 813–820. [Google Scholar] [CrossRef]
- Sundaram, H.; Cegielska, A.; Wojciechowska, A.; Delobel, P. Prospective, Randomized, Investigator-Blinded, Split-Face Evaluation of a Topical Crosslinked Hyaluronic Acid Serum for Post-Procedural Improvement of Skin Quality and Biomechanical Attributes. J. Drugs Dermatol. 2018, 17, 442–450. [Google Scholar] [PubMed]
- Bhardwaj, V.; Namkoong, J.; Tartar, O.; Diaz, I.; Mao, J.; Wu, J. In Vitro and Ex Vivo Mechanistic Understanding and Clinical Evidence of a Novel Anti-Wrinkle Technology in Single-Arm, Monocentric, Open-Label Observational Studies. Cosmetics 2022, 9, 80. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, R.; Yang, S.; Wu, Z.; Guo, S.; Dai, X.; Qiao, Y.; Shi, X. Influence of Temperature on Transdermal Penetration Enhancing Mechanism of Borneol: A Multi-Scale Study. Int. J. Mol. Sci. 2017, 18, 195. [Google Scholar] [CrossRef] [Green Version]
- Henry, L.; Delsuc, N.; Laugel, C.; Lambert, F.; Sandt, C.; Hostachy, S.; Bernard, A.-S.; Bertrand, H.C.; Grimaud, L.; Baillet-Guffroy, A.; et al. Labeling of Hyaluronic Acids with a Rhenium-tricarbonyl Tag and Percutaneous Penetration Studied by Multimodal Imaging. Bioconj. Chem. 2018, 29, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, K.; Zhang, J.; Ou, H.; Duan, J.; Zhang, S.; Wang, D.; Mitragotri, S.; Chen, M. Skin delivery of hyaluronic acid by the combined use of sponge spicules and flexible liposomes. Biomater. Sci. 2019, 7, 1299–1310. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Pavlík, V.; Machalová, V.; Čepa, M.; Šínová, R.; Šafránková, B.; Kulhánek, J.; Drmota, T.; Kubala, L.; Huerta-Ángeles, G.; Velebný, V.; et al. Retinoic Acid Grafted to Hyaluronic Acid Activates Retinoid Gene Expression and Removes Cholesterol from Cellular Membranes. Biomolecules 2022, 12, 200. [Google Scholar] [CrossRef]
- Albash, R.; Fahmy, A.M.; Hamed, M.I.A.; Darwish, K.M.; El-Dahmy, R.M. Spironolactone hyaluronic acid enriched cerosomes (HAECs) for topical management of hirsutism: In silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv. 2021, 28, 2289–2300. [Google Scholar] [CrossRef]
- Son, S.U.; Lim, J.-w.; Kang, T.; Jung, J.; Lim, E.-K. Hyaluronan-Based Nanohydrogels as Effective Carriers for Transdermal Delivery of Lipophilic Agents: Towards Transdermal Drug Administration in Neurological Disorders. Nanomaterials 2017, 7, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Qiu, D.; Liu, Y.; Chao, L. Topical anesthetic analgesic therapy using the combination of ropivacaine and dexmedetomidine: Hyaluronic acid modified long-acting nanostructured lipid carriers containing a skin penetration enhancer. Drug Des. Devel. Ther. 2019, 13, 3307–3319. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-H.; Chen, M.-C.; Wan, S.-W. Sodium Hyaluronate/Chitosan Composite Microneedles as a Single-Dose Intradermal Immunization System. Biomacromolecules 2018, 19, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, F.; Abourehab, M.A.S.; Hussain, Z. Hyaluronic acid decorated tacrolimus-loaded nanoparticles: Efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr. Polym. 2018, 197, 478–489. [Google Scholar] [CrossRef]
- El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Novel curcumin-loaded gel-core hyaluosomes with promising burn-wound healing potential: Development, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Elhalmoushy, P.M.; Elsheikh, M.A.; Matar, N.A.; El-Hadidy, W.F.; Kamel, M.A.; Omran, G.A.; Elnaggar, Y.S.R. Novel berberine-loaded hyalurosomes as a promising nanodermatological treatment for vitiligo: Biochemical, biological and gene expression studies. Int. J. Pharm. 2022, 615, 121523. [Google Scholar] [CrossRef] [PubMed]
- Wongprasert, P.; Dreiss, C.A.; Murray, G. Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol. Ther. 2022, 35, e15453. [Google Scholar] [CrossRef]
- Kühne, U.; Esmann, J.; von Heimburg, D.; Imhof, M.; Weissenberger, P.; Sattler, G. Safety and performance of cohesive polydensified matrix hyaluronic acid fillers with lidocaine in the clinical setting—An open-label, multicenter study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.W.; Moon, I.J.; Yun, W.J.; Chung, B.Y.; Kim, S.D.; Lee, G.Y.; Chang, S.E. A Randomized, Evaluator-Blinded, Split-Face Comparison Study of the Efficacy and Safety of a Novel Mannitol Containing Monophasic Hyaluronic Acid Dermal Filler for the Treatment of Moderate to Severe Nasolabial Folds. Ann. Dermatol. 2016, 28, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Van Dyke, S.; Hays, G.P.; Caglia, A.E.; Caglia, M. Severe Acute Local Reactions to a Hyaluronic Acid-derived Dermal Filler. J. Clin. Aesthetic Dermatol. 2010, 3, 32–35. [Google Scholar]
- Boen, M.; Alhaddad, M.; Wu, D.C.; Goldman, M.P. A Prospective Double-blind, Placebo-controlled Clinical Trial Evaluating the Efficacy of a Novel Combination of Hyaluronic Acid Serum and Antioxidant Cream for Rejuvenation of the Aging Neck. J. Clin. Aesthetic Dermatol. 2020, 13, 13–18. [Google Scholar]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.X.; Tan, W.D.; Srivastava, R.; Yow, A.P.; Wong, D.W.K.; Tey, H.L. Dissolving Triamcinolone-Embedded Microneedles for the Treatment of Keloids: A Single-Blinded Intra-Individual Controlled Clinical Trial. Dermatol. Ther. 2019, 9, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puviani, M.; Campione, E.; Offidani, A.M.; De Grandi, R.; Bianchi, L.; Bobyr, I.; Giannoni, M.; Campanati, A.; Bottagisio, M.; Bidossi, A.; et al. Effects of a cream containing 5% hyaluronic acid mixed with a bacterial-wall-derived glycoprotein, glycyrretinic acid, piroctone olamine and climbazole on signs, symptoms and skin bacterial microbiota in subjects with seborrheic dermatitis of the face. Clin. Cosmet. Investig. Dermatol. 2019, 12, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, T.; Rowland Powell, C. Efficacy and safety of a low-molecular weight hyaluronic Acid topical gel in the treatment of facial seborrheic dermatitis. J. Clin. Aesthetic Dermatol. 2012, 5, 20–23. [Google Scholar]
- Baldwin, H.; Alexis, A.F.; Andriessen, A.; Berson, D.S.; Farris, P.; Harper, J.; Lain, E.; Marchbein, S.; Stein Gold, L.; Tan, J. Evidence of Barrier Deficiency in Rosacea and the Importance of Integrating OTC Skincare Products into Treatment Regimens. J. Drugs Dermatol. 2021, 20, 384–392. [Google Scholar] [CrossRef]
- Lee, S.G.; Yoon, M.S.; Kim, D.H.; Shin, J.U.; Lee, H.J. Hyaluronan Oligosaccharides Improve Rosacea-Like Phenotype through Anti-Inflammatory and Epidermal Barrier-Improving Effects. Ann. Dermatol. 2020, 32, 189–196. [Google Scholar] [CrossRef]
- Maggioni, D.; Cimicata, A.; Praticò, A.; Villa, R.; Bianchi, F.M.; Busoli Badiale, S.; Angelinetta, C. A Preliminary Clinical Evaluation of a Topical Product for Reducing Slight Rosacea Imperfections. Clin. Cosmet. Investig. Dermatol. 2020, 13, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Bertolotti, A.; Leone, G.; Taïeb, A.; Soriano, E.; Pascal, M.; Maillard, H.; van Geel, N. Assessment of Non-cultured Autologous Epidermal Cell Grafting Resuspended in Hyaluronic Acid for Repigmenting Vitiligo and Piebaldism Lesions: A Randomized Clinical Trial. Acta Derm. Venereol. 2021, 101, adv00506. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šínová, R.; Pavlík, V.; Ondrej, M.; Velebný, V.; Nešporová, K. Hyaluronan: A key player or just a bystander in skin photoaging? Exp. Dermatol. 2022, 31, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Hergesell, K.; Valentová, K.; Velebný, V.; Vávrová, K.; Dolečková, I. Common Cosmetic Compounds Can Reduce Air Pollution-Induced Oxidative Stress and Pro-Inflammatory Response in the Skin. Skin Pharmacol. Physiol. 2022, 35, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Che Marzuki, N.H.; Wahab, R.A.; Abdul Hamid, M. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Kim, H.; Shin, H.; Jeong, H.; Kim, J.; Kim, D. Cosmetic effects of Prunus padus bark extract. Korean J. Chem. Eng. 2014, 31, 2280–2285. [Google Scholar] [CrossRef]
- Kupper, S.; Kłosowska-Chomiczewska, I.; Szumała, P. Collagen and hyaluronic acid hydrogel in water-in-oil microemulsion delivery systems. Carbohydr. Polym. 2017, 175, 347–354. [Google Scholar] [CrossRef]
- Kleinubing, S.A.; Outuki, P.M.; Hoscheid, J.; Pelegrini, B.L.; Antonio da Silva, E.; Renata de Almeida Canoff, J.; Miriam de Souza Lima, M.; Carvalho Cardoso, M.L. Hyaluronic acid incorporation into nanoemulsions containing Pterodon pubescens Benth. Fruit oil for topical drug delivery. Biocatal. Agric. Biotechnol. 2021, 32, 101939. [Google Scholar] [CrossRef]
- Kibbelaar, H.V.M.; Deblais, A.; Velikov, K.P.; Bonn, D.; Shahidzadeh, N. Stringiness of hyaluronic acid emulsions. Int. J. Cosmet. Sci. 2021, 43, 458–465. [Google Scholar] [CrossRef]
- Liu, W.; Ding, L.; Xu, J.; Shang, Y.; Wang, Z.; Liu, H. Synthesis of sinapic acid modified sodium hyaluronate particles and the one-step processing of multiple Pickering emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128785. [Google Scholar] [CrossRef]
- Dubuisson, P.; Picard, C.; Grisel, M.; Savary, G. How does composition influence the texture of cosmetic emulsions? Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 38–46. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT(1). Int. J. Toxicol. 2002, 21 (Suppl. S2), 19–94. [Google Scholar] [CrossRef] [PubMed]
- Wichayapreechar, P.; Anuchapreeda, S.; Phongpradist, R.; Rungseevijitprapa, W.; Ampasavate, C. Dermal targeting of Centella asiatica extract using hyaluronic acid surface modified niosomes. J. Liposome Res. 2020, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ghadi, Z.; Dinarvand, R.; Asemi, N.; Talebpour Amiri, F.; Ebrahimnejad, P. Preparation, characterization and in vivo evaluation of novel hyaluronan containing niosomes tailored by Box-Behnken design to co-encapsulate curcumin and quercetin. Eur. J. Pharm. Sci. 2019, 130, 234–246. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Huerta-Angeles, G.; Brandejsová, M.; Novotný, J.; Kopecká, K.; Šógorková, J.; Šmejkalová, D.; Velebný, V. Grafting of steroids to hyaluronan towards the design of delivery systems for antioxidants: The role of hydrophobic core. Carbohydr. Polym. 2018, 193, 383–392. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, S.; Lu, Y.; Lai, R.; Liu, Z.; Luo, W.; Xu, Y. Chitosan/hyaluronan nanogels co-delivering methotrexate and 5-aminolevulinic acid: A combined chemo-photodynamic therapy for psoriasis. Carbohydr. Polym. 2022, 277, 118819. [Google Scholar] [CrossRef] [PubMed]
- Franzé, S.; Rama, F.; Rocco, P.; Debernardi, M.; Bincoletto, V.; Arpicco, S.; Cilurzo, F. Rationalizing the Design of Hyaluronic Acid-Decorated Liposomes for Targeting Epidermal Layers: A Combination of Molecular Dynamics and Experimental Evidence. Mol. Pharm. 2021, 18, 3979–3989. [Google Scholar] [CrossRef]
- El Kechai, N.; Bochot, A.; Huang, N.; Nguyen, Y.; Ferrary, E.; Agnely, F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int. J. Pharm. 2015, 487, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Franzé, S.; Marengo, A.; Stella, B.; Minghetti, P.; Arpicco, S.; Cilurzo, F. Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int. J. Pharm. 2018, 535, 333–339. [Google Scholar] [CrossRef]
- Wan, T.; Pan, W.; Long, Y.; Yu, K.; Liu, S.; Ruan, W.; Pan, J.; Qin, M.; Wu, C.; Xu, Y. Effects of nanoparticles with hydrotropic nicotinamide on tacrolimus: Permeability through psoriatic skin and antipsoriatic and antiproliferative activities. Int. J. Nanomed. 2017, 12, 1485–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lee, S.; Ki, C.S. Modular formation of hyaluronic acid/β-glucan hybrid nanogels for topical dermal delivery targeting skin dendritic cells. Carbohydr. Polym. 2021, 252, 117132. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Z.; Niu, J.; Ma, H.-J.; Dad, H.A.; Shao, H.-T.; Yuan, T.-J.; Peng, L.-H. Transdermal siRNA delivery by pH-switchable micelles with targeting effect suppress skin melanoma progression. J. Control Release 2020, 322, 95–107. [Google Scholar] [CrossRef]
- Nasiri, M.I.; Vora, L.K.; Ershaid, J.A.; Peng, K.; Tekko, I.A.; Donnelly, R.F. Nanoemulsion-based dissolving microneedle arrays for enhanced intradermal and transdermal delivery. Drug Deliv. Transl. Res. 2021, 12, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, G.; Lee, H.; Bao, L.; Park, J.; Takama, N.; Kim, B. Comparison of polymers to enhance mechanical properties of microneedles for bio-medical applications. Micro Nano Syst. Lett. 2020, 8, 13. [Google Scholar] [CrossRef]
- Jang, M.; Baek, S.; Kang, G.; Yang, H.; Kim, S.; Jung, H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int. J. Cosmet. Sci. 2020, 42, 302–309. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Vilela, C.; Pinto, R.J.B.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 118, 111350. [Google Scholar] [CrossRef]
- Leone, M.; Romeijn, S.; Slütter, B.; O’Mahony, C.; Kersten, G.; Bouwstra, J.A. Hyaluronan molecular weight: Effects on dissolution time of dissolving microneedles in the skin and on immunogenicity of antigen. Eur. J. Pharm. Sci. 2020, 146, 105269. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, H.; Mao, J.; Li, Y.; Hussain, M.; Zhu, J.; Li, Y.; Zhang, L.; Tao, J.; Zhu, J. Enhanced in vitro efficacy for inhibiting hypertrophic scar by bleomycin-loaded dissolving hyaluronic acid microneedles. J. Mater. Chem. B 2019, 7, 6604–6611. [Google Scholar] [CrossRef]
- Han, S.-K.; Lee, S.-J.; Ha, H.-Y. Skin Moisturizing Effects of a Microneedle Patch Containing Hyaluronic Acid and Lonicerae flos. Processes 2021, 9, 321. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride. Pharmaceutics 2021, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bhattaccharjee, S.A.; Beck-Broichsitter, M.; Banga, A.K. Fabrication and characterization of hyaluronic acid microneedles to enhance delivery of magnesium ascorbyl phosphate into skin. Biomed. Microdevices 2019, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Wiraja, C.; Lio, D.C.S.; Xu, C. A Double-Layered Microneedle Platform Fabricated through Frozen Spray-Coating. Adv. Healthc. Mater. 2020, 9, 2000147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, Y.S.; Kim, G.M.; Bae, J.M. A hyaluronic acid-based microneedle patch to treat psoriatic plaques: A pilot open trial. Br. J. Dermatol. 2018, 178, e24–e25. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater. Sci. Eng. C 2017, 80, 187–196. [Google Scholar] [CrossRef]
- Chi, Y.; Huang, Y.; Kang, Y.; Dai, G.; Liu, Z.; Xu, K.; Zhong, W. The effects of molecular weight of hyaluronic acid on transdermal delivery efficiencies of dissolving microneedles. Eur. J. Pharm. Sci. 2022, 168, 106075. [Google Scholar] [CrossRef]
- Jung, H.S.; Kong, W.H.; Sung, D.K.; Lee, M.-Y.; Beack, S.E.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Nanographene Oxide–Hyaluronic Acid Conjugate for Photothermal Ablation Therapy of Skin Cancer. ACS Nano 2014, 8, 260–268. [Google Scholar] [CrossRef]
- Pan, W.; Qin, M.; Zhang, G.; Long, Y.; Ruan, W.; Pan, J.; Wu, Z.; Wan, T.; Wu, C.; Xu, Y. Combination of hydrotropic nicotinamide with nanoparticles for enhancing tacrolimus percutaneous delivery. Int. J. Nanomed. 2016, 11, 4037–4050. [Google Scholar]
- Maroda, M.; Bodnár, M.; Berkó, S.; Bakó, J.; Erős, G.; Csányi, E.; Szabó-Révész, P.; Hartmann, J.F.; Kemény, L.; Borbély, J. Preparation and investigation of a cross-linked hyaluronan nanoparticles system. Carbohydr. Polym. 2011, 83, 1322–1329. [Google Scholar] [CrossRef]
- Lin, L.-H.; Chen, C.-W.; Zhu, Y.-Q. Synthesis and cytotoxicity of quercetin/hyaluronic acid containing ether block segment. Colloids Surf. A 2020, 586, 124230. [Google Scholar] [CrossRef]
- Duan, Y.; Li, K.; Wang, H.; Wu, T.; Zhao, Y.; Li, H.; Tang, H.; Yang, W. Preparation and evaluation of curcumin grafted hyaluronic acid modified pullulan polymers as a functional wound dressing material. Carbohydr. Polym. 2020, 238, 116195. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Peng, K.; Sarode, A.; Prakash, S.; Zhao, Z.; Filippov, S.K.; Todorova, K.; Sell, B.R.; Lujano, O.; Bakre, S.; et al. Hyaluronic acid conjugates for topical treatment of skin cancer lesions. Sci. Adv. 2021, 7, eabe6627. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Lin, Y.-H.; Lin, S.-Y.; Li, Y.-N.; Chiang, C.-S.; Chang, C.-W. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics 2019, 9, 2411–2423. [Google Scholar] [CrossRef]
- Ho, Y.-J.; Hsu, H.-C.; Kang, S.-T.; Fan, C.-H.; Chang, C.-W.; Yeh, C.-K. Ultrasonic Transdermal Delivery System with Acid–Base Neutralization-Generated CO2 Microbubble Cavitation. ACS Appl. Bio Mater. 2020, 3, 1968–1975. [Google Scholar] [CrossRef] [PubMed]






| Status | Mw (kDa) | Type of Formulation | Advantages Given by the Presence of HA in the Formulation. | Ref. |
|---|---|---|---|---|
| In vitro/probands | 15 | Cream | Synergic activity of retinoids and HA. | [6] |
| In vivo (clinical). | HMW-HA (unknown) | Gel | Combining HA and diclofenac for treatment of atopic dermatitis in high-risk transplant patients, almost half of them remained free of lesions two years post-treatment. | [7] |
| In vitro and in vivo | <10 | Transfersomes | Changed the lipid fluidity | [16] |
| In vitro | 5, 100, 1000 | Hydrogel | Occluded the stratum corneum | [22] |
| In vitro and in vivo (clinical) | A mixture of 50 and 1000. | Serum | Demonstrated no irritation in vitro. Boosted skin hydration. | [27] |
| In vitro | 10 | Reverse micelle | Changed the lipids conformation increasing permeation of actives | [47] |
| Ex vivo | 10 | Liposomes | Boosted penetration by using amphiphilic HA | [48] |
| In vitro | <1000 | Nanoparticulation | Increased skin permeation more than chitosan | [49] |
| Ex vivo | Nanoparticulation | Increased both encapsulation efficiency and permeation efficiency of actives | [50] | |
| In vitro and in vivo | 300 | Nanostructured lipid carriers | Accumulation of the drug on the upper layer of the skin, increased and prolonged anesthetic effect. | [51] |
| In vivo (clinical) | Crosslinked HA | Serum | Retardance of skin ageing | [52] |
| In vivo (clinical), ex vivo (human skin explants/biopsies), and in vitro. | unknown | Cream and cream-gel | Significant wrinkle reduction after 28 and 56 days. Pro-collagen and overexpression of HA | [53] |
| In vitro | 150 | Ethosomes | Increased penetration efficiency | [54] |
| Preparation Method | Active | Mw (kDa) | Application | Size (nm) | Zeta Potential (mV) | Most Relevant Findings | Ref. |
|---|---|---|---|---|---|---|---|
| High-speed stirring | Azelaic Acid | Unknown | Whitening effect | ~419 ± 23 | 10.9 ± 0.44 | Boosted drug penetration | [5] |
| Ultra-turax® homogenization/second homogenization | Prunus padus extract | Unknown | Whitening effect | 360–430 | nd * | Enhanced anti-wrinkle and whitening effects | [86] |
| Stirring | Collagen | LMW-HA, Mw < 10 and HMW-HA, Mw ≥ 1000. | Skin regeneration | LMW (72.1 ± 5 to 120.8 ± 4.2) HMW (19.7 ± 3.8 to 85.3 ± 6.1) | nd * | Allowed skin penetration of HA/collagen as a function of HA concentration | [87] |
| Ultra-turax® homogenisation | Pterodon pubescens oil | Unknown | Anti-inflammatory | 16–22 | −14.2 ± 0.4 to −33.7 ± 1.9 | Improved spreading and increased stability. | [88] |
| Vortexing | Castor oil | HMW-HA 1500–1800 LMW-HA 21–40 | Cosmetics, sensorial study | nd * | nd * | Molecular weight affects the stringiness of the emulsion. | [89] |
| Formulation | Preparation Method | HA Mw (kDa) | Active | Application | EE (%) | Loading Capacity (%) | Size (nm) | Zeta Potential (mV) | Most Relevant Findings | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ethosomes | Stirring/sonification | 240 | Curcumin | Psoriasis | 0.1 | nr * | ~200 | −30 | Native HA-coated phospholipid vesicles reduced drug leakage, improved stability, and allowed the slow release of the loaded drug | [14] |
| Transfersomes | Thin film hydration technique/High pressure homogenization | nr * | Epigallocatechin-3-gallate (EGCGE) | UV radiation-protective | Up to 76.53 ± 2.68 and up to 48.57 ± 4.53 for HA | nr * | 101.2 ± 6.0 | −44.8 ± 5.24 | Native HA-based transferosomes capable of squeezing through the intercellular spaces of the SC. Enhanced EGCGE permeation. Enhanced free radical-scavenging properties and negligible cell toxicity. | [18] |
| Liposomes | Thin lipid film hydration and extrusion | 10 | 2,3-Di-(4-methoxyphenyl)-quinoxaline | Antileishmanial drug delivery | 20–60 | 2.36 ± 0.34 (HA), 1.53 ± 0.35 (HA-Chol) | 214.1 ± 4.3 (HA) and 238.1 ± 6.1 (HA-Chol) | −53.7 ± 1.8, −40.7 ± 5.7 | Amphiphilic HA-Chol derivative reached deeper layers of the skin due to higher affinity than native HA. | [48] |
| Nanostructured lipid carriers | Lipid melt emulsification/solvent injection | 300 | Bupivacaine | Antinociceptive | 88.9 ± 3.1 | 1.7 ± 0.2 | 154.6 ± 5.1 | −40 | Improved percutaneous penetration for the amphiphilic derivative HA-PEG-LOA (BPV/NLCs than non-modified BPV/NLCs or free BPV. | [51] |
| Solvent diffusion method | 3 | Ropivacaine/Dexmedetomidine | Anesthesia/antinociception effect | 90/88 | 16 ± 3 | 100 | −30 | Increased skin penetration by using the amphiphilic HA-PEG-DSPE derivative. | [63] | |
| Nanoparticles | High-pressure homogenization–evaporation method | 100 | Betamethasone | Atopic dermatitis | 73.43 ± 6.7 to 87.43 ± 9.1 | 27 ± 4.12 to 35 ± 6.39 | 279 ± 12 to 554 ± 23 | 61.5 ± 4.8 to 44.5 ± 4.6 | pH-controlled and sustained release. | [50] |
| High-pressure homogenization– evaporation method | 100 | Tacrolimus | Anti-dermatitis | 84.11 ± 4.93 | 29.34 ± 2.13 | 216 ± 16 to 389 ± 26 with HA% | 51 ± 4.67 to 34 ± 5.23 | Native HA improved thermal stability and decreased crystallinity. Sustained release. Possibility of targeting. | [65] | |
| Niosomes | Thin-film hydration method. | 30 | Centella asiatica extract | Herbal therapy for skin disorders (via eczema, wound healing) | 41.19 ± 0.61 to 73.54 ± 0.32 | 4.36 to 9.61 | 100–180 | −40 to −10 | Native HA enhanced bioactivity with potential as anti-psoriasis, eczema, anti-inflammation, or anti-ageing treatments. | [93] |
| Thin-film hydration method. | 200–400 | Curcumin/quercetin | Antioxidant and anti-inflammatory | 98.85 ± 0.55 93.13 ± 1.22 | 2 and 2.68 | 260.4 ± 6.6 | −34.97 ± 1.50 | Improved nanovesicle stability and allowed selective targeting. Native HA does not change the EE. | [94] | |
| Nanoparticulated gel | Lipid fusion, and ultrasound | 300 | Caffeic acid | Antioxidant | 87.8 ± 5.2 | 1.7 ± 0.05 | 230 ± 14 | nr | Increased spreadability of the formulation | [95] |
| Polymeric micelles | Dialysis | 44 and <10 | Curcumin | Antioxidant and anti-inflammatory | 43.10 ± 5.4 | 5.62 ± 1.7 | 172.6 ± 11.4 | −33.71 ± 0.45 mV | Bioconjugation with HA allowed the solubilization of curcumin in medium. | [20] |
| Solvent-evaporation method | 15 | Coenzyme Q10 | Cosmetic ingredient | 85.4 ± 0.4 | 9.8 ± 0.8 | 163 | −25.6 | The drug-loading capacity is controlled by chemical modification and structure of carrier. | [19,96] | |
| Nanogels | Ionotropic gelation | 10 | Methotrexate/5-aminolevulinic acid | Psoriasis | 75.42/71.94 | 0.05 and 10 | 141.43 ± 0.47 | 31.59 ± 0.44 | Complexed chitosan/HA complex reduced systemic toxicity of the drugs. | [97] |
| Preparation Method | Active | Mw (kDa) | Application | Length (μm) | Most Relevant Findings | Ref. |
|---|---|---|---|---|---|---|
| Two-step micromolding | Methotrexate (MTX) | 10 | Psoriasis | 650 ± 19 (h), 220 ± 7(b) | The mechanical properties were enough to pierce the skin and reached dermis | [4] |
| Micro-molding | 5-Aminolevulinic acid | 10 | Photodynamic therapy | 650 h | LMW-HA effectively protects the active from degradation | [8] |
| Micro-molding | Ovalbumin (OVA) | 7 and 200–500 | Immunization | 550 (h), 300 (b) | Stronger immune response by HA | [64] |
| Centrifugal lithography | Adenosine | HMW-HA 800 LMW-HA 39 | Cosmetics | 402.5 ± 12.9 81.2 ± 9.3 | HMW-HA presented better skin improvement effects, lower depth of wrinkles, and increased elasticity and dermal density | [107] |
| Casting-molding and centrifugation | Rutin | 403 | Cosmetics | 550 (h) 200 (b) | Combination of bacterial cellulose and HA | [108] |
| Micro-molding | Ovalbumin (OVA) | 4, 20, 150 and 1800 | Immunization | 300(h) | LMW-HA (150 kDa) showed a higher penetration efficiency (<96%) and dissolved faster than LMW-HA (4.8 kDa) or HMW-HA (1.8 MDa), which was unsuitable for the fabrication | [109] |
| Two-step casting | Bleomycin | 10 | Hypertrophic scars | 650 h | Fast dissolution ~10 min. Synergic effect of HA/bleomycin inhibited the proliferation of human hypertrophic scar fibroblasts. | [110] |
| Micro-molding | Lonicerae flos extract | nd * | Cosmetics | nd * | Native HA and the plant extract synergism’ improved skin moisturizing properties. | [111] |
| Micro-molding | Propranolol hydrochloride | 10 | Infantile hemangioma | 1200 (h), 300 (b) | LMW-HA was processed in an obelisk shape, giving higher cutaneous delivery than pyramidal or circular shapes. | [112] |
| Micro-molding and nanoparticulation | nd * | nd * | 600 (h), 250 (b) | Crosslinked HA presented higher mechanical strength due to the enhanced viscosity. | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhaščik, M.; Kováčik, A.; Huerta-Ángeles, G. Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications. Polymers 2022, 14, 4833. https://doi.org/10.3390/polym14224833
Juhaščik M, Kováčik A, Huerta-Ángeles G. Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications. Polymers. 2022; 14(22):4833. https://doi.org/10.3390/polym14224833
Chicago/Turabian StyleJuhaščik, Martin, Andrej Kováčik, and Gloria Huerta-Ángeles. 2022. "Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications" Polymers 14, no. 22: 4833. https://doi.org/10.3390/polym14224833