Iron (II) Metallo-Supramolecular Polymers Based on Thieno[3,2-b]thiophene for Electrochromic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of Unimers
3. Results and Discussion
3.1. Synthesis of Unimers
3.2. Self-Assembly of Fe-MSPs from Unimers and Fe2+ Ions in HFP/ACN Solutions
3.3. Preparation and Characterization of Fe-MSP Films
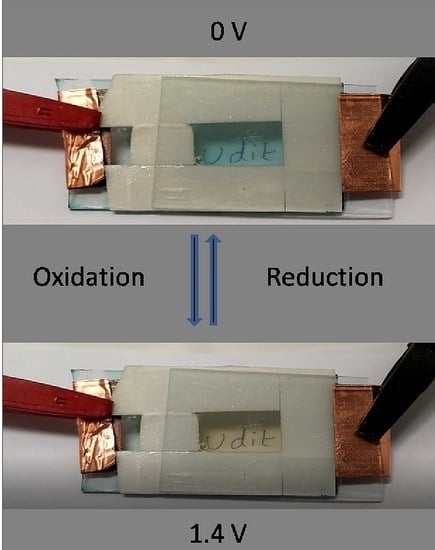
3.4. Electrochromism of Prepared MSPs
3.5. Fabrication of Electrochromic Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Invernale, M.A.; Acik, M.; Sotzing, G.A. Thiophene-Based Electrochromic Materials. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics—Chapter 20; Perepichka, I.F., Perepichka, D.F., Eds.; Wiley: Hoboken, NJ, USA, 2009; p. 757. [Google Scholar]
- Kim, J.; Remond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic Conjugated Polymers for Multifunctional Smart Windows with Integrative Functionalities. Adv. Mater. Technol. 2020, 5, 1900890. [Google Scholar] [CrossRef]
- Macher, S.; Schott, M.; Sassi, M.; Facchinetti, I.; Ruffo, R.; Patriarca, G.; Beverina, L.; Posset, U.; Giffin, G.A.; Loebmann, P. New Roll-to-Roll Processable PEDOT-Based Polymer with Colorless Bleached State for Flexible Electrochromic Devices. Adv. Funct. Mater. 2020, 30, 1906254. [Google Scholar] [CrossRef]
- Wu, W.; Wang, M.; Ma, J.; Cao, Y.; Deng, Y. Electrochromic Metal Oxides: Recent Progress and Prospect. Adv. Electron. Mater. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Celiesiute, R.; Ramanaviciene, A.; Gicevicius, M.; Ramanavicius, A. Electrochromic Sensors Based on Conducting Polymers, Metal Oxides, and Coordination Complexes. Crit. Rev. Anal. Chem. 2019, 49, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Laschuk, N.O.; Ebralidze, I.I.; Poisson, J.; Egan, J.G.; Quaranta, S.; Allan, J.T.S.; Cusden, H.; Gaspari, F.; Naumkin, F.Y.; Easton, E.B.; et al. Ligand Impact on Monolayer Electrochromic Material Properties. ACS Appl. Mater. Interfaces 2018, 10, 35334–35343. [Google Scholar] [CrossRef] [PubMed]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Navigating the Color Palette of Solution-Processable Electrochromic Polymers. Chem. Mater. 2011, 23, 397–415. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Wu, Y.; Li, C.; Ji, Y.; Li, L.; Hu, W.; Wang, Z.; Ma, W.; Li, W. Effect of Fluorination on Molecular Orientation of Conjugated Polymers in High Performance Field-Effect Transistors. Macromolecules 2016, 49, 6431–6438. [Google Scholar] [CrossRef]
- Otley, M.T.; Zhu, Y.; Zhang, X.; Li, M.; Sotzing, G.A. Color-Tuning Neutrality for Flexible Electrochromics Via a Single-Layer Dual Conjugated Polymer Approach. Adv. Mater. 2014, 26, 8004–8009. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, A. Supramolecular polymerizations. Macromol. Rapid Commun. 2002, 23, 511–529. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Synthesis and characterization of metallo-supramolecular polymers. Chem. Soc. Rev. 2016, 45, 5311–5357. [Google Scholar] [CrossRef] [PubMed]
- Vohlídal, J.; Hissler, M. Metallo-Supramolecular Polymers. In Smart Inorganic Polymers: Synthesis, Properties, and Emerging Applications in Materials and Life Sciences—Chapter 6; Hey-Hawkins, E., Hissler, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 141–162. [Google Scholar]
- Higuchi, M. Metallo-Supramolecular Polymers: Synthesis, Properties, and Device Applications; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Liang, Y.; Strohecker, D.; Lynch, V.; Holliday, B.J.; Jones, R.A. A Thiophene-Containing Conductive Metallopolymer Using an Fe(II) Bis(terpyridine) Core for Electrochromic Materials. ACS Appl. Mater. Interfaces. 2016, 8, 34568–34580. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Moos, M.; Schreck, M.H.; Lambert, C.; Kurth, D.G. Green to red electrochromic Fe(II) metallo-supramolecular polyelectrolytes self-assembled from fluorescent 2,6-bis(terpyridyl)pyrimidine bithiophene. Inorg. Chem. 2017, 56, 1418–1432. [Google Scholar] [CrossRef] [PubMed]
- Han, F.S.; Higuchi, M.; Kurth, D.G. Metallo-supramolecular polymers based on functionalized bis-terpyridines as novel electrochromic materials. Adv. Mater. 2007, 19, 3928–3931. [Google Scholar] [CrossRef]
- Pai, S.; Schott, M.; Niklaus, L.; Posset, U.; Kurth, D.G. A study of the effect of pyridine linkers on the viscosity and electrochromic properties of metallo-supramolecular coordination polymers. J. Mater. Chem. C 2018, 6, 3310–3321. [Google Scholar] [CrossRef]
- Takada, K.; Sakamoto, R.; Yi, S.-T.; Katagiri, S.; Kambe, T.; Nishihara, H. Electrochromic Bis(terpyridine)metal Complex Nanosheets. J. Am. Chem. Soc. 2015, 137, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-W.; Sato, T.; Zhang, J.; Moriyama, S.; Higuchi, M. Multi-colour electrochromic properties of Fe/Ru-based bimetallo-supramolecular polymers. J. Mater. Chem. C 2013, 1, 3408–3413. [Google Scholar] [CrossRef]
- Sato, T.; Higuchi, M. An alternately introduced heterometallo-supramolecular polymer: Synthesis and solid-state emission switching by electrochemical redox. Chem. Commun. 2013, 49, 5256. [Google Scholar] [CrossRef]
- Duerrbeck, A.; Gorelik, S.; Hobley, J.; Wu, J.E.; Hor, A.; Long, N. Highly emissive, solution-processable and dynamic Eu(III)-containing coordination polymers. Chem. Commun. 2015, 51, 8656–8659. [Google Scholar] [CrossRef]
- Svoboda, J.; Štenclová, P.; Uhlík, F.; Zedník, J.; Vohlídal, J. Synthesis and photophysical properties of α,ω-bis(terpyridine)oligothiophenes. Tetrahedron 2011, 67, 75–79. [Google Scholar] [CrossRef]
- Štenclová, P.; Šichová, K.; Šloufová, I.; Zedník, J.; Vohlídal, J.; Svoboda, J. Alcohol- and water-soluble bis(tpy)quaterthiophenes with phosphonium side groups: New conjugated units for metallo-supramolecular polymers. Dalton Trans. 2016, 45, 1208–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štenclová-Bláhová, P.; Svoboda, J.; Šloufová, I.; Vohlídal, J. Alcohol-soluble bis(tpy)thiophenes: New building units for constitutional dynamic conjugated polyelectrolytes. Phys. Chem. Chem. Phys. 2015, 17, 13743–13756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bláhová, P.; Zedník, J.; Šloufová, I.; Vohlídal, J.; Svoboda, J. Synthesis and photophysical properties of new α,ω-bis(tpy)oligothiophenes and their metallo-supramolecular polymers with Zn2+ ion couplers. Soft Matter. 2014, 12, 214–229. [Google Scholar] [CrossRef]
- Vitvarova, T.; Svoboda, J.; Hissler, M.; Vohlidal, J. Conjugated Metallo-Supramolecular Polymers Containing a Phosphole Unit. Organometallics 2017, 36, 777–786. [Google Scholar] [CrossRef]
- Hladysh, S.; Vaclavkova, D.; Vrbata, D.; Bondarev, D.; Havlicek, D.; Svoboda, J.; Zedník, J.; Vohlídal, J. Synthesis and characterization of metallo-supramolecular polymers from thiophene-based unimers bearing pybox ligands. RSC Adv. 2017, 7, 10718–10728. [Google Scholar] [CrossRef] [Green Version]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Li, P.; Ahrens, B.; Feeder, N.; Raithby, P.R.; Teat, S.J.; Khan, M.S. Luminescent digold ethynyl thienothiophene and dithienothiophene complexes; their synthesis and structural characterization. Dalton Trans. 2005, 5, 874–883. [Google Scholar] [CrossRef]
- Filler, R.; Schure, R.M. Highly acidic perhalogenated alcohols. A new synthesis of perfluoro-tert-butyl alcohol. J. Org. Chem. 1967, 32, 1217–1219. [Google Scholar] [CrossRef]
- Vitvarova, T.; Zednik, J.; Blaha, M.; Vohlidal, J.; Svoboda, J. Effect of Ethynyl and 2-Thienyl Substituents on the Complexation of 4′-Substituted 22′:6′,2″-Terpyridines with Zn2+ and Fe2+ Ions, and the Spectroscopic Properties of the Ligands and Formed Complex Species. Eur. J. Inorg. Chem. 2012, 2012, 3866–3874. [Google Scholar] [CrossRef]
- Schwarz, G.; Hasslauer, I.; Kurth, D.G. From terpyridine-based assemblies to metallo-supramolecular polyelectrolytes (MEPEs). Adv. Colloid Interface Sci. 2014, 207, 107–120. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murtaza, I.; Shi, J.; He, Y.; Xu, P.; Zhu, M.; Goto, O.; Meng, H. Thieno [3, 2-b]thiophene based electrochromic polymers: Experimental and theoretical appraisal of the EDOT position. RSC Adv. 2016, 6, 75522–75529. [Google Scholar] [CrossRef]
- Randles, J.E.B. Cathode-ray polarograph. II. Current-voltage curves. Trans. Faraday Soc. 1948, 44, 327–338. [Google Scholar] [CrossRef]
- Ševčík, A. Oscillographic polarography with periodical triangular voltage. Collect. Czech. Chem. Commun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Zhu, Y.; Otley, M.T.; Alamer, F.A.; Kumar, A.; Zhang, X.; Mamangun, D.M.D.; Li, M.; Arden, B.G.; Sotzing, G.A. Electrochromic properties as a function of electrolyte on the performance of electrochromic devices consisting of a single-layer polymer. Org. Electron. 2014, 15, 1378–1386. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Hsieh, T.-H.; Hsieh, C.-K.; Liao, J.-W.; Wu, T.-Y. Electrosynthesis and Characterization of Four Electrochromic Polymers Based on Carbazole and Indole-6-Carboxylic Acid and Their Applications in High-Contrast Electrochromic Devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]
- Nie, G.; Wang, L.; Liu, C. High performance electrochromic devices based on a polyindole derivative, poly(1H-benzo[g] indole). J. Mater. Chem. C 2015, 3, 11318–11325. [Google Scholar] [CrossRef]
- Mondal, S.; Ninomiya, Y.; Yoshida, T.; Mori, T.; Bera, M.K.; Ariga, K.; Higuchi, M. Dual-Branched Dense Hexagonal Fe(II)-Based Coordination Nanosheets with Red-to-Colorless Electrochromism and Durable Device Fabrication. ACS Appl. Mater. Interfaces 2020, 12, 31896–31903. [Google Scholar] [CrossRef] [PubMed]
- Kuai, Y.; Li, W.; Dong, Y.; Wong, W.-Y.; Yan, S.; Dai, Y.; Zhang, C. Multi-color electrochromism from coordination nanosheets based on a terpyridine-Fe(II) complex. Dalton Trans. 2019, 48, 15121–15126. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006. [Google Scholar]












| MSP | λU * Solution nm | λU (nm) Solid State 0 V (Fe2+) 1.4 V (Fe3+) | λMLCT Solution nm | λMLCT Solid State nm | |
|---|---|---|---|---|---|
| Fe-Tt | 391 → 412 | 420 | 475 | 614 | 632 |
| Fe-TtE | 406 → 426 | 432 | 483 | 594 | 612 |
| Fe-TtPh | 395 → 418 | 428 | 452 | 572 | 590 |
| Fe-TtB | 470 → 495 | 502 | (520)WEAK 1045NIR | 610 | 620 |
| Fe-MSP | Surface Area Scanned μm2 | Rq nm | Ra nm | Rmax nm | Thickness nm |
|---|---|---|---|---|---|
| Fe-Tt | 4.1 | 2.8 | 2.2 | 22.9 | 40 ± 5 |
| Fe-TtE | 4.4 | 3.2 | 2.5 | 48.8 | 40 ± 5 |
| Fe-TtPh | 4.1 | 3.9 | 3.0 | 40.6 | 35 ± 5 |
| Fe-TtB | 4.3 | 5.6 | 4.1 | 50.0 | 35 ± 3 |
| MSP | λMLCT nm | Δ T (%) | ΔOD | tb s | tc s | CE cm2 C−1 | Oxidation Peak Slope | Reduction Peak Slope |
|---|---|---|---|---|---|---|---|---|
| Fe-Tt | 632 | 64.5 | 0.82 | 1.1 | 1.8 | 641 | 2.12 | −2.29 |
| Fe-TtE | 612 | 54.5 | 0.42 | 3.7 | 1.8 | 229 | 2.2 | −2.14 |
| Fe-TtPh | 590 | 43.0 | 0.44 | 1.8 | 1.8 | 332 | 1.79 | −1.74 |
| Fe-TtB | 618 | 29.0 | 0.25 | 4.6 | 1.7 | 173 | 0.8 | −0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernyshev, A.; Acharya, U.; Pfleger, J.; Trhlíková, O.; Zedník, J.; Vohlídal, J. Iron (II) Metallo-Supramolecular Polymers Based on Thieno[3,2-b]thiophene for Electrochromic Applications. Polymers 2021, 13, 362. https://doi.org/10.3390/polym13030362
Chernyshev A, Acharya U, Pfleger J, Trhlíková O, Zedník J, Vohlídal J. Iron (II) Metallo-Supramolecular Polymers Based on Thieno[3,2-b]thiophene for Electrochromic Applications. Polymers. 2021; 13(3):362. https://doi.org/10.3390/polym13030362
Chicago/Turabian StyleChernyshev, Andrei, Udit Acharya, Jiří Pfleger, Olga Trhlíková, Jiří Zedník, and Jiří Vohlídal. 2021. "Iron (II) Metallo-Supramolecular Polymers Based on Thieno[3,2-b]thiophene for Electrochromic Applications" Polymers 13, no. 3: 362. https://doi.org/10.3390/polym13030362