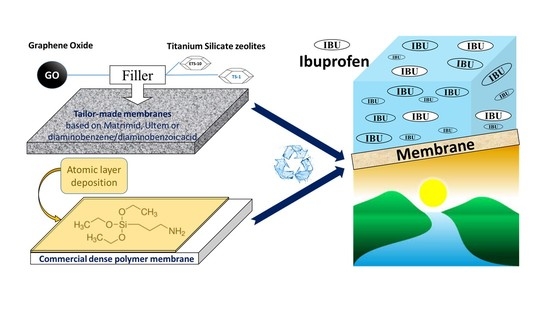
Removal of Ibuprofen from Water by Different Types Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Commercial Membranes
2.1.2. Tailor-Made Membranes
2.1.3. Materials Used for Atomic Layer Deposition
2.1.4. Atomic Layer Deposition Silane-Modified PDMS Membrane
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Sorption Measurements
2.2.3. Pertraction Measurement
2.2.4. Analytics
2.2.5. Characterisation Methods
3. Results
3.1. Sorption
3.1.1. Sorption of IBU from Water by Tailor-Made Membranes
3.1.2. Sorption of IBU by Commercial Membranes
3.2. Results of Pertraction Measurements
3.2.1. Characterisation of an ALD-Modified PDMS Membrane and Its Use in the Pertraction of IBU from Water
3.2.2. Point of Zero Charge
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALD | Atomic Layer Deposition |
| DABA | 3,5-diaminobenzoic acid |
| DAM | 1,3-diaminobenzene |
| ED | Electrodialysis |
| ETS-10 | Engelhard Corporation titanosilicate (Na2TiSi5O13) |
| GO | graphene oxide |
| IBU | ibuprofen |
| logKow | n-octanol/water partition coefficient |
| PDMS | polydimethylsiloxane |
| PET | polyethylene terephthalate |
| pKa | negative decimal logarithm of the dissociation constant of an acid at 25 °C |
| PP | polypropylene |
| PS | polystyrene |
| SWCNT | single-walled carbon nanotubes |
| TS-1 | titanium silicate |
| WCA | water contact angle |
| wt. % | weight percent |
References
- Buser, H.R.; Poiger, T.; Muller, M.D. Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Rozman, D.; Hrkal, Z.; Váňa, M.; Vymazal, J.; Boukalová, Z. Occurrence of Pharmaceuticals in Wastewater and Their Interaction with Shallow Aquifers: A Case Study of Horní Beřkovice, Czech Republic. Water 2017, 9, 218. [Google Scholar] [CrossRef]
- Brozinski, J.-M.; Lahti, M.; Meierjohann, A.; Oikari, A.; Kronberg, L. The Anti-Inflammatory Drugs Diclofenac, Naproxen and Ibuprofen are found in the Bile of Wild Fish Caught Downstream of a Wastewater Treatment Plant. Environ. Sci. Technol. 2013, 47, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Removal and seasonal variability of selected analgesics/anti-inflammatory, anti-hypertensive/cardiovascular pharmaceuticals and UV filters in wastewater treatment plant. Environ. Sci. Pollut. Res. 2014, 21, 7578–7585. [Google Scholar] [CrossRef]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Dvořáková Březinova, T.; Vymazal, J.; Koželuh, M.; Kule, L. Occurrence and removal of ibuprofen and its metabolites in full-scale constructed wetlands treating municipal wastewater. Ecol. Eng. 2018, 120, 1–5. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Teva, F.; Leal, A.I. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration. Chem. Eng. J. 2010, 163, 264–272. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Maeng, S.K.; Fujioka, T.; Kennedy, M.; Amy, G. Proposing nanofiltration as acceptable barrier for organic contaminants in water reuse. J. Membr. Sci. 2010, 362, 334–345. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Urtiaga, A.M.; Pérez, G.; Ibáñez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Yangali Quintanilla, V.; Maeng, S.K.; Fujioka, T.; Kennedy, M.; Li, Z.; Amy, G. Nanofiltration vs. reverse osmosis for the removal of emerging organic contaminants in water reuse. Desalination Water Treat. 2011, 34, 50–56. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (PhACs): Mechanisms and role of membrane pore size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Sagawa, N.; Shikata, T. Are all polar molecules hydrophilic? Hydration numbers of nitro compounds and nitriles in aqueous solution. Phys. Chem. Chem. Phys. 2014, 16, 13262–13270. [Google Scholar] [CrossRef]
- Arola, K.; Ward, A.; Mänttäri, M.; Kallioinen, M.; Batstone, D. Transport of pharmaceuticals during electrodialysis treatment of wastewater. Water Res. 2019, 161, 496–504. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cai, X.; Chen, K.; Zheng, H.; Wang, C.; Zhang, K.; Lin, D.; Kong, L.; Liu, J. Study on the removal of organic micropollutants from aqueous and ethanol solutions by HAP membranes with tunable hydrophilicity and hydrophobicity. Chemosphere 2017, 174, 380–389. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Boller, M.; Schäfer, A.I. The role of NOM fouling for the retention of estradiol and ibuprofen during ultrafiltration. J. Membr. Sci. 2009, 329, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Kárászová, M.; Bourassi, M.; Gaálová, J. Membrane Removal of Emerging Contaminants from Water: Which Kind of Membranes Should We Use? Membranes 2020, 10, 305. [Google Scholar] [CrossRef]
- Căprărescu, S.; Zgârian, R.G.; Tihan, G.T.; Purcar, V.; Eftimie Totu, E.; Modrogan, C.; Chiriac, A.-L.; Nicolae, C.A. Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal. Polymers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Caprarescu, S.; Radu, A.-L.; Purcar, V.; Sarbu, A.; Vaireanu, D.-I.; Ianchis, R.; Ghiurea, M. Removal of Copper Ions from Simulated Wastewaters Using Different Bicomponent Polymer Membranes. Water Air Soil Pollut. 2014, 225, 2079. [Google Scholar] [CrossRef]
- José, C.; Briand, L.E.; Michlig, N.; Repetti, M.R.; Benedetich, C.; Cornaglia, L.M.; Bosko, M.L. Isolation of ibuprofen enantiomers and racemic esters through electrodialysis. J. Membr. Sci. 2021, 618, 118714. [Google Scholar] [CrossRef]
- Tristán, C.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Reverse Electrodialysis: Potential Reduction in Energy and Emissions of Desalination. Appl. Sci. 2020, 10, 7317. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Q.; Lu, H.; Wang, J.; Zhao, J.; Li, P. Electrodialysis with porous membrane for bioproduct separation: Technology, features, and progress. Food Res. Int. 2020, 137, 109343. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gutierrez, L.; Van Vooren, T.; Vanoppen, M.; Kazemabad, M.; Verliefde, A.; Cornelissen, E. Fate of organic micropollutants in reverse electrodialysis: Influence of membrane fouling and channel clogging. Desalination 2021, 512, 115114. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Comparison of the removal of hydrophobic trace organic contaminants by forward osmosis and reverse osmosis. Water Res. 2012, 46, 2683–2692. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Heiranian, M.; Elimelech, M. True driving force and characteristics of water transport in osmotic membranes. Desalination 2021, 520, 115360. [Google Scholar] [CrossRef]
- Zuo, K.; Wang, K.; DuChanois, R.M.; Fang, Q.; Deemer, E.M.; Huang, X.; Xin, R.; Said, I.A.; He, Z.; Feng, Y.; et al. Selective membranes in water and wastewater treatment: Role of advanced materials. Mater. Today 2021. [Google Scholar] [CrossRef]
- Gaálová, J.; Bourassi, M.; Soukup, K.; Trávníčková, T.; Bouša, D.; Sundararajan, S.; Losada, O.; Kasher, R.; Friess, K.; Sofer, Z. Modified Single-Walled Carbon Nanotube Membranes for the Elimination of Antibiotics from Water. Membranes 2021, 11, 720. [Google Scholar] [CrossRef]
- Gaálová, J.; Michel, M.; Bourassi, M.; Ladewig, B.P.; Kasal, P.; Jindřich, J.; Izák, P. Nafion membranes modified by cationic cyclodextrin derivatives for enantioselective separation. Sep. Purif. Technol. 2021, 266, 118538. [Google Scholar] [CrossRef]
- Bourassi, M.; Pasichnyk, M.; Oesch, O.; Sundararajan, S.; Trávničková, T.; Soukup, K.; Kasher, R.; Gaálová, J. Glycidyl and Methyl Methacrylate UV-Grafted PDMS Membrane Modification toward Tramadol Membrane Selectivity. Membranes 2021, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Bourassi, M.; Martin, E.; Bourre, M.; Fila, V.; Gaálová, J. Separation of Diethyl Phthalate From Water by Pervaporation. WSEAS Trans. Environ. Dev. 2021, 17, 81–87. [Google Scholar] [CrossRef]
- Xiong, G.; Cao, Y.; Guo, Z.; Jia, Q.; Tian, F.; Liu, L. The roles of different titanium species in TS-1 zeolite in propylene epoxidation studied by in situ UV Raman spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Das, P.; Zaman, A.; Das, P. Application of graphene oxide nanoplatelets for adsorption of Ibuprofen from aqueous solutions: Evaluation of process kinetics and thermodynamics. Process Saf. Environ. Prot. 2016, 101, 45–53. [Google Scholar] [CrossRef]
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.E.; Elimelech, M. Relating Nanofiltration Membrane Performance to Membrane Charge (Electrokinetic) Characteristics. Environ. Sci. Technol. 2000, 34, 3710–3716. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Role of electrostatic interactions in the retention of pharmaceutically active contaminants by a loose nanofiltration membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.-H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J. The role of membrane surface charge and solute physic-chemical properties in the rejection of organic acids by NF membranes. J. Membr. Sci. 2005, 249, 227–234. [Google Scholar] [CrossRef]
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Elam, J.W.; Routkevitch, D.; Mardilovich, P.P.; George, S.M. Conformal Coating on Ultrahigh-Aspect-Ratio Nanopores of Anodic Alumina by Atomic Layer Deposition. Chem. Mater. 2003, 15, 3507–3517. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Brožová, L.; Zazpe, R.; Otmar, M.; Přikryl, J.; Bulánek, R.; Žitka, J.; Krejčíková, S.; Izák, P.; Macak, J.M. Chiral Templating of Polycarbonate Membranes by Pinene Using the Modified Atomic Layer Deposition Approach. Langmuir 2020, 36, 12723–12734. [Google Scholar] [CrossRef]
- Martin-Gil, V.; López, A.; Hrabanek, P.; Mallada, R.; Vankelecom, I.F.J.; Fila, V. Study of different titanosilicate (TS-1 and ETS-10) as fillers for Mixed Matrix Membranes for CO2/CH4 gas separation applications. J. Membr. Sci. 2017, 523, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.Z.; Martin-Gil, V.; Supinkova, T.; Lambert, P.; Castro-Muñoz, R.; Hrabanek, P.; Kocirik, M.; Fila, V. Novel MMM using CO2 selective SSZ-16 and high-performance 6FDA-polyimide for CO2/CH4 separation. Sep. Purif. Technol. 2021, 254, 117582. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Dung, C.T. Mixed Matrix Membranes Based on PIMs for Gas Permeation: Principles, Synthesis, and Current Status. Chem. Eng. Commun. 2017, 204, 295–309. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; de la Iglesia, Ó.; Fíla, V.; Téllez, C.; Coronas, J.; Figoli, A. Graphene oxide—Filled polyimide membranes in pervaporative separation of azeotropic methanol–MTBE mixtures. Sep. Purif. Technol. 2019, 224, 265–272. [Google Scholar] [CrossRef]
- Jain, A.; Ahmad, M.Z.; Linkès, A.; Martin-Gil, V.; Castro-Muñoz, R.; Izak, P.; Sofer, Z.; Hintz, W.; Fila, V. 6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation. Nanomaterials 2021, 11, 668. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.-S.; O’Brien, G.S.; Kosar, W. Hydrophobic/hydrophilic PVDF/Ultem® dual-layer hollow fiber membranes with enhanced mechanical properties for vacuum membrane distillation. J. Membr. Sci. 2017, 523, 103–110. [Google Scholar] [CrossRef]
- Kim, R.I.; Shin, J.H.; Lee, J.S.; Lee, J.-H.; Lee, A.S.; Hwang, S.S. Tunable Crystalline Phases in UV-Curable PEG-Grafted Ladder-Structured Silsesquioxane/Polyimide Composites. Materials 2020, 13, 2295. [Google Scholar] [CrossRef]
- Fatyeyeva, K.; Dahi, A.; Chappey, C.; Langevin, D.; Valleton, J.-M.; Poncin-Epaillard, F.; Marais, S. Effect of cold plasma treatment on surface properties and gas permeability of polyimide films. RSC Adv. 2014, 4, 31036–31046. [Google Scholar] [CrossRef]
- Fouladivanda, M.; Karimi-Sabet, J.; Abbasi, F.; Moosavian, M.A. Step-by-step improvement of mixed-matrix nanofiber membrane with functionalized graphene oxide for desalination via air-gap membrane distillation. Sep. Purif. Technol. 2021, 256, 117809. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Botas, J.A.; Gutierrez, F.J. Characterization of adsorptive and hydrophobic properties of silicalite-1, ZSM-5, TS-1 and Beta zeolites by TPD techniques. Sep. Purif. Technol. 2007, 54, 1–9. [Google Scholar] [CrossRef]
- Paul, S.C.; Githinji, L.J.; Ankumah, R.O.; Willian, K.R.; Pritchett, G. Sorption Behavior of Ibuprofen and Naproxen in Simulated Domestic Wastewater. Water Air Soil Pollut. 2014, 225, 1–11. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Hwang, Y.; Heo, Y.; Yoo, Y.; Kim, J. The addition of functionalized graphene oxide to polyetherimide to improve its thermal conductivity and mechanical properties. Polym. Adv. Technol. 2014, 25, 1155–1162. [Google Scholar] [CrossRef]
- Moretti, G.; Salvi, A.M.; Guascito, M.R.; Langerame, F. An XPS study of microporous and mesoporous titanosilicates. Surf. Interface Anal. 2004, 36, 1402–1412. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Progress on Incorporating Zeolites in Matrimid(®)5218 Mixed Matrix Membranes towards Gas Separation. Membranes 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- De Luca, P.; Bernaudo, I.; Elliani, R.; Tagarelli, A.; Nagy, J.B.; Macario, A. Industrial Waste Treatment by ETS-10 Ion Exchanger Material. Materials 2018, 11, 2316. [Google Scholar] [CrossRef] [Green Version]
- Jedynak, K.; Szczepanik, B.; Rędzia, N.; Slomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water 2019, 11, 1099. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.-F.; Jennifer, R.; Dominic, S.; Eccleston, G. A Comparative Study of Transmembrane Diffusion and Permeation of Ibuprofen across Synthetic Membranes Using Franz Diffusion Cells. Pharmaceutics 2010, 2, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarveiya, V.; Templeton, J.F.; Benson, H.A.E. Ion-pairs of ibuprofen: Increased membrane diffusion. J. Pharm. Pharmacol. 2004, 56, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Bandpei, A.; Eslami, A.; Kazemian, H.; Zarrabi, M.; Al-Musawi, T.J. A high density 3-aminopropyltriethoxysilane grafted pumice-derived silica aerogel as an efficient adsorbent for ibuprofen: Characterization and optimization of the adsorption data using response surface methodology. Environ. Technol. Innov. 2020, 18, 100642. [Google Scholar] [CrossRef]
- Farrington, K.; Regan, F. Molecularly imprinted sol gel for ibuprofen: An analytical study of the factors influencing selectivity. Talanta 2009, 78, 653–659. [Google Scholar] [CrossRef] [PubMed]

















 | Molar mass (g/mol) | 206.3 |
| logKow 1 | 3.97 | |
| Melting point (°C) | 76 | |
| Pressure sat (25 °C) Pa | 0.0063 | |
| pKa | 4.9 | |
| Water solubility (mg/L; 25 °C) | 21 |
| Membrane | Diameter (mm) | Thickness (µm) | Weight (mg) | Surface (cm2) |
|---|---|---|---|---|
| PDMS | 30 | 200 | 95.9 | 7.07 |
| PP | 38 | 4 | 4.2 | 11.34 |
| PS | 38 | 29 | 34.7 | 11.34 |
| Membrane | Material | Filler |
|---|---|---|
| Ultem | Polyetherimide | None |
| Graphene oxide 0.5 wt. % | ||
| Graphene oxide 2.5 wt. % | ||
| Graphene oxide 5 wt. % | ||
| Matrimid | Polyimide | None |
| TS-1 zeolite 10 wt. % | ||
| TS-1 zeolite 20 wt. % | ||
| TS-1 zeolite 30 wt. % | ||
| ETS-10 zeolite 30 wt. % | ||
| 6FDA-DAM:DABA 3:1 | sub-Tg thermally annealed 6FDA-2,4,6-trimethyl-1,3-diaminobenzene (DAM): 3,5-diaminobenzoic acid (DABA)(3:1) | None Graphene oxide 2.8 wt. % |
| Graphene oxide 5 wt. % |
| Membrane | Atomic concentration (%) | |||||
|---|---|---|---|---|---|---|
| Carbon | Oxygen | Nitrogen | Silicon | Sodium | Chlorine | |
| PDMS | 70.32 | 8.25 | 13.76 | 6.82 | 0.85 | - |
| Blank * | 69.64 | 7.44 | 14.81 | 6.64 | 0.84 | 0.64 |
| ALD modified | 60.44 | 16.23 | 4.62 | 16.36 | 1.20 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourassi, M.; Kárászová, M.; Pasichnyk, M.; Zazpe, R.; Herciková, J.; Fíla, V.; Macak, J.M.; Gaálová, J. Removal of Ibuprofen from Water by Different Types Membranes. Polymers 2021, 13, 4082. https://doi.org/10.3390/polym13234082
Bourassi M, Kárászová M, Pasichnyk M, Zazpe R, Herciková J, Fíla V, Macak JM, Gaálová J. Removal of Ibuprofen from Water by Different Types Membranes. Polymers. 2021; 13(23):4082. https://doi.org/10.3390/polym13234082
Chicago/Turabian StyleBourassi, Mahdi, Magda Kárászová, Mariia Pasichnyk, Raul Zazpe, Jana Herciková, Vlastimil Fíla, Jan M. Macak, and Jana Gaálová. 2021. "Removal of Ibuprofen from Water by Different Types Membranes" Polymers 13, no. 23: 4082. https://doi.org/10.3390/polym13234082