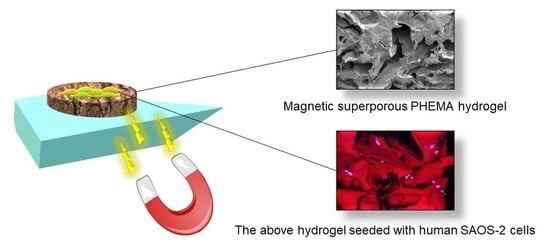
Magnetic Superporous Poly(2-hydroxyethyl methacrylate) Hydrogel Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of γ-Fe2O3 Nanoparticles
2.3. Preparation of Nonmagnetic and Magnetic Superporous Poly(2-Hydroxyethyl Methacrylate) (PHEMA) and Poly(2-Hydroxyethyl Methacrylate-co-2-Dimethylaminoethyl Methacrylate) [P(HEMA-DMAEMA)] Hydrogels
2.4. Characterization of Hydrogels
2.5. Tissue Culture Experiments
2.6. Cell Metabolic Activity
2.7. Confocal Microscopy
3. Results and Discussion
3.1. Magnetic Superporous PHEMA Hydrogel Scaffolds
3.2. In Vitro Testing of PHEMA-Based Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matos, A.M.; Gonçalves, A.I.; El Haj, A.J.; Gomes, M.E. Magnetic biomaterials and nano-instructive tools as mediators of tendon mechanotransduction. Nanoscale Adv. 2020, 2, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Ortolani, A.; Bianchi, M.; Mosca, M.; Caravelli, S.; Fuiano, M.; Marcacci, M.; Russo, A. The prospective opportunities offered by magnetic scaffolds for bone tissue engineering: A review. Joints 2017, 4, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Panseri, S.; Russo, A.; Giavaresi, G.; Sartori, M.; Veronesi, F.; Fini, M.; Salter, D.; Ortolani, A.; Strazzari, A.; Visani, A.; et al. Innovative magnetic scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 2278–2286. [Google Scholar] [CrossRef]
- Zhao, X.H.; Kim, J.; Cezar, C.A.; Huebsch, N.; Lee, K.; Bouhadir, K.; Mooney, D.J. Active scaffolds for on-demand drug and cell delivery. Proc. Natl. Acad. Sci. USA 2011, 108, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.H.; Liu, T.Y.; Tsai, C.H.; Chen, S.Y. Preparation and characterization of magnetic ferroscaffolds for tissue engineering. J. Magn. Magn. Mater. 2007, 310, 2871–2873. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, B.Q.; Zhou, Y.; Jia, D.C. Chitosan-induced synthesis of magnetite nanoparticles via iron ions assembly. Polym. Adv. Technol. 2008, 19, 1256–1261. [Google Scholar] [CrossRef]
- Liu, H.X.; Wang, C.Y.; Gao, Q.X.; Liu, X.X.; Tong, Z. Magnetic hydrogels with supracolloidal structures prepared by suspension polymerization stabilized by Fe2O3 nanoparticles. Acta Biomater. 2010, 6, 275–281. [Google Scholar] [CrossRef]
- Bannerman, A.D.; Li, X.Y.; Wan, W.K. A “degradable” poly(vinyl alcohol) iron oxide nanoparticle hydrogel. Acta Biomater. 2017, 58, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Xu, W. Magneto-mechanical properties of polydimethylsiloxane composites with a binary magnetic filler system. Polym. Compos. 2019, 40, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xia, J.Y.; Pang, X.L.; Zhao, M.; Wang, B.Q.; Yang, L.L.; Wan, H.S.; Wu, J.B.; Fu, S.Z. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C 2016, 73, 537–543. [Google Scholar] [CrossRef]
- Horák, D. Application of poly(2-hydroxyethyl methacrylate) in medicine. In Polymers and Composites: Synthesis, Properties, and Applications, Polymer Yearbook; Pethrick, R.A., Zaikov, G.E., Horák, D., Eds.; Nova Science Publishers: New York, NY, USA, 2007; Volume 21, pp. 1–33. [Google Scholar]
- Kopeček, J. Hydrogels from soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. A 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Nam, S.H.; Koh, W.-G. Preparation of collagen-immobilized poly(ethylene glycol)/poly(2-hydroxyethyl methacrylate) interpenetrating network hydrogels for potential application of artificial cornea. J. Appl. Polym. Sci. 2012, 123, 637–645. [Google Scholar] [CrossRef]
- Hidzir, N.M.; Radzali, N.A.M.; Rahman, I.A.; Shamsudin, S.A. Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl. Eng. Technol. 2020, 52, 2320–2327. [Google Scholar] [CrossRef]
- Passos, M.F.; Dias, D.R.C.; Bastos, G.N.T.; Jardini, A.L.; Benatti, A.C.B.; Dias, C.G.B.T.; Maciel Filho, R. pHEMA hydrogels: Synthesis, kinetics and in vitro tests. J. Therm. Anal. Calorim. 2016, 125, 361–368. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Park, K.; Chen, J.; Park, H. Superporous hydrogel composites: A new generation of hydrogels with fast swelling kinetics, high swelling ratio and high mechanical strength. In Polymeric Drugs and Drug Delivery Systems; Ottenbrite, R.M., Kim, S.W., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 145–156. [Google Scholar]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. B 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Bao, G.; Jiang, T.; Ravanbakhsh, H.; Reyes, A.; Ma, Z.; Strong, M.; Wang, H.; Kinsella, J.M.; Li, J.; Mongeau, L. Triggered micropore-forming bioprinting of porous viscoelastic hydrogels. Mater. Horiz. 2020, 7, 2336–2347. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, G.; Du, G.; Zhan, D.; Cong, Y.; Cheng, Y.; Fu, J. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf. B 2013, 103, 318–325. [Google Scholar]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darouie, S.; Majd, S.A.; Rahimi, F.; Hashemi, E.; Kabirsalmani, M.; Dolatshahi-Pirouz, A.; Arpanaei, A. The fate of mesenchymal stem cells is greatly influenced by the surface chemistry of silica nanoparticles in 3D hydrogel-based culture systems. Mater. Sci. Eng. C 2020, 106, 110259. [Google Scholar] [CrossRef]
- Macková, H.; Plichta, Z.; Proks, V.; Kotelnikov, I.; Kučka, J.; Hlídková, H.; Horák, D.; Kubinová, Š.; Jiráková, K. RGDS- and SIKVAVS-modified superporous poly(2-hydroxyethyl methacrylate) scaffolds for tissue engineering applications. Macromol. Biosci. 2016, 16, 1621–1631. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Wang, J.H.C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomech. Model Mechanobiol. 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Mokhtari-Jafari, F.; Amoabediny, G.; Dehghan, M.M. Role of biomechanics in vascularization of tissue-engineered bones. J. Biomech. 2020, 110, 109920. [Google Scholar] [CrossRef] [PubMed]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic bone tissue engineering graft substitutes: What is the future? Injury 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Zasońska, B.A.; Boiko, N.; Horák, D.; Klyuchivska, O.; Macková, H.; Beneš, M.J.; Babič, M.; Trchová, M.; Hromádková, J.; Stoika, R. The use of hydrophilic poly(N,N-dimethylacrylamide) for promoting engulfment of magnetic γ-Fe2O3 nanoparticles by mammalian cells. J. Biomed. Nanotechnol. 2013, 9, 479–491. [Google Scholar] [CrossRef]
- Dunlop, D.; Özdemir, Ö. Rock Magnetism: Fundamentals and Frontiers; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Porosimeter Pascal 140 and Pascal 440, Instruction Manual; ThermoFinnigan: Rodano, Italy, 1996; p. 8.
- Rigby, S.P.; Barwick, D.; Fletcher, R.S.; Riley, S.N. Interpreting mercury porosimetry data for catalyst supports using semi-empirical alternatives to the Washburn equation. Appl. Catal. A 2003, 238, 303–318. [Google Scholar] [CrossRef]
- Taktak, F.; Yildiz, M.; Sert, H.; Soykan, C. A novel triple-responsive hydrogels based on 2-(dimethylamino) ethyl methacrylate by copolymerization with 2-(N-morpholino) ethyl methacrylate. J. Macromol. Sci. A 2015, 52, 39–46. [Google Scholar] [CrossRef]
- Horák, D.; Hlídková, H.; Hradil, J.; Lapčíková, M.; Šlouf, M. Superporous poly(2-hydroxyethyl methacrylate) based scaffolds: Preparation and characterization. Polymer 2008, 49, 2046–2054. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Salazar, J.S.; Perez, L.; de Abril, O.; Phuoc, L.T.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.-M.; Begin-Colin, S.; Pourroy, G. Magnetic iron oxide nanoparticles in 10-40 nm range: Composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Davardoostmanesh, M.; Hossein, A.; Elaheh, K.G.; Azadeh, M.; Elnaz, S. Graphitic carbon nitride nanosheets prepared by electrophoretic size fractionation as an anticancer agent against human bone carcinoma. Mater. Sci. Eng. C 2020, 111, 110803. [Google Scholar] [CrossRef]
- Abdelrahman, R.M.; Abdel-Mohsen, A.M.; Zboncak, M.; Frankova, J.; Lepcio, P.; Kobera, L.; Steinhart, M.; Pavlinak, D.; Spotaz, Z.; Sklenářová, R.; et al. Hyaluronan biofilms reinforced with partially deacetylated chitin nanowhiskers: Extraction, fabrication, in-vitro and antibacterial properties of advanced nanocomposites. Carbohydr. Polym. 2020, 235, 115951. [Google Scholar] [CrossRef] [PubMed]
- Çetin, D.; Kahraman, A.S.; Gümüşderelioğlu, M. Novel scaffolds based on poly(2-hydroxyethyl methacrylate) superporous hydrogels for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 1157–1178. [Google Scholar]
- Hanak, B.W.; Hsieh, C.Y.; Donaldson, W.; Browd, S.R.; Lau, K.K.S.; Shain, W. Reduced cell attachment to poly(2-hydroxyethyl methacrylate)-coated ventricular catheters in vitro. J. Biomed. Mater. Res. B 2017, 106, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta Bioenerg. 1999, 1413, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-X.; Tang, W.-L.; Wang, X.-X. Superparamagnetic iron oxide nanoparticles may affect endothelial progenitor cell migration ability and adhesion capacity. Cytotherapy 2010, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.C.; Layrolle, P.; Libouban, H.; Filmon, R.; Legeay, G.; Cincu, C.; Baslé, M.F.; Chappard, D. Preparation of macroporous poly(2-hydroxyethyl methacrylate) with interconnected porosity. J. Optoelectron. Adv. Mater. 2007, 9, 2125–2129. [Google Scholar]






| Hydrogel | Most Frequent Pore Diameter a (µm) | Pore Volume a (ml/g) | Porosity a (%) | Compressive Modulus (kPa) | Compressive Strength (kPa) | Toughness (mJ/mm3) |
|---|---|---|---|---|---|---|
| PHEMA | 84 | 0.23 | 22 | 88.7 ± 15.9 | 46.5 ± 4.9 | 0.122 ± 0.02 |
| P(HEMA-DMAEMA) | 102 | 0.63 | 42 | 45.9 ± 8.5 | 16.5 ± 0.8 | 0.055 ± 0.01 |
| PHEMA@γ-Fe2O3 | 93 | 0.40 | 32 | 98.5 ± 7.3 | 61.7 ± 8.0 | 0.154 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasońska, B.A.; Brož, A.; Šlouf, M.; Hodan, J.; Petrovský, E.; Hlídková, H.; Horák, D. Magnetic Superporous Poly(2-hydroxyethyl methacrylate) Hydrogel Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 1871. https://doi.org/10.3390/polym13111871
Zasońska BA, Brož A, Šlouf M, Hodan J, Petrovský E, Hlídková H, Horák D. Magnetic Superporous Poly(2-hydroxyethyl methacrylate) Hydrogel Scaffolds for Bone Tissue Engineering. Polymers. 2021; 13(11):1871. https://doi.org/10.3390/polym13111871
Chicago/Turabian StyleZasońska, Beata A., Antonín Brož, Miroslav Šlouf, Jiří Hodan, Eduard Petrovský, Helena Hlídková, and Daniel Horák. 2021. "Magnetic Superporous Poly(2-hydroxyethyl methacrylate) Hydrogel Scaffolds for Bone Tissue Engineering" Polymers 13, no. 11: 1871. https://doi.org/10.3390/polym13111871